Titanium Implant Osseointegration Problems with Alternate Solutions Using Epoxy/Carbon-Fiber-Reinforced Composite
Abstract
:1. Introduction
2. Corrosion
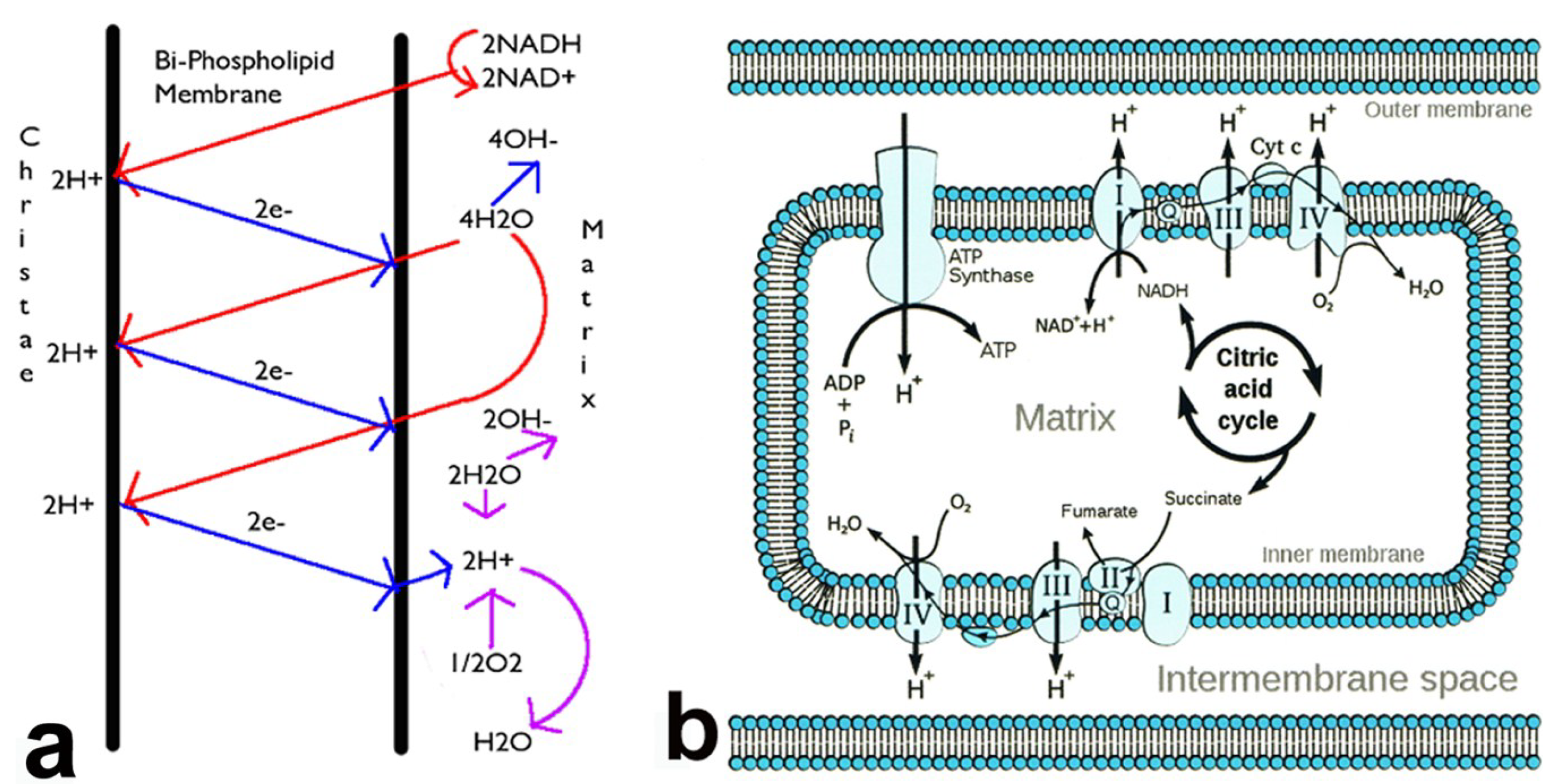
3. Infection
4. Coatings
5. Polymer Matrix Composites (PMCs)
5.1. Results for PMC Biocompatibility
| Material | Density (g/cm3) | Resistivity a (Ω m) | Tensile strength (MPa) | Yield strength (MPa) | Modulus (GPa) |
|---|---|---|---|---|---|
| Bone longitudinal (Ω m radial-longitudinal 100% wet) [1,2] | 1.8–2.1 | 45–150 | 90–149 | 114 | 15.2–18.6 |
| Titanium grades 1–4 [1,2,14] | 4.5–4.51 | 10−7 | 240–550 | 170–485 | 104–110 |
| Titanium-6-4aluminum vanadium alloy [1,2,14] | 4.4–5.0 | 10−8 | 860–1103 | 795–1034 | 116–120 |
| Bisphenyl Unidirectional CF b [2,14,53,54] | 1.6 | 5 | 780–1850 | 140–325 | |
| Bisphenyl Unidirectional CF b 4-pt. bend [2,19,29] | 1.6 | 5 | 660–1800 | 64–255 | |
| Bisphenyl/CF b Exp.Uni-woven laminate 4-pt. bend [19,29] | 1.49 (±0.01) c | 5 | 963 (±240) c | 774 (±176) c | 64 (±14.4) c |
| Bisphenyl 3-D Woven E-Glass 3-pt. Bend X-Y planes [19] | 576 (±129) c | 441 (±75) c | 26 (±18) c | ||
| Unidirectional Photocure 3-pt Bend QF b [29] | 1118.8 (±207.6) c | 76.6 (±13.3) c | |||
| Polymer Acrylic Bone Cement (PMMA) 4 pt Bend [14,29] | 1.17–1.20 | >1012 | 54.8 (±3.8) c | 43.2 (±3.6) c | 1.7 (±0.1) c |

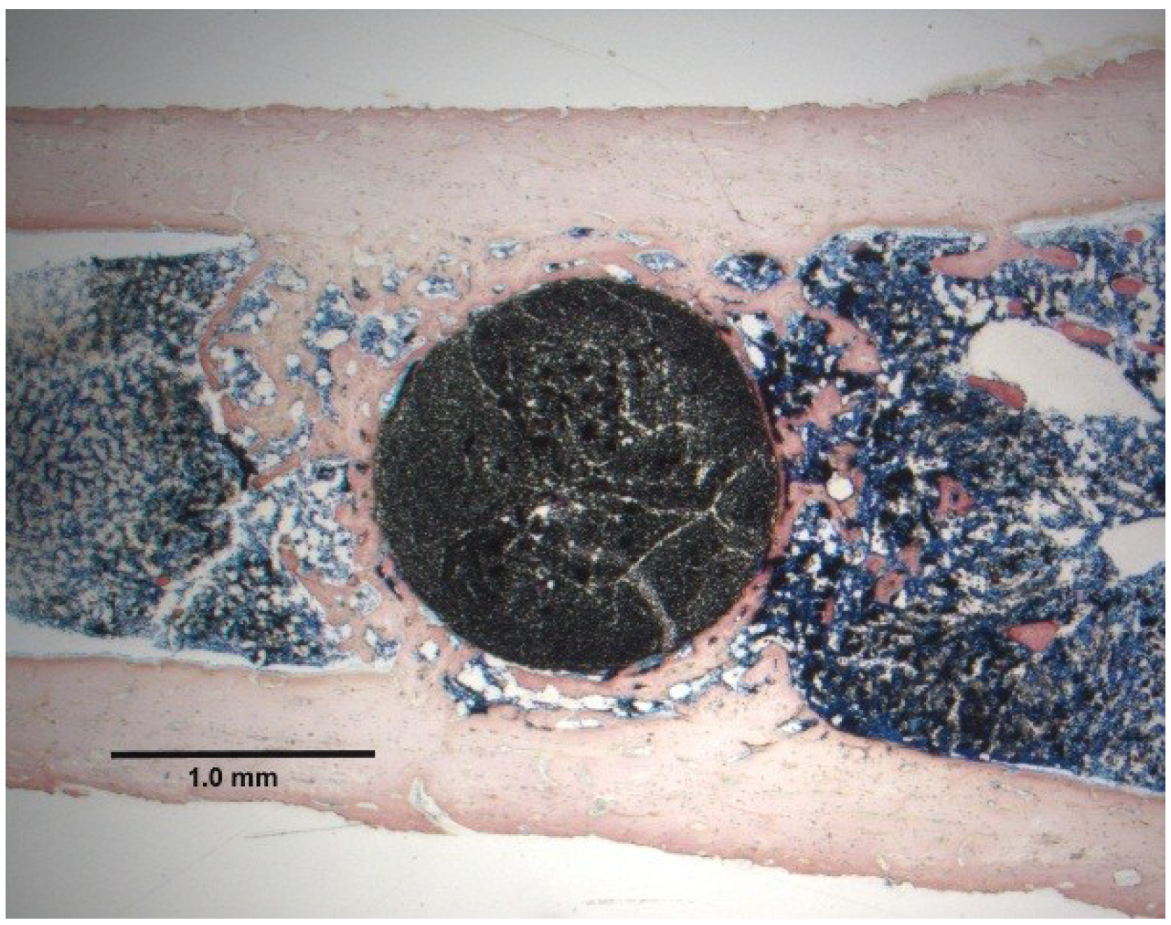
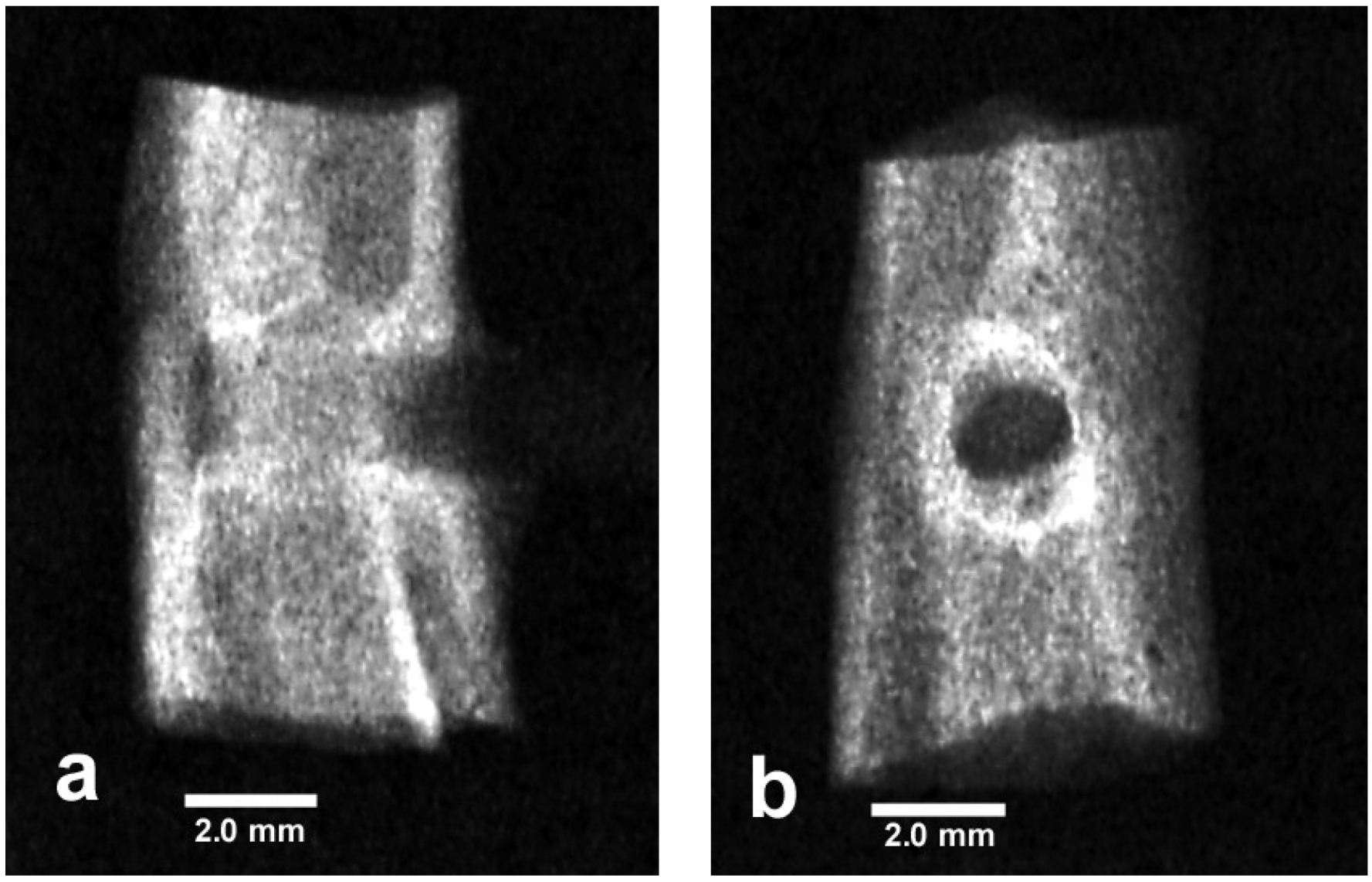
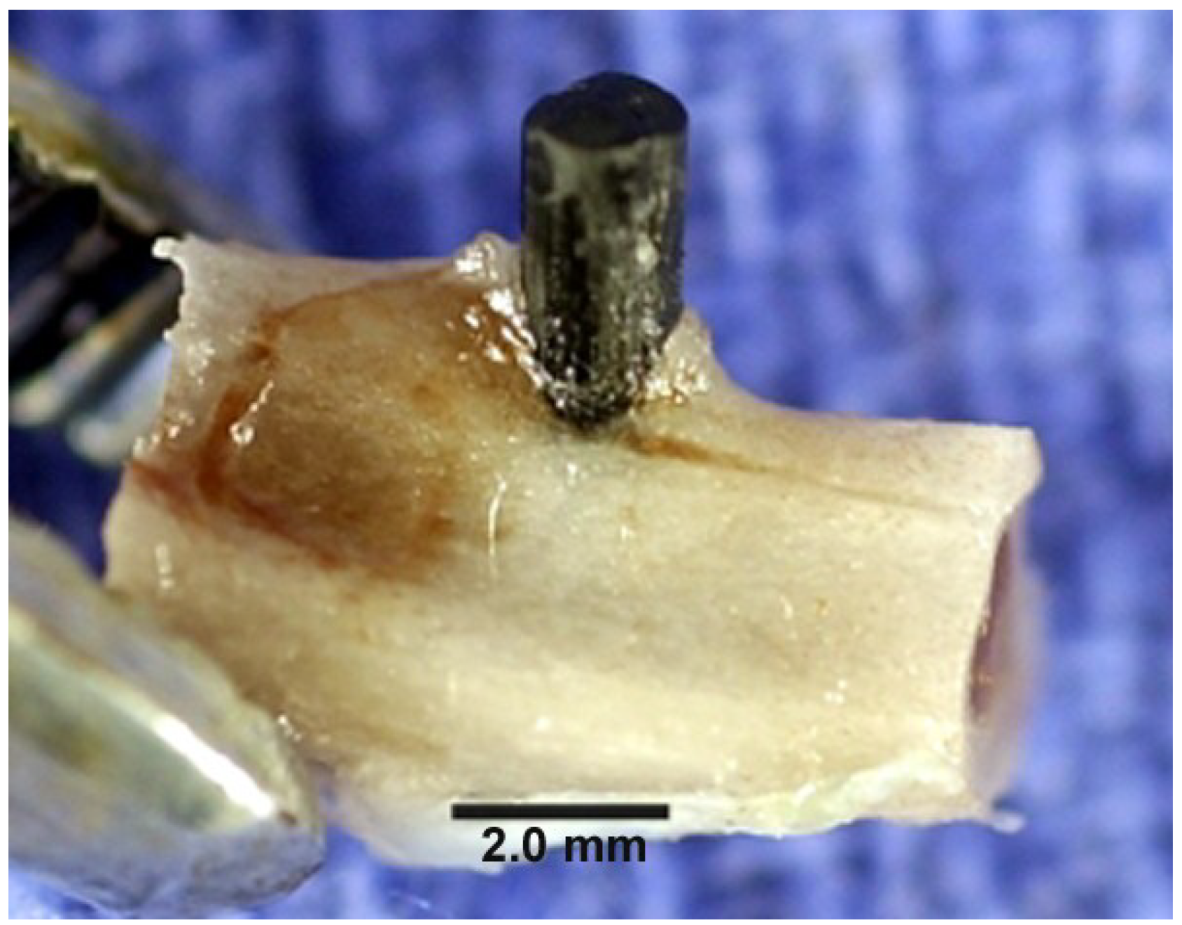
5.2. Nonpolar Molecular Attractions with Secondary Bonding
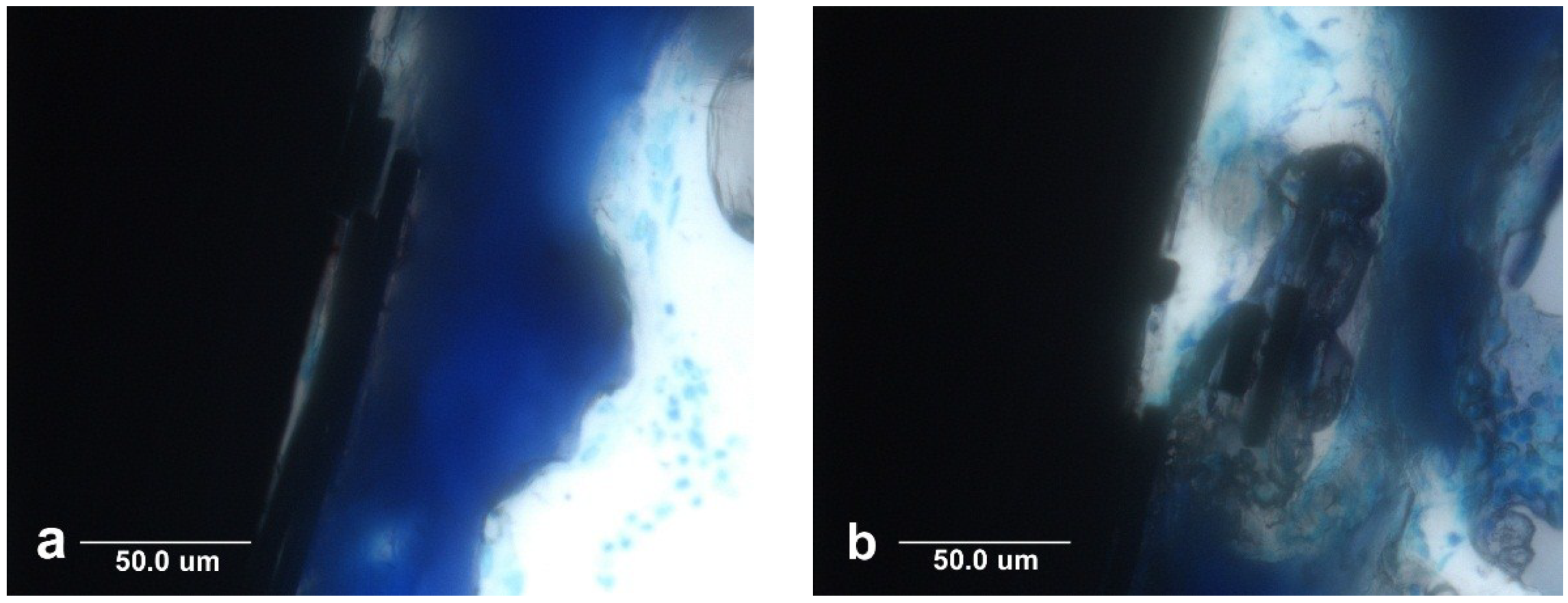
5.3. Carbon Fiber Biocompatibility

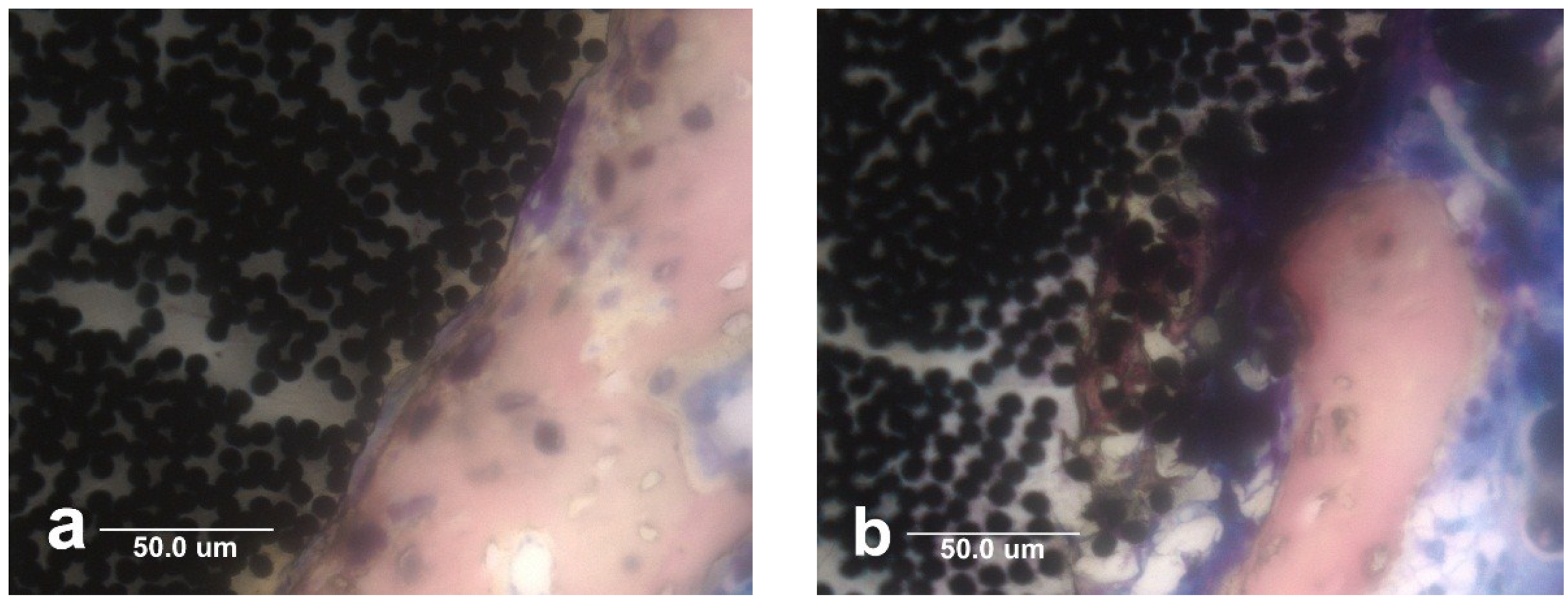
5.4. Electrical Biocompatibility and Semiconducting Properties
| Material | Type | Resistivity (Ωm) |
|---|---|---|
| Titanium pure | Conductor | 4.2–5.2 × 10−7 [14] |
| Titanium-6Al-4V alloy | Conductor | 1.7 × 10−8 [14] |
| Titanium dioxide (rutile) | Semiconductor | 29–910 [65] |
| Bisphenol-polymer/carbon fiber composite | Semiconductor | 5 [19] |
| Bone longitudinal | Semiconductor | 45–46 [2] |
| Bone radial | Semiconductor | 150 [2] |
| Physiologic saline | Semiconductor | 0.72 [2] |
| Silicon pure | Semiconductor | 3000 [66] |
| Silicon phosphorous doped | Semiconductor | 20–80 [67] |
| Lipid phosphate headgroup/water interface | Semiconductor | 100 [64] |
| Carbon fibers | Conductor | 9.5–18 × 10−6 [14] |
