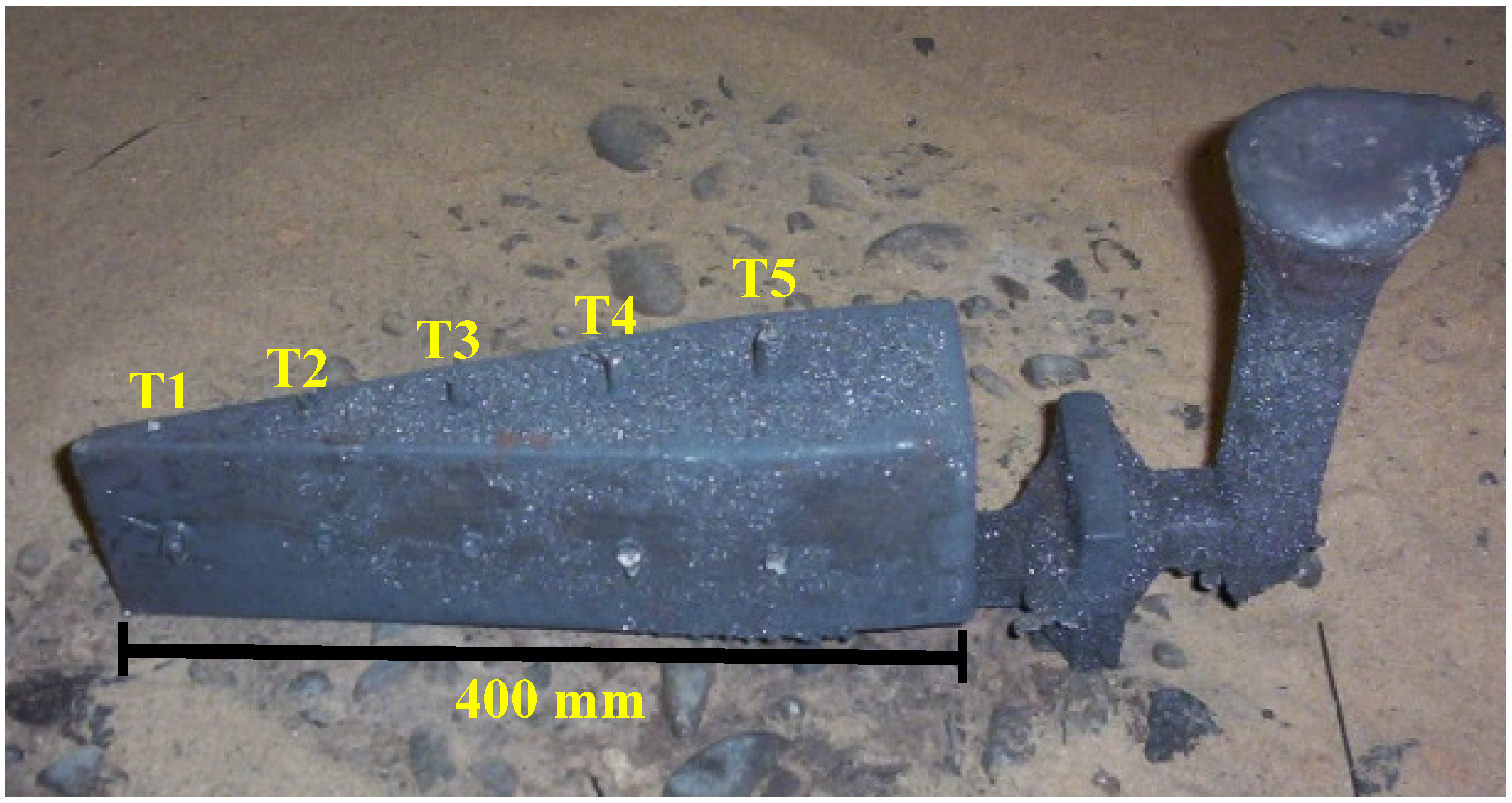
4.1. Experimental
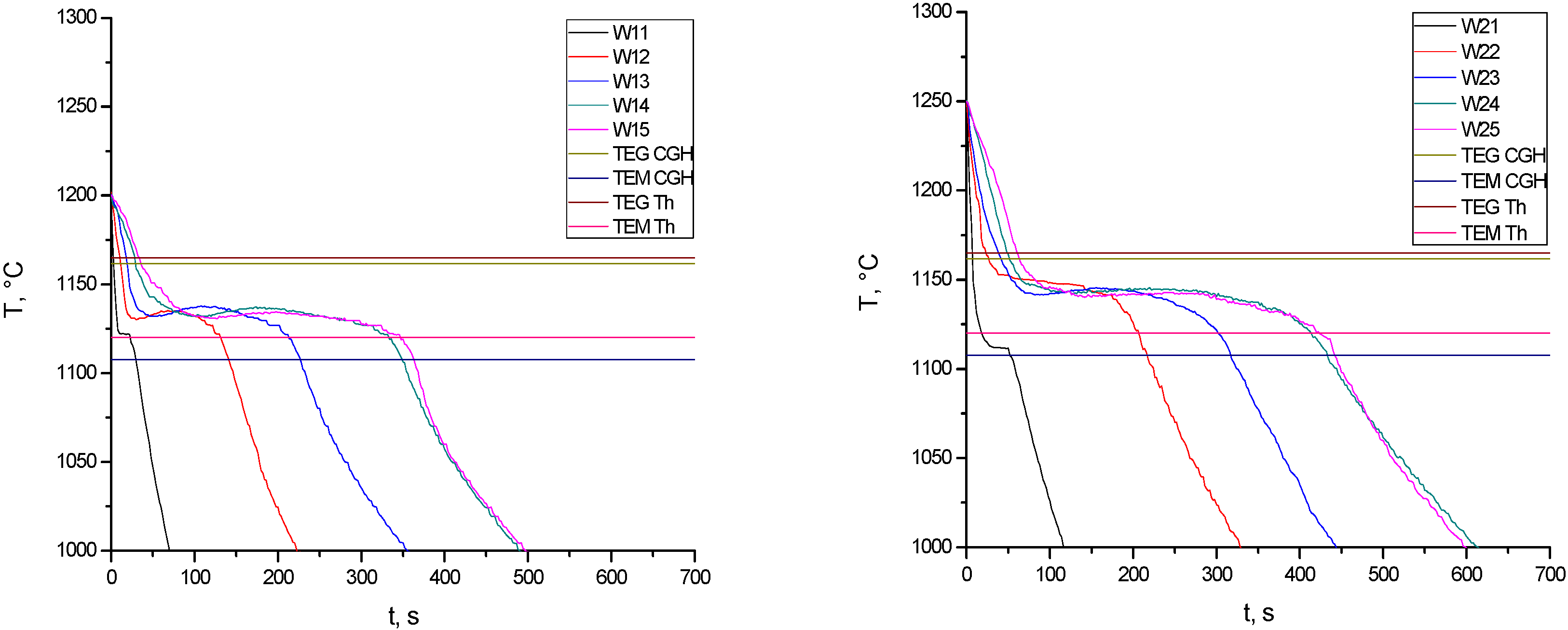
Figure 4 shows the experimental cooling curves of the five analyzed points in experiences W1 and W2 together with the stable (
TEG) and metastable (
TEM) eutectic temperatures. These temperatures were obtained using an experimental correlation derived from the works of Chipman [
25], Gustafson [
26] and Henschel [
27], among others; this correlation is identified as CGH. The same temperatures were obtained with the software Thermocalc [
28], labeled Th, which does not consider the phosphorus content in its database. When comparing the cooling process of each point in both experiences, it is observed that the higher pouring temperature of W2 produces longer solidification times than those of W1 and, in addition, the temperatures of the different reactions in experience W2 are slightly higher than those of W1. In both experiences, it is also seen that, particularly in the first cooling stage, as the thickness of the part increases, the cooling rate decreases. As a consequence of this, a continuous increase in the solidification time of the five points along the part is observed. As expected, the points located at the thicker part has a longer solidification time than the ones located at the thinner parts, which evidences the effect of wedge thickness on the cooling process. This effect is also influenced by the pouring temperature,
i.e., as the pouring temperature increases, the solidification times increases as well.
As mentioned above, the stable and metastable eutectic temperatures are included in
