Analysis of Heavy Metal in Electrocoagulated Metal Hydroxide Sludge (EMHS) from the Textile Industry by Energy Dispersive X-Ray Fluorescence (EDXRF)
Abstract
:1. Introduction
2. Results and Discussion
| Metal | Reference Value (mg/kg) | Analysis Value (mg/kg) | Metal | Reference Value (mg/kg) | Analysis Value (mg/kg) |
|---|---|---|---|---|---|
| Cd | 12.78 | 11.2 | Ni | 80.2 | 87.3 |
| Cr | 202 | 214.1 | Pb | 202.1 | 214 |
| Cu | 627.4 | 664.2 | Hg | 3.64 | 3.3 |
| Mo | 46.7 | 39.3 | Zn | 1273 | 1301.4 |
| As | 7.82 | 8.8 | Ag | 98 | 94.3 |
| Mn | - | - | Se | 16 | 16.1 |
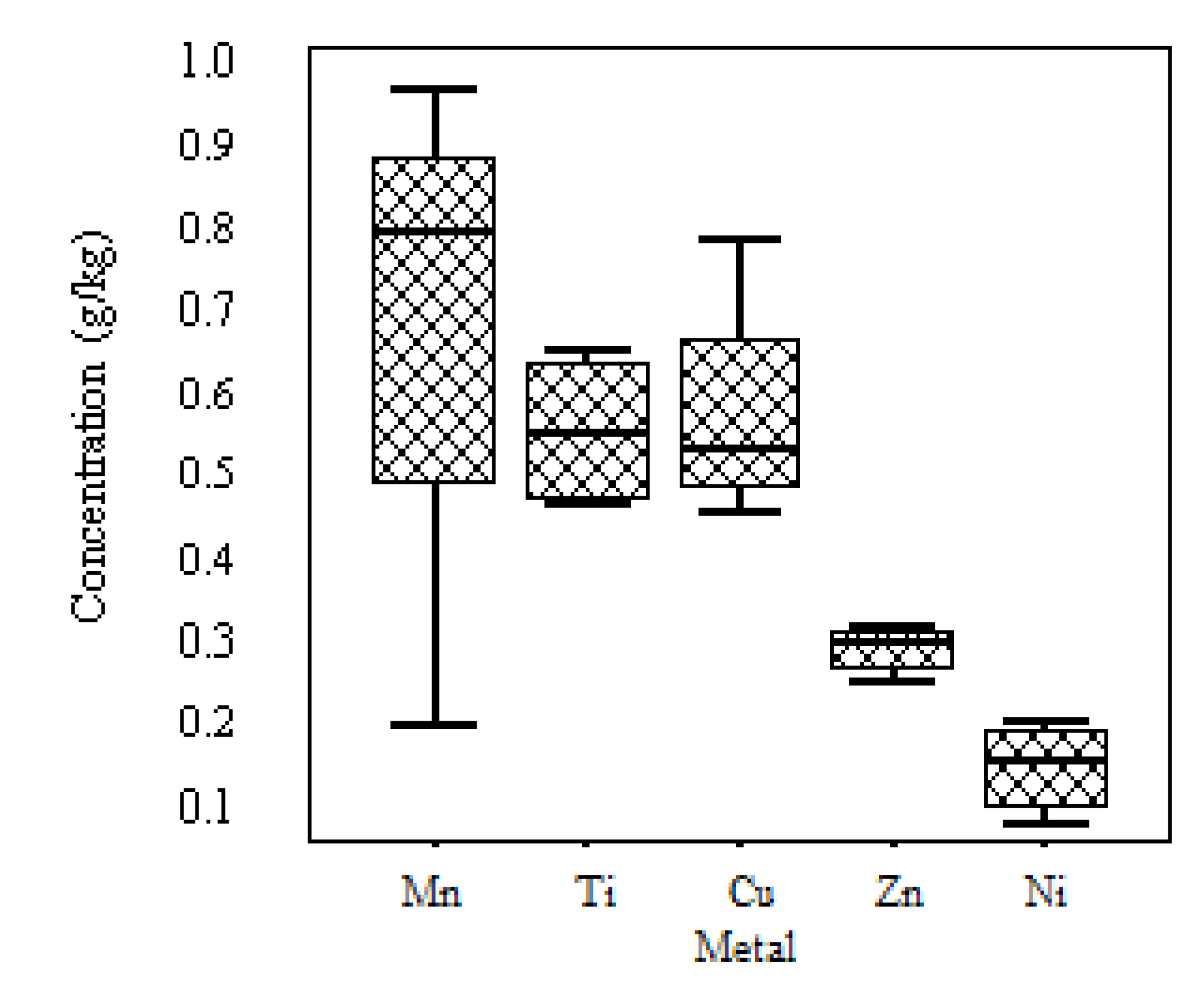
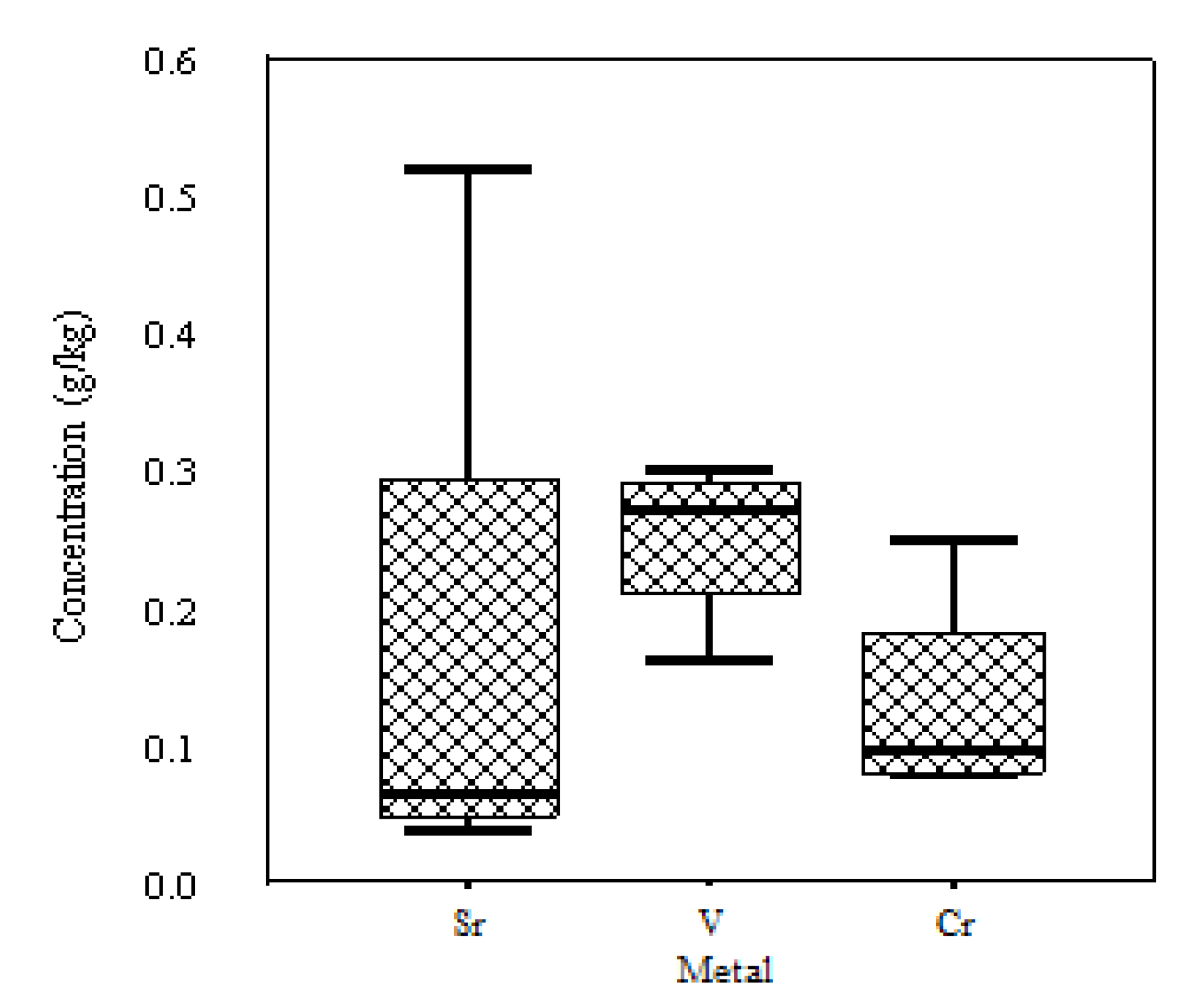
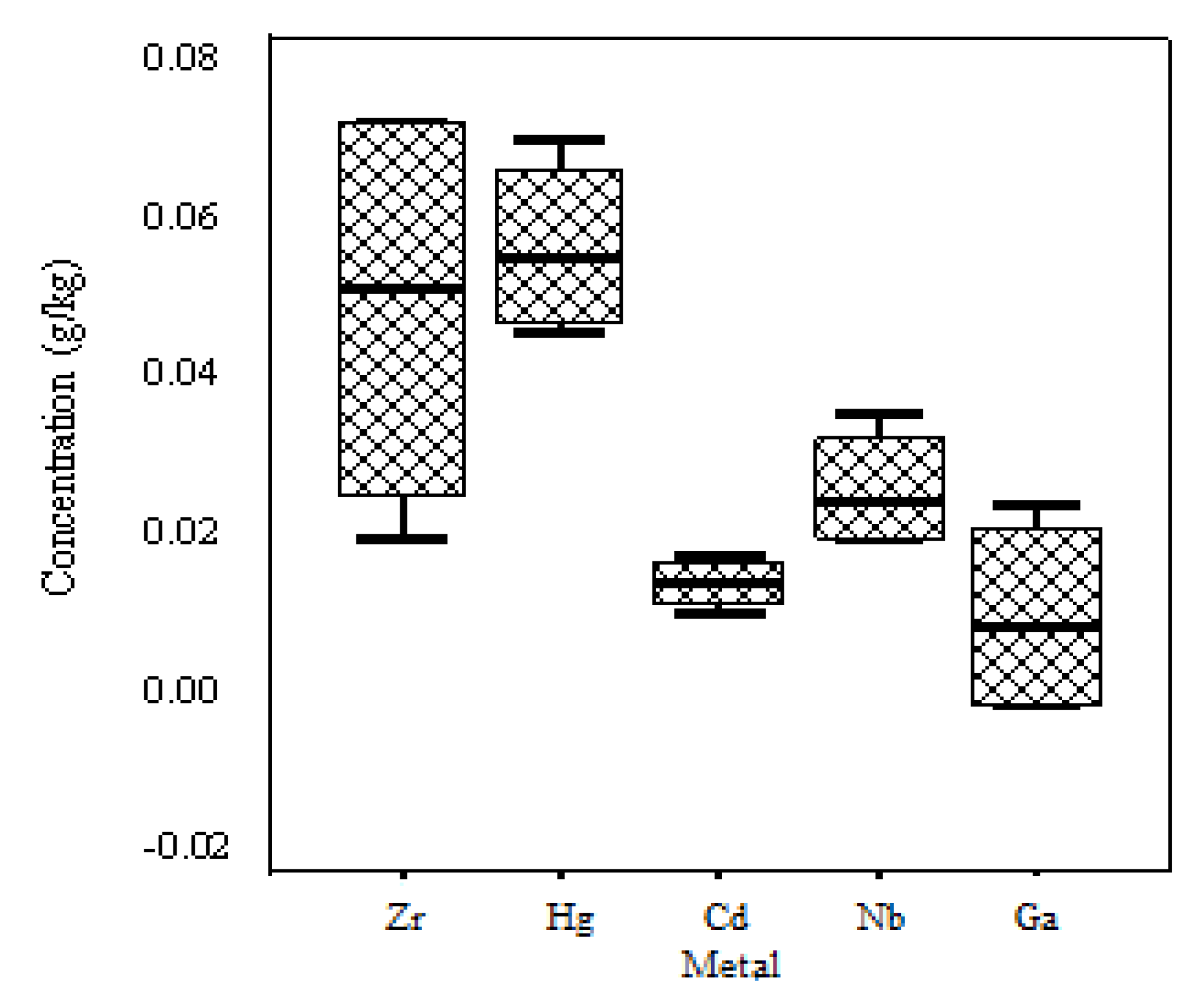
| Mn | Ti | Cu | Zn | Ni | Sr | Zr | V | Cr | Hg | Cd | Nb | Ga | |
| g/kg | mg/kg | ||||||||||||