| General metals | Conductors | ~10−6–10−9 [14] |
| Thermoset bisphenyl epoxy polymer | Insulator | 1010–1013 [14] |
| Acrylic bone cement polymer | Insulator | >1012 [14] |
| Pure quartz fiber | Insulator | 1020 [68] |
5.5. Stress Transfer
5.6. Additives for Low Thermal PMC Processing
5.7. Biocompatibility Coatings
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Ratner, B.D.; Hoffman, A.S.; Schoen, F.J.; Lemons, J.E. Chapters 1 Properties of Materials, 2.9 Metals-Titanium, 4.1 Host Reactions-Introduction-Infection, 4.8 Biofilm, Biomaterials and Infections, 6.3 Degradative Effects of the Biological Environment on Metals and Ceramics, 7.7-Orthopedics and 7.8-Tissue Interfaces. In Biomaterials Science, 2nd ed.; Elsevier: San Diego, CA, USA, 2004; pp. 23–65, 148–149, 295–296, 345–348, 430–439, 526–555, 566. [Google Scholar]
- Park, B.J.; Lakes, R.S. Chapters 3 Characterization of Materials I and II, 5 Metallic Implant Materials, 6.6 Carbons and 9 Biologic Materials. In Biomaterials an Introductions, 2nd ed.; Plenum Press: New York, NY, USA, 1992; pp. 47–48, 64, 89–115, 131–134, 197, 203–204. [Google Scholar]
- Anusavice, K.J. Chapters 3 Electrochemical Corrosion, 19 Dental Casting and Soldering Alloys and 23 Dental Implants. In Phillips’ Science of Dental Materials, 11th ed.; Saunders: St. Louis, MO, USA, 2003; pp. 58–59, 579–580, 768–778. [Google Scholar]
- Actis, L.; Gaviria, L.; Guda, T.; Ong, J.L. Antimicrobial surfaces for craniofacial implants: State of the art. J. Korean Assoc. Oral Maxillofac. Surg. 2013, 39, 43–54. [Google Scholar] [CrossRef]
- Petersen, R.C. Bisphenyl-polymer/carbon-fiber-reinforced composite compared to titanium alloy bone implant. Int. J. Polym. Sci. 2011. [Google Scholar] [CrossRef]
- Sakka, S.; Coulthard, P. Implant failure: Etiology and complications. Med. Oral Patol. Oral y Cir. Bucal 2011, 16, e2–e4. [Google Scholar]
- Hickok, N.J.; Shapiro, I.M. Immobilized antibiotics to prevent orthopedic implant infections. Adv. Drug Deliv. Rev. 2012, 64, 1165–1176. [Google Scholar] [CrossRef] [PubMed]
- Dodds, E.C.; Lawson, W. Synthetic estrogenic agents without the phenanthrene nucleus. Nature 1936, 137, 996. [Google Scholar] [CrossRef]