Figure 4 with the aim of analyzing and evaluating the cementite nucleation in each point along the part. The different temperatures obtained with both methods (
i.e., correlations CGH and Th) can clearly be appreciated. It is observed that, according to the values obtained with the correlation Th in the thinner parts of the wedge (locations W11 and W21), the metastable reaction would take place since the cooling curves of these points intersect the metastable eutectic temperature. On the other hand, it should be noted that this effect is not predicted in the same way with the CGH correlation. Considering this last correlation, it is seen that all the temperature-time curves fall within the range
TEG-
TEM, which means that, regardless of what is predicted by the Th correlation, all points of the wedge would solidify according to the stable Fe-graphite diagram. However, from the metallographic analysis carried out at the different points, the validity of the prediction given by the Th correlation is confirmed since, as mentioned below, a high fraction of white eutectic was found in locations W11 and W21.
Figure 4.
Experimental cooling curves of experiences W1 and W2.
Table 3 summarizes the results obtained from the standard procedure carried out to determine the characteristic times and temperatures of the solidification experiences. The eutectic undercooling ∆
TEG =
TEG −
TEU, the recalescence amplitude ∆
Tr =
TER −
TEU and the elapsed time from the eutectic reaction until the solidification ending ∆
tES =
t(
TES) –
t(
TEG) are also included. It can be seen that, as the pouring temperature increases, ∆
Tr decreases and ∆
tES increases.
Table 3.
Experimental cooling curves characterization for experiences W1 and W2.
