Experimental Investigations on the Influence of Adhesive Oxides on the Metal-Ceramic Bond
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
| Material | Trade Name | Manufacturer | Composition as Percent Mass |
|---|---|---|---|
| Alloy | Primallor 3 | DeguDent Hanau, Germany | Au: 70.00; Pd 15.00; Pt: 7.50; Ag: 7.68; Rh: 3.22; Ir: 0.43 |
| Degutan | DeguDent Hanau, Germany | Au: 80.20; Pd: 13.50; Pt: 4.00; Ir: 0.20; Sn: 2.10 | |
| Dental ceramic | Symbio ceram | Ducera Rosbach, Germany | SiO2: 60–70; Al2O3: 10–15; K2O: 5–10; Na2O: 10–15; CaO: 0–0.2; SnO2: 0–0.2; F: 0–0.2; B2O3: 0–1; CeO2: 0–1; |
| Series No. | Series | Alloy | Treatment |
|---|---|---|---|
| 1 | P St | Primallor 3 | —Air abrasion (110 µm Al2O3, 2 bar) |
| —Oxidation in air (980 °C, 10 min) | |||
| —Air abrasion (110 µm Al2O3, 2 bar) | |||
| 2 | P | Primallor 3 | —Preparation with 1200 grit abrasive paper |
| 3 | P Al I | Primallor 3 | —Preparation with 1200 grit abrasive paper |
| —Implantation of Al ions (~ 5 at%) | |||
| 4 | P Al II | Primallor 3 | —Preparation with 1200 grit abrasive paper |
| —Implantation of Al ions (~ 15 at%) | |||
| 5 | P Cu I | Primallor 3 | —Preparation with 1200 grit abrasive paper |
| —Implantation of Cu ions (~ 5 at%) | |||
| 6 | P Cu II | Primallor 3 | —Preparation with 1200 grit abrasive paper |
| —Implantation of Cu ions (~ 5 at%) | |||
| —Oxidation in air (980 °C, 10 min) | |||
| 7 | P In | Primallor 3 | —Preparation with 1200 grit abrasive paper |
| —Implantation of In ions (~ 5 at%) | |||
| 8 | Dg | Degutan | —Preparation with 1200 grit abrasive paper |
| 9 | Dg St | Degutan | —Air abrasion (110 µm Al2O3, 2 bar) |
| —Oxidation in air (980 °C, 10 min) | |||
| —Air abrasion (110 µm Al2O3, 2 bar) |
- -
- Air abrasion with Al2O3 (particle size: 110 µm) at 2 bar pressure;
- -
- Oxidation in air at 980 °C for 10 min;
- -
- Air abrasion again (as above).
| Series | Implanted Ion | Ion Energy (keV) | Application Rate (ions/cm−2) |
|---|---|---|---|
| P Al I | Al | 200 | 4 × 1016 |
| P Al II | Al | 200 | 2 × 1017 |
| P Cu I | Cu | 200 | 4 × 1016 |
| P Cu II | Cu | 200 | 4 × 1016 |
| P In | In | 200 | 4 × 1016 |
2.2. Testing
- -
- Mechanical testing of the metal-ceramic bond was conducted using the Schwickerath method. The specimen preparation for this was in accordance with ISO 9693 and ISO 9693-1 [15,16]. Three-point bending tests to determine the shear bond strength were performed with a universal strength testing machine, TIRA test 2720 (Industriegerätewerk, Rauenstein, Germany). The testing speed was 1.5 mm/min. The evaluation of the results for the shear bond strength was done in accordance with ISO 9693 and ISO 9693-1 (Young’s modulus of Primallor 3: 97 kN/mm²; Degutan: 83 kN/mm2) [15,16].
- -
- Surface roughness measurement of the test specimens was performed with a profilometer of the type Hommel-Tester T6000 (Hommelwerke, Schwennigen, Germany) in roughness mode (TKE 100/17 probe). The direction of measurement was at a right angle to the direction of abrasion. The error of measurement was determined prior to each series with a surface roughness reference calibration standard (No. 230747/3511, Hommelwerke, Schwennigen, Germany). The mean error was 3.5%, which is within the allowed tolerances. Measurements were in accordance with ISO 4287, 4288 and 25178 [18,19,20].