- Lewis, J.B.; Rueggeberg, F.A.; Lapp, C.A.; Ergle, J.W.; Schuster, G.S. Identification and characterization of estrogenlike components in commercial resin-based dental restorative materials. Clin. Oral Investig. 1999, 3, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Gennari, L.; Nuti, R.; Bilezikian, J.P. Aromatase activity and bone homeostasis in men. J. Clin. Endocrinol. Metab. 2004, 89, 5898–5907. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, R.; Inoue, D.; Shibata, M.; Saika, M.; Kido, S.; Ooka, H.; Tomitama, H.; Sakamoto, Y.; Matsumoto, T. Estrogen promotes early osteoblast differentiation and inhibits adipocyte differentiation in mouse bone marrow stromal cell lines that express estrogen receptor (ER) α or β. Endocrinology 2002, 143, 2349–2356. [Google Scholar] [PubMed]
- Weitzmann, M.N.; Pacifici, R. Estrogen deficiency and bone loss: An inflammatory tale. J. Clin. Investig. 2006, 116, 1186–1194. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.T. Chapters 3.2 Epoxy Resins and 49.1–49.2 Composite Sporting Goods General Descriptions-Introduction-Manufacturing. In Handbook of Composites, 2nd ed.; Chapman and Hall: New York, NY, USA, 1998; pp. 48–50, 1045–1049. [Google Scholar]
- Callister, W.D. Chapters 17 Composites, 19 Electrical Properties and Appendix C Properties of Selected Materials. In Materials Science and Engineering, 4th ed.; John Wiley & Sons: New York, NY, USA, 1997; pp. 510–531, 593–624, 774–800. [Google Scholar]
- Akpinar, I.; Anil, N.; Parnas, L.A. Natural tooth’s stress distribution in occlusion with a dental implant. J. Oral Rehabil. 2000, 27, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Tanimoto, Y.; Hayakawa, T.; Nemoto, K. Mode superposition transient dynamic analysis for dental implants with stress-adsorbing elements: A finite element analysis. Dent. Mater. J. 2006, 25, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Chung, D.D.L. Structural composite materials tailored for damping. J. Alloys Compd. 2003, 355, 216–223. [Google Scholar] [CrossRef]
- Duyck, J.; Ronold, J.J.; van Oosterwyck, H.; Naert, I.; Vander Sloten, J.; Ellingsen, J.E. The influence of static and dynamic loading on marginal bone reactions around osseointegrated implants: An animal study. Clin. Oral Implants Res. 2001, 12, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Ramadin, Y.; Jawad, S.A.; Musameh, S.M.; Ahmad, M.; Zihlif, A.M.; Paesano, A.; Martuscelli, E.; Ragosta, G. Electrical and electromagnetic shielding behavior of laminated epoxy carbon fiber composite. Polym. Int. 1994, 34, 145–150. [Google Scholar] [CrossRef]