| Point | tTEG, s | TEG, °C | tTEU, s | TEU, °C | tTER, s | TER, °C | tTES, s | TES, °C | ∆Tr | ∆TEG | ∆tES | dT/dt, °C/s |
|---|
| W11 | 2.1 | 1172.8 | 11.8 | 1121.9 | 15.6 | 1122.5 | 37.1 | 1091.3 | 0.5 | 50.9 | 54.1 | 13.9 |
| W12 | 3.8 | 1188.9 | 31.5 | 1130.1 | 77.8 | 1135.0 | 150.1 | 1096.4 | 5.0 | 58.8 | 174.5 | 3.7 |
| W13 | 10.8 | 1181.6 | 51.4 | 1131.9 | 113.9 | 1137.6 | 238.0 | 1094.8 | 5.7 | 49.7 | 241.5 | 2.1 |
| W14 | 15.7 | 1181.9 | 101.9 | 1132.1 | 175.9 | 1137.2 | 356.0 | 1100.8 | 5.2 | 49.9 | 338.5 | 1.3 |
| W15 | 20.0 | 1183.2 | 127.4 | 1130.9 | 198.4 | 1134.3 | 372.9 | 1095.3 | 3.5 | 52.3 | 355.9 | 1.2 |
| W21 | 5.9 | 1180.4 | 35.0 | 1111.5 | 49.8 | 1111.9 | 65.8 | 1087.0 | 0.4 | 68.9 | 59.9 | 12.1 |
| W22 | 17.4 | 1180.6 | ---- | ---- | ---- | ---- | 224.6 | 1098.7 | ---- | ---- | 207.2 | 3.3 |
| W23 | 20.2 | 1190.1 | 88.4 | 1141.6 | 157.0 | 1145.3 | 316.3 | 1108.8 | 3.7 | 48.6 | 296.1 | 1.7 |
| W24 | 34.3 | 1191.4 | 129.8 | 1142.5 | 219.9 | 1145.5 | 433.0 | 1107.9 | 3.0 | 48.9 | 398.6 | 1.5 |
| W25 | 50.4 | 1182.4 | 141.1 | 1140.4 | 244.7 | 1142.9 | 438.3 | 1113.9 | 2.5 | 42.0 | 387.9 | 1.3 |
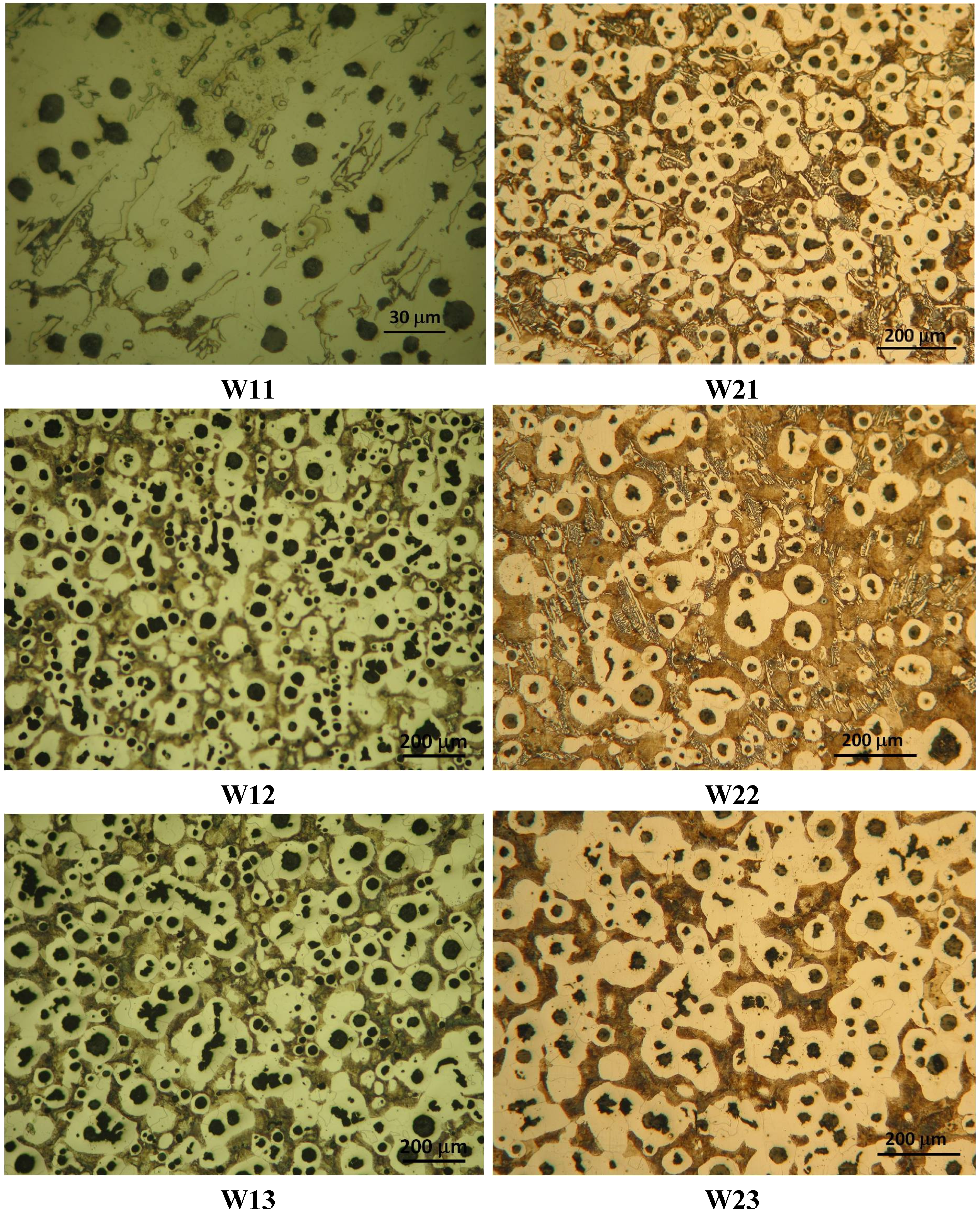
The as-cast microstructures at the different locations in experiences W1 and W2 are shown in
Figure 5. The experimental phase fractions for each of those points are summarized in
Table 4. In both cases it is observed that, as the pouring temperature increases, the nodule density decreases. This would be due to the effect of the high pouring temperature and the longer time that the molten metal is submitted to this high temperature, producing the growth of the graphite nodules which relates to a lower nodule density. As can be seen in
Figure 5, a continuous growth of the graphite nodules is produced from the thinner to the thicker parts of the wedges because of the effect of both cooling rate and pouring temperature on the nucleation and growth time of a graphite particle during the solidification process. As also can be seen in this figure, in the thinner parts of both wedges, random carbides distributed in the structure are observed which, unlike the experimental correlation CGH, is also predicted by Thermocalc. This can be seen in
Figure 4, where the cooling curve of points W11 and W21 do not intersect the metastable eutectic temperature curves calculated with this correlation (CGH), thus the white eutectic nucleation is not allowed to succeed. It is important to point out that the presence of carbides is also observed in point W22, a fact that according to
Figure 4 would not be plausible. Despite this, it is necessary to take into account the presence of alloying elements in the alloy, mainly manganese and chromium. These elements are strong carbide promoters [