- -
- Raster electron microscopy (REM) and SEM (XL 30 ESEM, Phillips, Eindhoven, The Netherlands) were used in order to analyze the surface microstructure combined with the chemical composition of specimens. One specimen surface was evaluated for each treatment condition. The effects of ion implantations were studied by comparing implanted and non-implanted surfaces.
- -
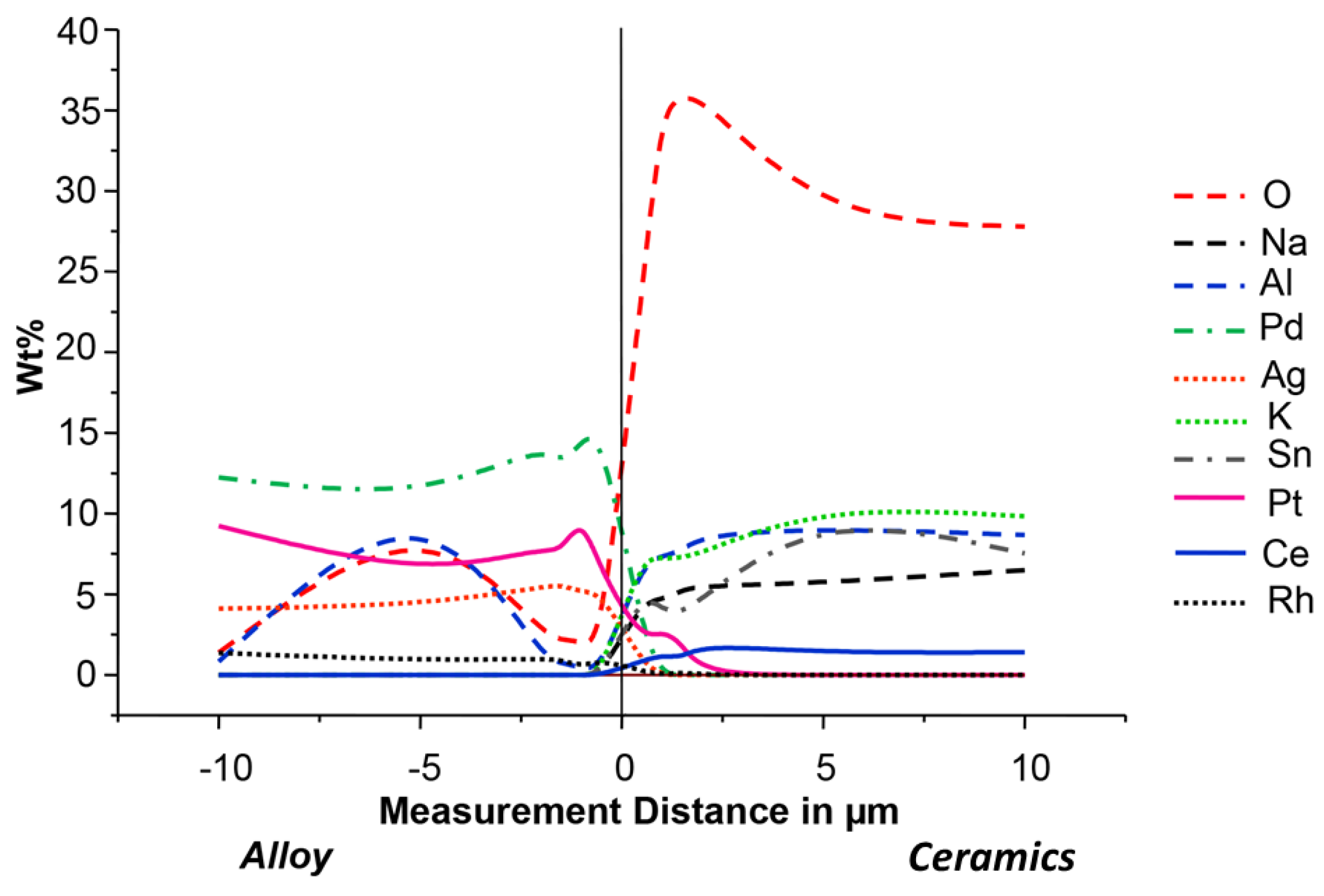
- The element distribution was analyzed using AES, applying low-energy electrons [21]. The depth profile of the implanted elements was produced with a microlab 310 F (Fissons Instruments, Uckfield, UK). The depth profiles of individual elements were plotted directly after implantation, after implantation and oxidation in air, as well as after application and firing of the dental ceramic. Using Profile-Codes™, a computer program (Implant Sciences Inc., Wilmington, WA, USA), the concentration profiles for each of the ions to implant was calculated beforehand, in order to derive the target implantation depth in relation to the initial implantation conditions mentioned.