- Jones, D.A. Chapter 15.1.6 Alloy Selection-Titanium Alloys. In Principles and Prevention of Corrosion, 2nd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 1996; pp. 524–526. [Google Scholar]
- Gittens, R.A.; Olivares-Navarrete, R.; Tannenbaum, R.; Boyan, B.D.; Schwartz, Z. Electrical implications of corrosion for osseointegration of titanium implants. J. Dent. Res. 2011, 90, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraghavan, V.; Sabane, A.V.; Tejas, K. Hypersensitivity to titanium: A less explored area of research. J. Indian Prosthodont. Soc. 2012, 12, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Zumdahl, S.S. Chapters 14.11 the Lewis Acid-Base Model, 19.3 the Chemistry of Phosphorous and 20.2 First Row Transition Metals. In Chemistry; D.C. Heath and Company: Lexington, MA, USA, 1993; pp. 680–682, 904–907, 943–944. [Google Scholar]
- McMurry, J. Chapters 1.5–1.6 the Nature of Chemical Bonds-Valence Bond Theory, 2 Polar Covalent Bonds; Acids and Bases. In Organic Chemistry; Brooks/Cole-Thomson: Belmont, CA, USA, 2004; pp. 7–11, 29–60. [Google Scholar]
- Trethewey, K.R.; Chamberlain, J. Chapter 15.6 Titanium. In Corrosion for Science and Engineering, 2nd ed.; Longman: Essex, UK, 1995; pp. 356–361. [Google Scholar]
- Helmlinger, G.; Yuan, F.; Dellian, M.; Jain, R.K. Interstitial pH and pO2 gradients in solid tumors in vivo: High-resolution measurements reveal a lack of correlation. Nat. Med. 1997, 3, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Gillies, R.J. Introduction. In The Tumour Microenvironment: Causes and Consequences of Hypoxia and Acidity, Proceedings of the Novartis Foundation Symposium 240, London, UK, 10–12 October 2001; John Wiley and Sons: New York, NY, USA, 2001; pp. 1–6. [Google Scholar]
- Weinberg, R.A. Chapter 13.6. In The Biology of Cancer; Garland Science Taylor and Francis Group: New York, NY, USA, 2007; pp. 556–557. [Google Scholar]
- Petersen, R.C. Chapter 8 Micromechanical Test Data, 19 Mitochondria and 20 Structural Plasma Membrane for Nanocircuits. In Micromechanics/Electron Interactions for Advanced Biomedical Research; Lambert: Saarbrücken, Germany, 2011; pp. 121–126, 316–322, 323–327. [Google Scholar]