29] which, together with the high cooling rate in the thinner sections of the part, could be the reason for the presence of this hard and brittle phase in those zones, even in the 20 mm thick part of wedge W2 (point W21). Towards the thicker parts of the wedges the cementite phase is no longer observed. In these positions, the microstructure is mainly composed by variable quantities of pearlite, ferrite and graphite nodules that depend on the pouring temperature, the cooling rate and the chemical composition of the alloy. As a consequence, when comparing the microstructure of the thinner parts of the wedges to the other locations, there is a change in the graphite fraction, since part of the available carbon forms carbides, producing the stable phase fraction to be lowered; see
Table 4. Regarding the phases present in each point, it can be seen that in both cases the carbides fraction is around 5% and part of a ferritic-pearlitic matrix with variables quantities according to the cooling conditions in each point. Towards the thicker parts of the wedges (from W12 upwards for W1 and from W23 upwards for W2), no carbides are observed in the final solidification microstructure of the parts.
Figure 5.
Solidification microstructure at the five points in experiences W1 and W2.
Table 4.
Experimental phase fraction at different locations in experiences W1 and W2.
| Point | Pouring T, °C | % F | % G | % P | % Fe3C |
|---|
| W11 | 1200 | 76.9 | 6.9 | 11.6 | 4.6 |
| W12 | 1200 | 59.9 | 12.0 | 28.1 | 0 |
| W13 | 1200 | 59.2 | 13.2 | 27.6 | 0 |
| W14 | 1200 | 61.6 | 12.4 | 26.0 | 0 |
| W15 | 1200 | 68.6 | 12.8 | 18.6 | 0 |
| W21 | 1250 | 42.3 | 9.4 | 42.5 | 5.8 |
| W22 | 1250 | 36.9 | 5.6 | 51.2 | 6.3 |
| W23 | 1250 | 62.7 | 7.7 | 29.6 | 0 |
| W24 | 1250 | 62.8 | 6.8 | 30.4 | 0 |
| W25 | 1250 | 53.0 | 9.4 | 37.6 | 0 |
4.2. Numerical Simulation and Experimental Validation
The nucleation parameters
b and
c were chosen to be the ones with which the better numerical-experimental adjustment was achieved,
i.e., those exhibiting the lower experimental-numerical discrepancy for both the cooling curves and the graphite nodule counts. The parameters that simultaneously minimize this error for experiences W1 and W2 were
b = 4.0 × 10
12 nuclei/(m °C s) and
c = 340 °C. The numerical results obtained with these parameters are compared to the experimental measurements presented in the previous section. They are summarized in
Table 5, considering the total nodule count at each point together with the numerical-experimental error, computed as:
It can be seen that, as the pouring temperature increases, the nodule density decreases. This means that bigger nodules are produced because of the effect of a prolonged growth time of these particles in contact with the liquid phase giving rise to a lower quantity of large graphite particles.
Table 5.
Error between the average experimental and numerical nodule counts obtained with b = 4.0 × 1012 nuclei/(m °C s) and c = 340 °C.