- -
- EDX was used to analyze the composition of the alloy surfaces. One specimen coated with ceramic was studied for each series. The specimen was embedded in a synthetic resin of the type Speci Fix 20 (Struers, Rødovre, Denmark). After hardening, the specimen resin block was sectioned using an Accutom 50 (Struers, Rødovre, Denmark) and prepared for EDX analysis. An Edwards Sputter Coater S 158 B (Edwards High Vacuum International, Crawby, West Sussex, UK) was used for carbon sputtering. The EDX analyses were performed with the aforementioned scanning electron microscope. In both the alloy and the ceramics, chemical composition was analyzed in 6 points situated on a line with various distance from the alloy-ceramic interface, 0.5, 1.0, 1.5, 2.0, 5.0 and 10 (mm) micrometers, respectively. An elemental analysis was derived from each measurement.
2.3. Statistics
3. Results
3.1. Shear Bond Strength
| Series | Primallor 3 | Degutan | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| P St | P | P Al I | P Al II | P Cu I | P Cu II | P In | Dg St | Dg | ||
| Shear Bond Strength (MPa) | Mean | 41.5 | 29.0 | 31.4 | 19.2 | 30.3 | 29.1 | 29.0 | 47.8 | 36.6 |
| Standard Deviation | 2.6 | 5.0 | 4.6 | 3.2 | 4.6 | 3.2 | 5.2 | 1.7 | 3.8 | |
| Maximum Roughness Profile Height (Rz) (µm) | Mean | 9.5 | 1.3 | 1.4 | 1.4 | 1.6 | 2.6 | 0.5 | 12.7 | 1.2 |
| Standard Deviation | 0.9 | 0.2 | 0.1 | 0.1 | 0.3 | 1.1 | 0.1 | 0.6 | 0.2 | |
| Series | P Al I | P Al II | P Cu I | P Cu II | P In |
|---|---|---|---|---|---|
| P St | s. (0.002) | s. (0.001) | s. (0.001) | s. (0.001) | s. (0.001) |
| P | n.s. (0.172) | s. (0.002) | n.s. (0.600) | n.s. (1.000) | n.s. (0.834) |
| Dg St | s. (0.001) | s. (0.001) | s. (0.001) | s. (0.001) | s. (0.001) |
| Dg | n.s. (0.003) | s. (0.001) | n.s. (0.003) | n.s. (0.004) | n.s. (0.004) |
3.2. Roughness
| Series | P Al I | P Al II | P Cu I | P Cu II | P In |
|---|---|---|---|---|---|