- Sircar, S. Chapters 2 the Cell Membrane-Composition, Structure, 6 Resting Membrane Potential, 74 Metabolic Pathways and 82 Ovarian Hormones-Physiologic Actions. In Fundamentals of Medical Physiology; Joel, M., Ed.; Thieme: New York, NY, USA, 2011; pp. 9–11, 33–35, 467–469, 535. [Google Scholar]
- Denaro, V.; Cittadini, A.; Barnaba, S.A.; Ruzzini, L.; Denaro, L.; Rettino, A.; de Paola, B.; Papapietro, N.; Sgambato, A. Static electromagnetic fields generated by corrosion currents inhibit human osteoblast differentiation. Spine 2008, 33, 955–959. [Google Scholar] [CrossRef] [PubMed]
- Shahgaldi, B.F.; Heatley, F.W.; Dewar, A.; Corrin, B. In vivo corrosion of cobalt-chromium and titanium wear particles. J. Bone Jt. Surg. 1995, 77-B, 962–966. [Google Scholar]
- Nautiyal, V.P.; Mittal, A.; Agarwal, A.; Pandey, A. Tissue response to titanium implant using scanning electron microscope. Natl. J. Maxillofac. Surg. 2013, 4, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, M.; Watanabe, H.; Higashi, H.; Kubosawa, H. Titanium alloy stem as a cause for adverse reaction to metal debris after bipolar hemiarthroplasty. In Case Reports in Orthopedics; Hindawi Publishing Corporation: Cairo, Egypt, 2014; Volume 2014. [Google Scholar] [CrossRef]
- Kim, K.H.; Ramaswamy, N. Electrochemical surface modification of titanium in dentistry. Dent. Mater. J. 2009, 28, 20–36. [Google Scholar] [CrossRef] [PubMed]
- Tanner, A.; Maiden, M.F.J.; Lee, K.; Shulman, L.B.; Weber, H.P. Dental implant infections. Clin. Infect. Dis. 1997, 25, S213–S217. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.; Monteiro, F.J.; Ferraz, M.P. Infection of orthopedic implants with emphasis on bacterial adhesion process and techniques used in studying bacterial-material interactions. Biomatter 2012, 2, 176–194. [Google Scholar] [CrossRef] [PubMed]
- Rugg-Gunn, A. Dental caries: Strategies to control this preventable disease. Acta Med. Acad. 2013, 42, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Stużycka, I. The oral microbiome in dental caries. Pol. J. Microbiol. 2014, 63, 127–135. [Google Scholar] [PubMed]
- Kalesinskas, P.; Kačerguis, T.; Ambrozaitis, A.; Peciulienė, V.; Ericson, D. Reducing dental plaque formation and caries development. A review of current methods and implications for novel pharmaceuticals. Stomatol. Balt. Dent. Maxillofac. J. 2014, 16, 44–52. [Google Scholar]