| Point | Experimental nodule count NV (nuclei/m3) | Numerical nodule count NV (nuclei/m3) | Error |
|---|
| W11 | 2.47 × 1013 | 1.12 × 1013 | 0.30 |
| W12 | 5.45 × 1012 | 1.01 × 1013 | 0.73 |
| W13 | 6.53 × 1012 | 8.23 × 1012 | 0.07 |
| W14 | 6.75 × 1012 | 6.82 × 1012 | 0.00 |
| W15 | 7.62 × 1012 | 8.22 × 1012 | 0.01 |
| W21 | 6.28 × 1012 | 1.14 × 1013 | 0.66 |
| W22 | 3.47 × 1012 | 9.38 × 1012 | 2.90 |
| W23 | 1.46 × 1012 | 7.96 × 1012 | 19.82 |
| W24 | 2.00 × 1012 | 6.48 × 1012 | 5.02 |
| W25 | 1.23 × 1012 | 7.82 × 1012 | 28.71 |
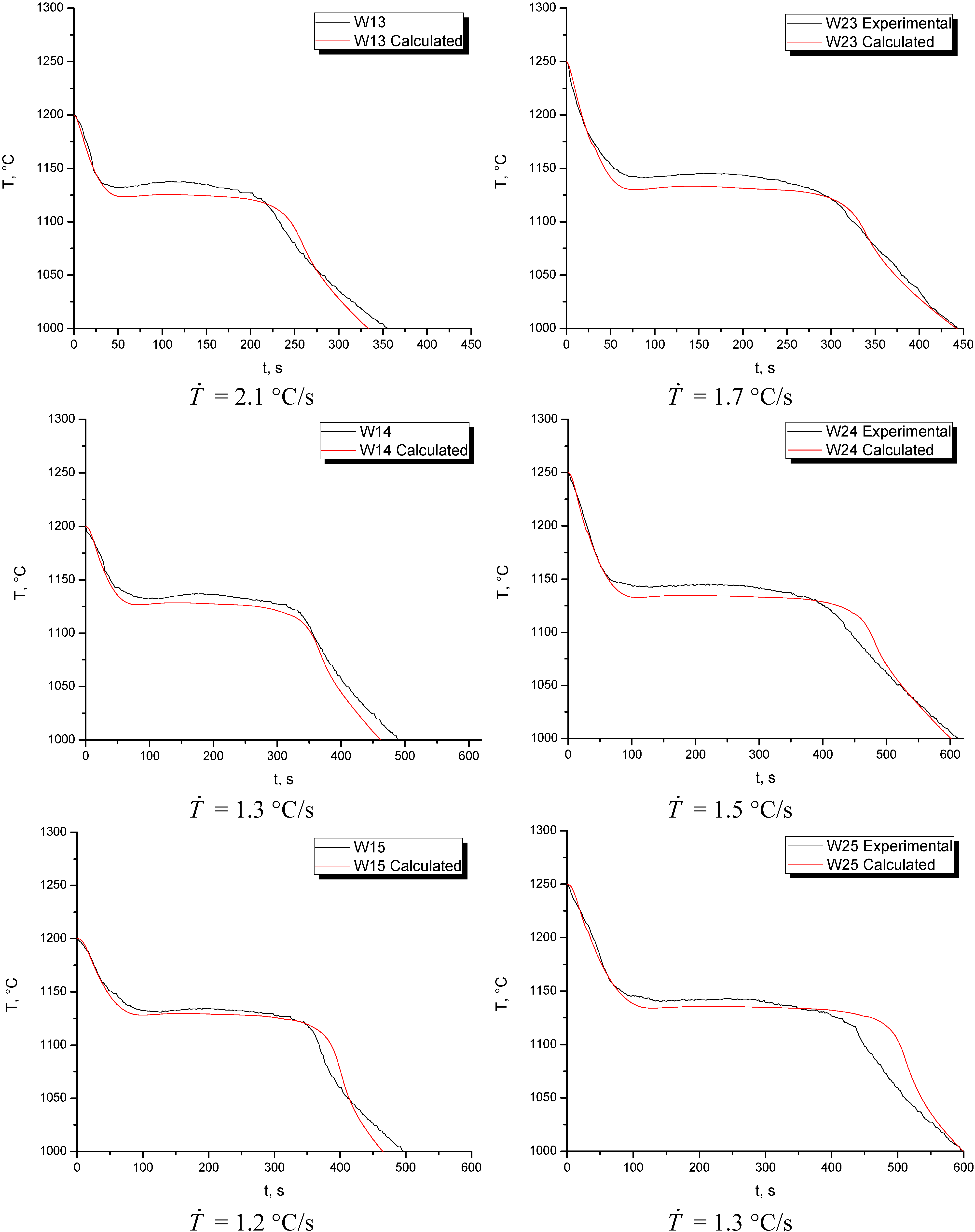
Once the nucleation parameters were determined,
Figure 6 plots the experimental and numerical cooling curves for the five points in experiences W1 and W2 together with the experimental cooling rate at the initial stage of cooling. It can be seen that in all the locations, because of the lower pouring temperature, the initial cooling rates are higher in W1 than in W2, which produces the mentioned difference in the graphite nodules size. In both cases, it is observed that in the thinner points of the wedge, the numerical-experimental fit is not as desired. However, the fit improves towards the thicker parts. It is also observed that, as the pouring temperature increases, the solidification time increases as well. In all the locations for experience W1, the computed cooling curves predict a solidification time very similar to the experimental one. It is also notable that in all the locations for experience W1, the calculated cooling curves are located below the experimental ones. The temperature for the eutectic reaction is not well-simulated from point W11 to W13 as in points W14 and W15 where the fit improves slightly. Besides this, although in all the locations the experimental and numerical plateaus differ slightly in temperature, the time extension of such plateaus is very similar, especially in the thick part of the wedge,
i.e., points W14 and W15. At the end of the solidification process, the slopes of the experimental and numerical cooling curves are very similar which means that, in general, the numerical-experimental fit is very acceptable. At this point, it can be established that the computed results improve in the thicker parts of the wedge. For experience W2, it can be seen that in all points the calculated cooling curves fall below the experimental ones, with the exception of point W21, where the opposite is seen. In this experience, the best and worse fits were found in points W23 and W22, respectively. For point W23, the numerical and experimental cooling curves are practically superimposed. Regarding characteristic times, the numerical-experimental fit for the eutectic reaction is very acceptable, especially in the thicker parts of the wedge. Besides this, the extensions of the thermal plateaus and the slopes of the calculated and experimental cooling curves at the initial and final stages of the cooling process are very similar, which further improve at the points where the cooling rate is lower. In both experiences, the numerical simulation also predicts longer solidification times for the points located in the thicker part of the sample compared to those located in the thinner part of it.