| Average | 0.76 | 0.56 | 0.53 | 0.31 | 0.18 | 69.33 | 0.05 | 166.5 | 130 | 55 | 14.5 | 26 | 10.5 |
| Standard Deviation | 0.35 | 0.08 | 0.18 | 0.03 | 0.05 | 18.04 | 0.03 | 112.3 | 81.2 | 10.9 | 3.1 | 7.4 | 12.4 |
| Maximum | 0.98 | 0.67 | 0.78 | 0.34 | 0.24 | 88 | 0.07 | 300 | 250 | 68 | 18 | 35 | 24 |
| Minimum | 0.23 | 0.48 | 0.34 | 0.28 | 0.12 | 52 | 0.02 | 26 | 80 | 45 | 11 | 20 | 0 |
| Landfill Legislation (Class I) (g/kg) [37] | NA | NA | 0–6 | 0–8 | 0–2 | NA | NA | NA | 0-3 | NA | NA | NA | NA |
| Landfill Legislation (Class II) (g/kg) [37] | NA | NA | 6–60 | 8–75 | 2–50 | NA | NA | NA | 3–50 | NA | NA | NA | NA |
| Permissible limit in India (mg/kg) [31] | 400 | NA | 0.2–0.3 | 300 | NA | NA | NA | NA | NA | NA | 3–6 | NA | NA |
| SEPAC limit in China (mg/kg) [33] | 688 | NA | 0.1 | 300 | 26.6 | NA | NA | NA | 250 | NA | 0.6 | NA | NA |