- Du, J.; Peng, Y.; Zhang, T.; Ding, X.; Aheng, Z. Study on pH-sensitive and thermosensitive polymer networks containing polyacetal segments. J. Appl. Polym. Sci. 2002, 83, 3002–3006. [Google Scholar] [CrossRef]
- Jain, R.; Standley, S.M.; Fréchet, M.J. Synthesis and degradation of pH-sensitive linear poly(amidoamines)s. Macromolecules 2007, 40, 452–457. [Google Scholar] [CrossRef]
- Li, J.; Guoqiang, J.; Ding, F. The effect of pH on the polymer degradation and drug release from PLGA-mPET microparticles. J. Appl. Polym. Sci. 2008, 109, 475–482. [Google Scholar] [CrossRef]
- De Gruyter, W. Analysis of acids and degradation products related to iron and sulfur in the Swedish warship Vasa. Holzforschung 2008, 62, 694–703. [Google Scholar]
- Gu, W.; Simon Ting, S.R.; Stenzel, M.H. Synthesis of pH-responsive and thiol-degradable hollow microspheres. Polymer 2013, 54, 1010–1017. [Google Scholar] [CrossRef]
- Li, R.; Feng, F.; Wang, Y.; Yang, X.; Yang, X.; Yang, V.C. Folic acid-conjugated pH/temperature/redox multi-stimuli responsive polymer microspheres for delivery of anti-cancer drug. J. Colloid Interface Sci. 2014, 429, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Shida, T.; Koseki, H.; Yoda, I.; Horiuchi, H.; Sakoda, H.; Osaki, M. Adherence ability of Staphylococcus epidermidis on prosthetic biomaterials: An in vitro study. Int. J. Nanomed. 2013, 8, 3955–3961. [Google Scholar]
- Shapiro, I.M.; Hickok, N.J.; Parvizi, J.; Stewart, S.; Schaer, T.P. Molecular engineering of an orthopaedic implant: From bench to bedside. Eur. Cells Mater. 2012, 23, 362–370. [Google Scholar]
- Letíc-Gavrilovíc, A.; Scandurra, R.; Abe, K. Genetic potential of interfacial guided osteogenesis in implant devices. Dent. Mater. J. 2000, 19, 99–132. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Cuik, D.Z.; Jeon, H.R.; Chung, H.J.; Park, Y.J.; Kim, O.S.; Kim, Y.J. Surface characteristics of thermally treated titanium surfaces. J. Periodontal Implant Sci. 2012, 42, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H.; Horng, C.J.; Okazaki, M.; Takahashi, J. Oxidation effects on porcelain-titanium interface reactions and bond strength. Dent. Mater. J. 1990, 9, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Hanawa, T. An overview of biofuntionalization of metal in Japan. J. R. Soc. Interface 2009, 6, S361–S369. [Google Scholar] [CrossRef] [PubMed]