Figure 6.
Numerical and experimental cooling curves in experiences W1 and W2.
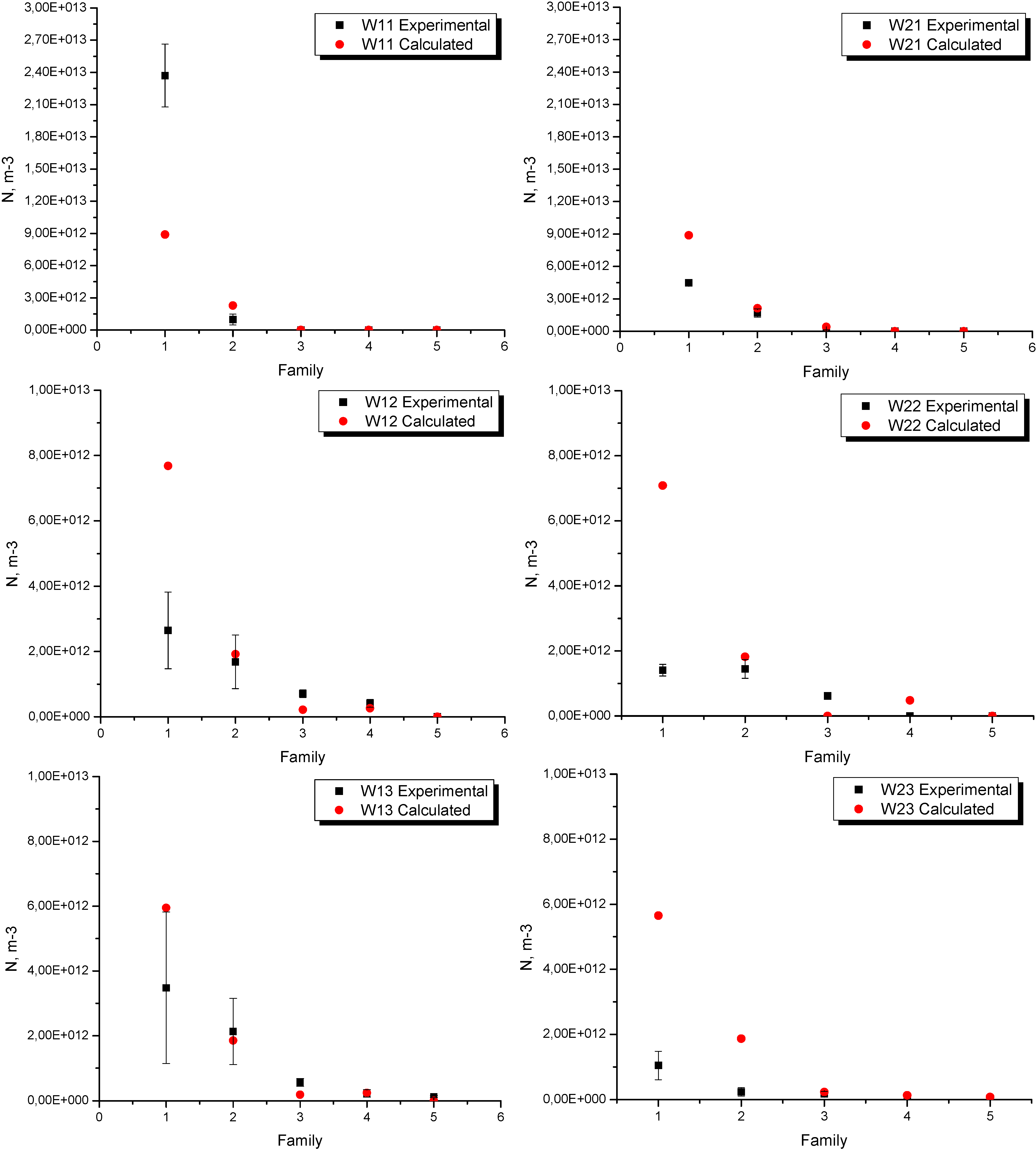
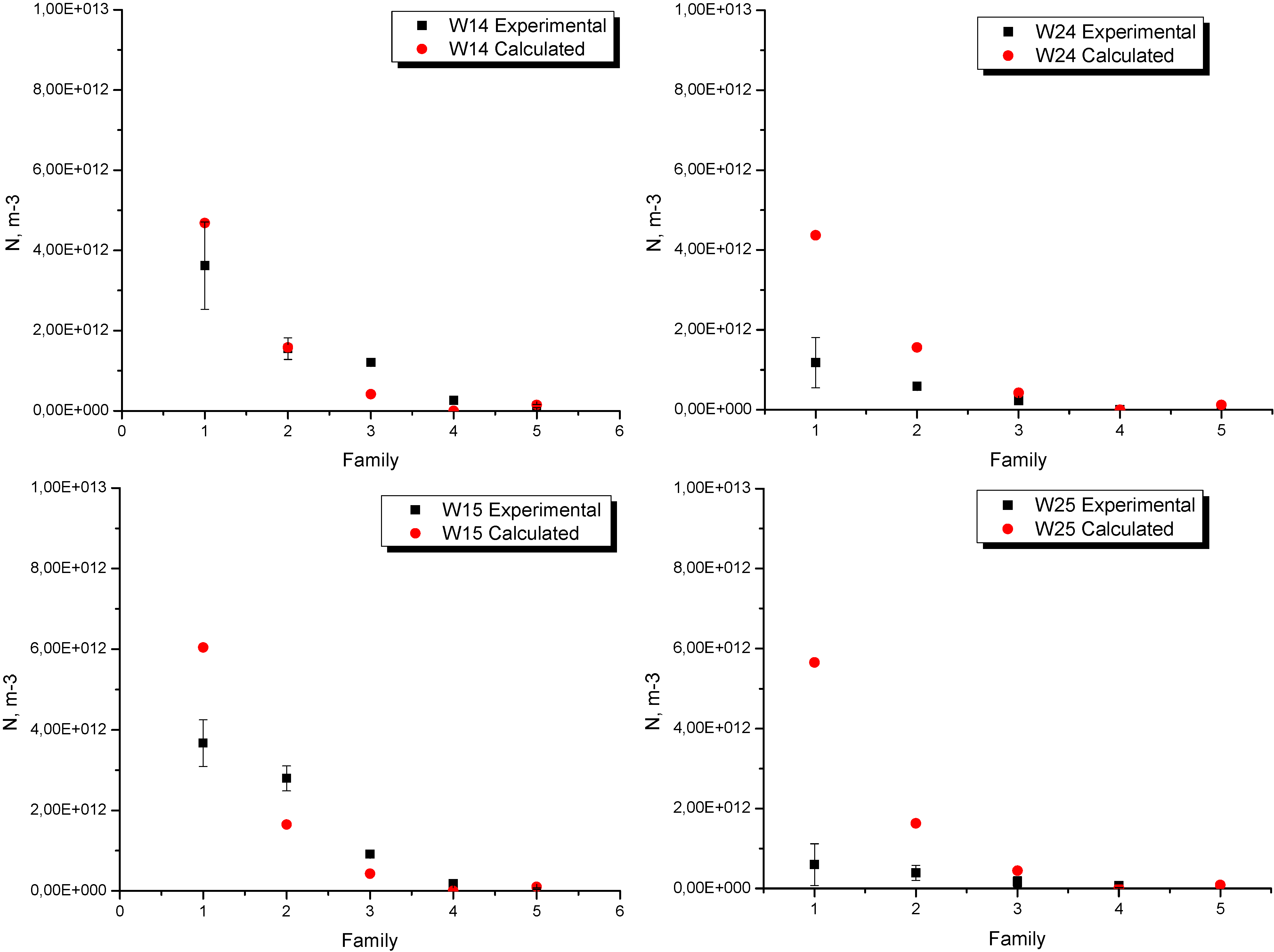
The experimental and calculated nodule counts for each location in experiences W1 and W2 are shown in
Figure 7. Three measurements close to the thermocouples location were made (center of each sample). As can be seen, in both experiences the higher numerical-experimental discrepancies were found in the smaller family,
i.e., in the small size nodules. This difference is more significant in W2, not only for family 1 but for the five families considered in the analysis. Despite these differences, in both cases the trend is the same, i.e., the nodule density decreases towards the larger families. As mentioned above, it is also notable in this figure that an increase in the pouring temperature produces a decrease in the nodule density in all the families considered in the analysis. This fact is well-reproduced by the numerical model.
Figure 7.
Experimental and numerical nodule count in experiences W1 and W2.
Table 6 and
Table 7 summarize the experimental and numerical total nodule count and solidification times for experiences W1 and W2. The effect of the pouring temperature on both the microstructure and the solidification times at each location along the part are apparent where, in addition, it is notable that both effects are well-predicted by the numerical model. In particular, it is seen in experience W1 that, considering the extreme values, the nodule