| P St | s. (0.002) | s. (0.002) | s. (0.002) | s. (0.002) | s. (0.001) |
| P | n.s. (0.010) | n.s. (0.010) | n.s. (0.008) | s. (0.004) | s. (0.001) |
| Dg St | s. (0.001) | s. (0.002) | s. (0.002) | s. (0.002) | s. (0.001) |
| Dg | n.s. (0.007) | n.s. (0.005) | n.s. (0.012) | s. (0.0019) | s. (0.001) |
3.3. Correlation Analysis Shear Bond Strength: Roughness
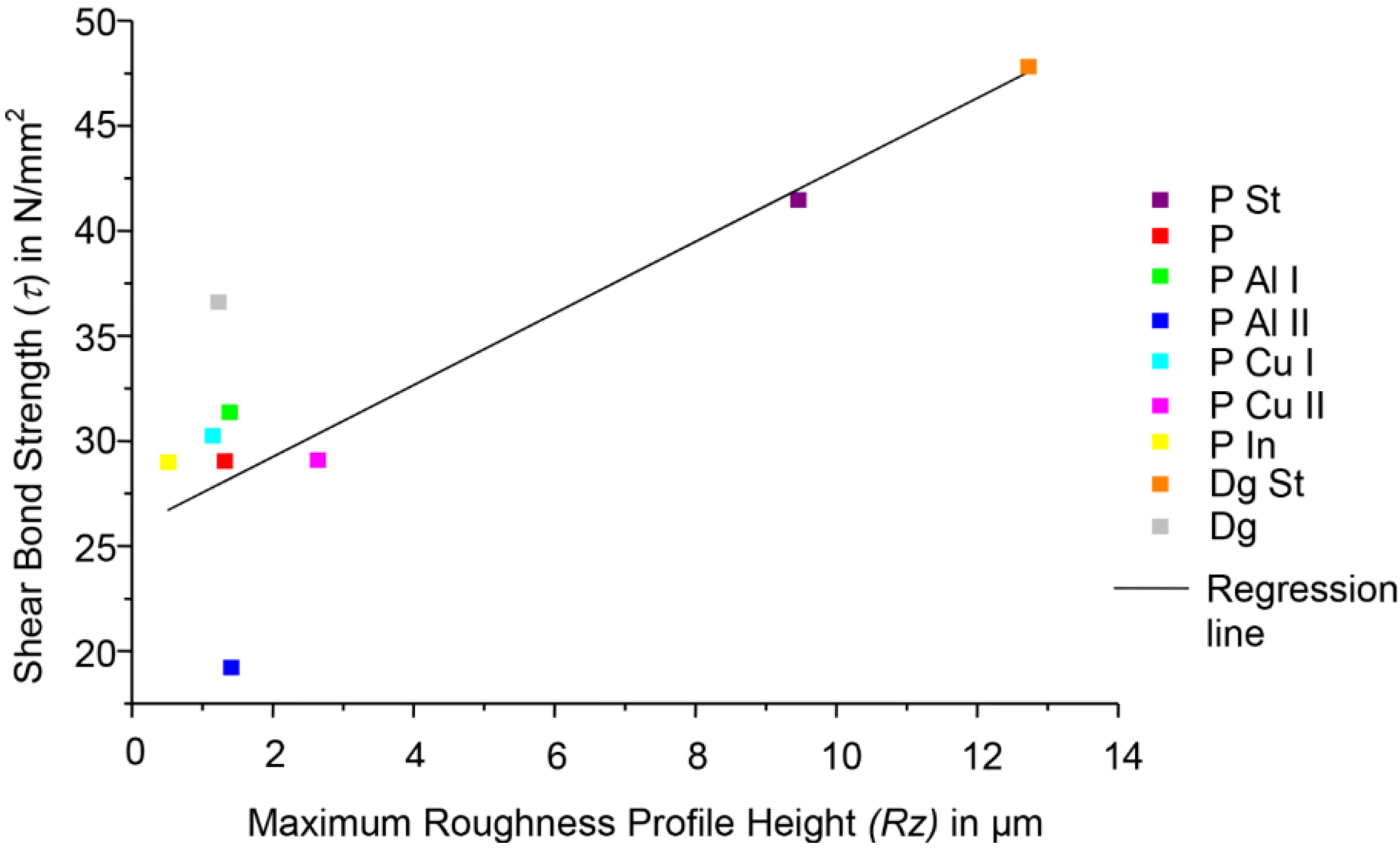
3.4. Concentration Analyses

4. Discussion
4.1. Shear Bond Strength and Roughness
4.2. Chemical Bonding: Mechanical Bonding
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Coornaert, J.; Adriaens, P.; De Boever, J. Long-term clinical study of porcelain-fused-to-gold restorations. J. Prosthet. Dent. 1984, 51, 338–342. [Google Scholar]
- Walter, M.; Reppel, P.D.; Böning, K.; Freesmeyer, W.B. Six-year follow-up of titanium and high-gold porcelain-fused-to-metal fixed partial dentures. J. Oral. Rehabil. 1999, 26, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Walton, T.R. An up to 15-year longitudinal study of 515 metal-ceramics FDPs: Part 2. Modes of failure and influence of various clinical characteristics. Int. J. Prosthodont. 2003, 16, 177–182. [Google Scholar] [PubMed]
- Holm, C.; Tidehag, P.; Tillberg, A.; Molin, M. Longevity and quality of FDPs: A retrospectivestudy of restorations 30, 20, and 10 years after insertion. Int. J. Prosthodont. 2003, 16, 283–289. [Google Scholar]
- Napankangas, R.; Raustia, A. An 18-year retrospective analysis of treatment outcomes with metal-ceramic fixed partial dentures. Int. J. Prosthodont. 2011, 24, 314–319. [Google Scholar] [PubMed]
- Eichner, K. Gegenwärtiger Stand der werkstoffkundlichen und klinischen Metallkeramik. ZWR 1997, 97, 477–485. [Google Scholar]