- Chawla, K.K. Chapter 2 Reinforcements, 8 Carbon Fiber Composites, 10.2 Micromechanics-Mechanical Properties. In Composite Materials, 2nd ed.; Springer: New York, NY, USA, 1998; pp. 6–71, 252–262, 308–317. [Google Scholar]
- Toda, K.; Miyaura, C.; Okada, T.; Shizuta, Y. Dietary bisphenol A prevent ovarian degeneration and bone loss in female mice lacking the aromatase gene (Cyp 19). Eur. J. Biochem. 2002, 269, 2214–2222. [Google Scholar] [CrossRef] [PubMed]
- Pelch, K.E.; Carleton, S.M.; Phillips, C.L.; Nagel, S.C. Developmental exposure to xenoestrogens at low doses alters femur length and tensile strength in adult mice. Biol. Reprod. 2011, 86, 1–9. [Google Scholar]
- Radzikowska, J.; Gajowik, A.; Dorzyńska, M. Induction of micronuclei in peripheral blood and bone marrow reticulocytes of male mice after subchronic exposure to X-rays and bisphenol A. Rocz. Panstw. Zakl. Hig. 2012, 63, 17–23. [Google Scholar] [PubMed]
- Atkins, P. Chapters Introduction and 22.3 Interaction between Dipoles-Intermolecular Forces. In Physical Chemistry, 5th ed.; W.H. Freeman and Company: New York, NY, USA, 1994; pp. 12, 763–771. [Google Scholar]
- Ali, M.S.; French, T.A.; Hastings, G.W.; Rae, T.; Rushton, N.; Ross, E.R.S.; Wynn-Jones, C.H. Carbon fibre composite bone plates. Development, Evaluation and early clinical experience. J. Bone Jt. Surg. 1990, 72-B, 586–591. [Google Scholar]
- Youxian, D.; Dianxun, W.; Mujin, S.A. Study of the surface of carbon fiber by means of X-ray photoelectron spectroscopy-III. Compos. Sci. Technol. 1987, 30, 119–126. [Google Scholar] [CrossRef]
- Mendes, D.G.; Angel, D.; Grishkan, A.; Boss, J. Histological response to carbon fibre. J. Bone Jt. Surg. 1985, 67-B, 645–649. [Google Scholar]
- Gartner, L.P.; Hiatt, J.L. Chapter 7 Cartilage and Bone-Osteocytes. In Color Textbook of Histology, 3rd ed.; Saunders Elsevier: Philadelphia, PA, USA, 2007; p. 140. [Google Scholar]
- Petersen, R.C. Reactive secondary sequence oxidative pathology polymer model and antioxidant tests. Int. Res. J. Pure Appl. Chem. 2012, 2, 247–285. [Google Scholar] [CrossRef]
- Marcus, R.A.; Sutin, N. Electron transfers in chemistry and biology. Biochim. Biophys. Acta 1985, 811, 265–322. [Google Scholar] [CrossRef]