count increases with the cooling rate, from the thicker to the thinner part of the wedge. The nodule count and the cooling rate of the points W12 to W14 are similar. Thus, it is possible to affirm that a high cooling rate will produce a high quantity of graphite nodules, which is also well-predicted by the numerical simulation which, in turn, also overestimates the nodule count in W15. In general, the computed solidification time is close to the experimental values, which agree with the graphical presentation in
Figure 6. For experience W2, like in W1, as the cooling rate increases, the nodule count increases as well. However, the effect of this parameter is less marked than in W1, where the difference between the tip and the back of the wedge is more significant. Furthermore, in all points for experience W2, the calculated nodule density is higher than the experimental one, even though the calculated solidification times are very similar to the experimental ones.
Table 6.
Experimental and numerical total nodule count and solidification times in experience W1.
| Point | NV (nuclei/m3) Experimental | NV (nuclei/m3) Calculated | t(TES)(s) Experimental | t(TES)(s) Calculated |
|---|
| W11 | 2.47 × 1013 ± 3.43 × 1012 | 1.12 × 1013 | 56.2 | 57.6 |
| W12 | 5.45 × 1012 ± 2.23 × 1012 | 1.01 × 1013 | 178.3 | 156.3 |
| W13 | 6.53 × 1012 ± 3.62 × 1012 | 8.23 × 1012 | 252.3 | 258.6 |
| W14 | 6.75 × 1012 ± 1.50 × 1012 | 6.82 × 1012 | 354.2 | 366.7 |
| W15 | 7.62 × 1012 ± 1.01 × 1012 | 8.22 × 1012 | 375.9 | 402.1 |
Table 7.