- Bumgardner, J.D.; Lucas, L.C. Corrosion and cell culture evaluations of nickel-chromium dental casting alloys. J. Appl. Biomater. 1994, 5, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Wataha, J.C.; Hanks, C.T. Biological effects of palladium and risk of using palladium in dental casting alloys. J. Oral. Rehabil. 1996, 23, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Wataha, J.C. Biocompatibility of dental casting alloys: A review. J. Prosthet. Dent. 2000, 83, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Al-Hiyasat, A.S.; Bashabsheh, O.M.; Darmani, H. An investigation of the cytotoxic effects of dental casting alloys. Int. J. Prosthodont. 2003, 16, 8–12. [Google Scholar]
- Wataha, J.C. Alloys for prosthodontics restorations. J. Prosthet. Dent. 2002, 87, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Möller, W.; Richter, E. Praktische Anwendungen der Ionenimplantation. Galvanotechnik 1989, 89, 3–11. [Google Scholar]
- Mihoc, R.; Hobkirk, J.A.; Armitage, D.; Jones, F.H. Influence of ion implantation on physicochemical processes at titanium surfaces. Int. J. Prosthodont. 2006, 19, 24–28. [Google Scholar]
- Hohmann, W. Dentalkeramik Auf Der Basis Hydrothermaler Gläser. Quintessenz: Berlin, Germany, 1993. [Google Scholar]
- Metal-Ceramic Dental Restorative Systems; Beuth: Berlin, Germany, 1999; ISO 9693.
- Dentistry—Compatibility Testing, Metal-Ceramic Systems; Beuth: Berlin, Germany, 2012; ISO 9693–1.
- Dentistry—Casting Gold Alloys; Beuth: Berlin, Germany, 2004; ISO 1562.
- Geometrical Product Specifications (GPS)—Surface Texture : Profile Method—Terms, Definitions and Surface Texture Parameters; Beuth: Berlin, Germany, 2010; ISO 4287.
- Geometrical Product Specifications (GPS)—Surface Texture: Profile Method—Rules and procedures for the assessment of surface texture; Beuth: Berlin, Germany, 1998; ISO 4288.
- Geometrical Product Specifications (GPS)—Surface Texture: Areal—Part 2: Terms, Definitions and Surface Texture Parameters; Surface Texture: Areal—Part 3: Specification Operators; Surface Texture: Areal—Part 6: Classification of Methods For Measuring Surface Texture; Beuth: Berlin, Germany, 2012; ISO 25178.