- Jendrasiak, G.L.; Smith, R.L. The interaction of water with the phospholipid head group and its relationship to the lipid electrical conductivity. Chem. Phys. Lipids 2004, 131, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Berger, L.I.; Pamplin, B.R. Resistivity of semiconductors-table V. In Handbook of Chemistry and Physics, 77th ed.; Lide, D.R., Ed.; CRC Press: Boca Raton, FL, USA, 1996–1997; pp. 12–99. [Google Scholar]
- Halliday, D.; Resnick, R.; Walker, J. Fundamentals of Physics, 4th ed.; John Wiley & Sons: New York, NY, USA, 1993. [Google Scholar]
- Avset, B.S. Evaluation of silicon diodes made on a variety of high-resistivity phosphorus-doped substrates. Nucl. Instrum. Methods Phys. Res. A 1997, 385, 137–144. [Google Scholar] [CrossRef]
- Kossuth. In Quartzel Fused Quartz Textiles Technical Brochure; Saint Gobain: Paris, France, 1998.
- Petersen, R.C. Free-Radical Polymer Science Structural Cancer Model: A Review. Scientifica 2013, 2013. [Google Scholar] [CrossRef]
- Torosantucci, R.; Mozziconacci, O.; Sharov, V.; Schoneich, C.; Jiskoot, W. Chemical modifications in aggregates of recombinant human insulin induced by metal-catalyzed oxidation: Covalent cross-linking via michael addition to tyrosine oxidation products. Pharm. Res. 2012, 29, 2276–2293. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, R.A.; Dean, R.T.; Rodgers, K.J. The impact of specific oxidized amino acids on protein turnover in J774 cells. Biochem. J. 2008, 410, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.C. Computational conformational antimicrobial analysis developing mechanomolecular theory for polymer biomaterials in materials science and engineering. Int. J. Comput. Mater. Sci. Eng. 2014, 3. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petersen, R.C. Titanium Implant Osseointegration Problems with Alternate Solutions Using Epoxy/Carbon-Fiber-Reinforced Composite. Metals 2014, 4, 549-569. https://doi.org/10.3390/met4040549
Petersen RC. Titanium Implant Osseointegration Problems with Alternate Solutions Using Epoxy/Carbon-Fiber-Reinforced Composite. Metals. 2014; 4(4):549-569. https://doi.org/10.3390/met4040549
Chicago/Turabian StylePetersen, Richard C. 2014. "Titanium Implant Osseointegration Problems with Alternate Solutions Using Epoxy/Carbon-Fiber-Reinforced Composite" Metals 4, no. 4: 549-569. https://doi.org/10.3390/met4040549