Experimental and numerical total nodule count and solidification times in experience W2.
| Point | NV (nuclei/m3) Experimental | NV (nuclei/m3) Calculated | t(TES)(s) Experimental | t(TES)(s) Calculated |
|---|
| W21 | 6.28 × 1012 ± 3.94 × 1011 | 1.14 × 1013 | 65.8 | 68.9 |
| W22 | 3.47 × 1012 ± 1.56 × 1011 | 9.38 × 1012 | 224.6 | 202.8 |
| W23 | 1.46 × 1012 ± 6.03 × 1011 | 7.96 × 1012 | 316.3 | 341.4 |
| W24 | 2.00 × 1012 ± 7.13 × 1011 | 6.48 × 1012 | 433.0 | 480.7 |
| W25 | 1.23 × 1012 ±7.26 × 1011 | 7.82 × 1012 | 438.3 | 513.8 |
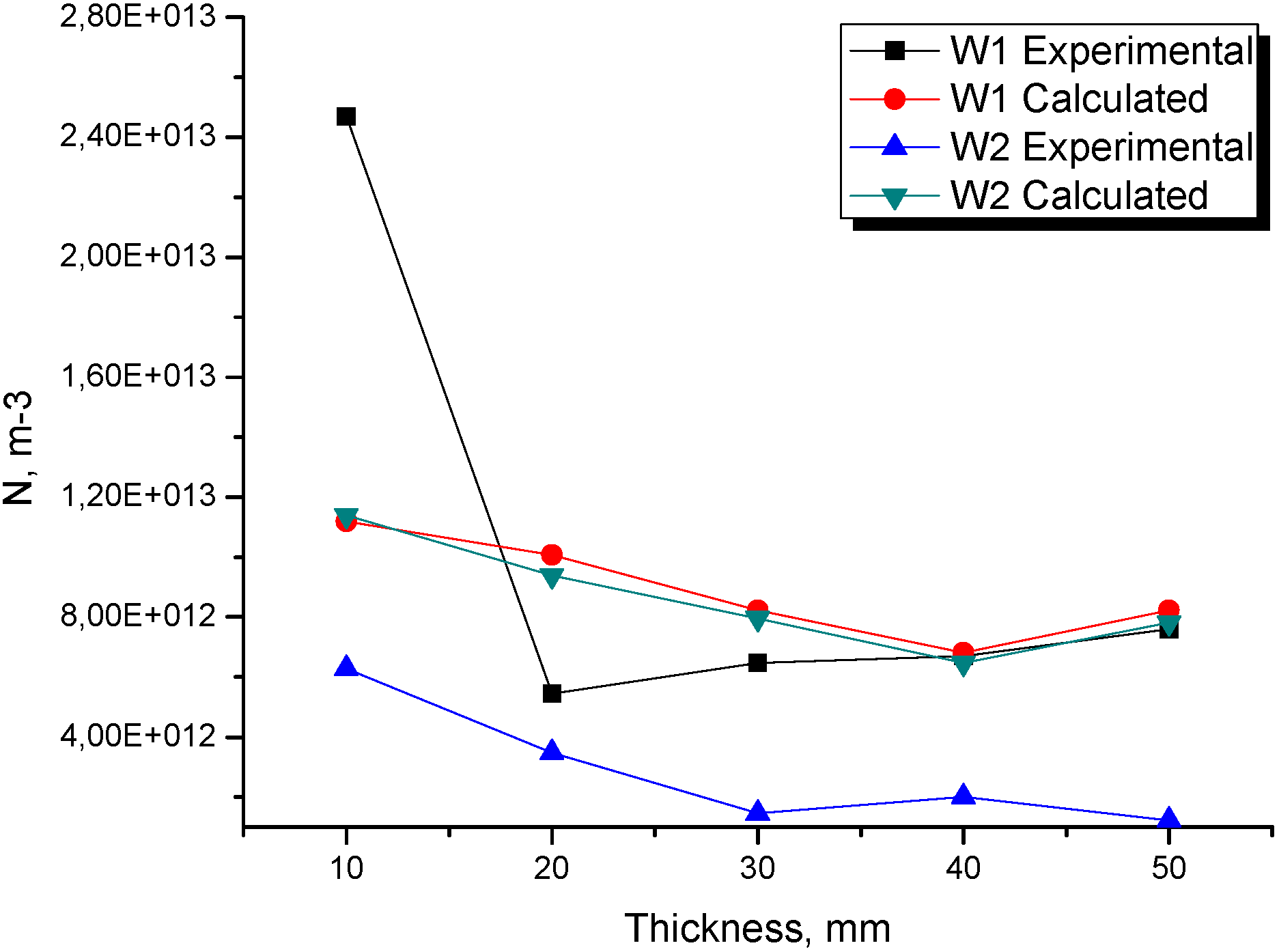
Figure 8 shows the numerical and experimental relationship between the total nodule count and the wall thickness for experiences W1 and W2. It is observed that, as the wall thickness increases, the nodule density decreases, which is also well-predicted by the simulation, especially in experience W1. It is both numerically and experimentally seen that the nodule density does not greatly vary along the wedge, which is directly related to the similar cooling conditions of the points located from 20 mm upwards (from W12 and W22). With these results, it is possible to infer that the thermal contribution of the thicker parts plays an important role on these solidification experiences' morphologies.
Figure 8.
Relationship between numerical and experimental total nodule count and wall thickness for experiences W1 and W2.