- Briggs, D.; Seah, M.P. Practical Surface Analysis: Vol. 1. In Auger and X-ray Photoelectron Spectroscopy; Wiley: Chichester, UK, 1990. [Google Scholar]
- Holm, S. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 1979, 6, 65–70. [Google Scholar]
- Armitage, P.; Colton, T. Encyclopaedia of Biostatistics, 2nd ed.; Wiley: Chichester, UK, 2005; pp. 7872–7878. [Google Scholar]
- Gilbert, J.L.; Covey, D.A.; Lautenschläger, E.P. Bond characteristics of porcelain fused to milled titanium. Dent. Mater. 1994, 10, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Hautaniemi, J.A. The effect of indium on porcelain bonding between porcelain and Au-Pd-In alloy. J. Sci. Mat. Med. 1995, 66, 46–50. [Google Scholar] [CrossRef]
- Petridis, H.; Garefis, P.; Hirayama, H.; Kafantaris, N.M; Koidis, P.T. Bonding indirect resin composites to metal: Part I. Comparison of shear bond strengths between different metal-resin bonding systems and a metal-ceramic system. Int. J. Prosthodont. 2003, 16, 635–639. [Google Scholar]
- Derfert, B. Vergleichende Untersuchungen zur Verbundfestigkeit von verschiedenen Metall-Keramik-Kombinationen auf der Basis von EM- und NEM-Legierungen in Verbindung mit herkömmlicher und niedrigschmelzender Keramik. Ph.D. Thesis, Freie Universität Berlin, Berlin, Germany, 12 December 2003. [Google Scholar]
- Mc Lean, J.W.; Sced, I.R. Bonding of Dental Porcelain to metal I. The Gold Alloy Porcelain Bond. Trans. J. Brit. Ceram. Soc. 1973, 72, 229–233. [Google Scholar]
- Charnay, R.; Guiraldenq, P.; Enriore, J.M.; Brugirard, J.; Heberard, X. Mechanical properties of the ceramic-metal bonding of a gold-platinum dental alloy and chemical characterization of the interface. Mem. Etud. Rev. Met. 1992, 12, 797–803. [Google Scholar]
- Mc Lean, J.W. The Science and Art of Dental Ceramics; Quintessence: Chicago, IL, USA, 1980; pp. 65–90. [Google Scholar]
- Anusavice, K.J. Phillip’s Science of Dental Materials, 12th ed.; Saunders: Philadelphia, PA, USA, 2012. [Google Scholar]
- Schwickerath, H. Das Festigkeitsverhalten von aufbrennfähigen Keramiken. Dtsch. Zahnärztl. Z 1985, 40, 996–1003. [Google Scholar] [PubMed]
- Karlsson, S. Failures and length of service in fixed Prosthodontics after Long-term function. A longitudinal clinical study. Swed. Dent. J. 1989, 13, 185–192. [Google Scholar] [PubMed]
- Jokstad, A.; Esposito, M.; Coulthard, P.; Worthington, H.V. The reporting of randomised controlled trials in prosthodontics. Int. J. Prosthodont. 2002, 15, 230–242. [Google Scholar] [PubMed]
- Glantz, P.O.; Nilner, K.; Jendresen, M.D.; Sundberg, H. Quality of fixed prosthodontics after twenty two years. Acta. Odontol. Scand. 2002, 60, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Bullard, J.T.; Dill, R.E.; Marker, V.A.; Payne, E.V. Effects of sputtered metal oxide films on the ceramic-to-metal-bond. J. Prosthet. Dent. 1985, 54, 776–778. [Google Scholar] [CrossRef] [PubMed]
- Walter, M. Zur Porenbildung in der keramischen Verblendung von Palladium-Silber-Legierungen. Dtsch. Zahnärztl. Z 1988, 43, 145–149. [Google Scholar] [PubMed]
- Reitemeier, B.; Hänsel, K.; Kastner, C.; Weber, A.; Walter, M.H. A prospective 10-year study of metal ceramic single crowns and fixed dental prosthesis retainers in private practice settings. J. Prosthet. Dent. 2013, 109, 149–155. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enghardt, S.; Richter, G.; Richter, E.; Reitemeier, B.; Walter, M.H. Experimental Investigations on the Influence of Adhesive Oxides on the Metal-Ceramic Bond. Metals 2015, 5, 119-130. https://doi.org/10.3390/met5010119
Enghardt S, Richter G, Richter E, Reitemeier B, Walter MH. Experimental Investigations on the Influence of Adhesive Oxides on the Metal-Ceramic Bond. Metals. 2015; 5(1):119-130. https://doi.org/10.3390/met5010119
Chicago/Turabian StyleEnghardt, Susanne, Gert Richter, Edgar Richter, Bernd Reitemeier, and Michael H. Walter. 2015. "Experimental Investigations on the Influence of Adhesive Oxides on the Metal-Ceramic Bond" Metals 5, no. 1: 119-130. https://doi.org/10.3390/met5010119