| Mn | Ti | Cu | Zn | Ni | Sr | V | Cr | Zr | Hg | Cd | Nb | Ga | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mn | 1 | ||||||||||||
| Ti | −0.498 | 1 | |||||||||||
| Cu | 0.071 | 0.827 | 1 | ||||||||||
| Zn | 0.948 | −0.738 | −0.231 | 1 | |||||||||
| Ni | −0.438 | −0.532 | −0.872 | −0.130 | 1 | ||||||||
| Sr | −0.968 * | 0.397 | −0.184 | −0.907 | 0.453 | 1 | |||||||
| V | 0.886 | −0.238 | 0.327 | 0.802 | −0.492 | −0.973 * | 1 | ||||||
| Cr | −0.973 * | 0.593 | 0.040 | −0.977 * | 0.270 | 0.975 * | −0.911 | 1 | |||||
| Zr | −0.598 | 0.986 * | 0.739 | −0.819 | −0.451 | 0.526 | −0.387 | 0.702 | 1 | ||||
| Hg | −0.624 | 0.193 | −0.241 | −0.614 | 0.203 | 0.794 | −0.893 | 0.740 | 0.348 | 1 | |||
| Cd | 0.343 | −0.978 * | −0.891 | 0.621 | 0.686 | −0.271 | 0.138 | −0.475 | −0.958 * | −0.186 | 1 | ||
| Nb | 0.676 | −0.897 | −0.623 | 0.810 | 0.176 | −0.510 | 0.306 | −0.670 | −0.880 | −0.062 | 0.788 | 1 | |
| Ga | 0.583 | −0.983 * | −0.741 | 0.810 | 0.471 | −0.521 | 0.390 | −0.697 | −0.999 ** | −0.370 | 0.962 * | 0.858 | 1 |
3. Experimental Section
3.1. Samples Collection and Preparation
3.2. Experiment
4. Conclusions
Acknowledgements
References
- Rahman, S.H.; Neelormi, S.; Tareq, S.M. Environmental impact assessments of textile and dyeing industries on ecosystem of karnopara canal at Savar, Bangladesh. Jahangirnagar Univ. J. Sci. 2008, 31, 19–32. [Google Scholar]
- Islam, M.M.; Halim, M.A.; Safiullah, S.; Hoque, S.A.M.W.; Islam, M.S. Heavy metal (Pb, Cd, Zn, Cu, Cr, Fe and Mn) content in textile sludge in Gazipur, Bangladesh. Res. J. Environ. Sci. 2009, 3, 311–315. [Google Scholar] [CrossRef]
- Golder, A.K.; Samanta, A.N.; Ray, S. Anionic reactive dye removal from aqueous solution using a new adsorbent-sludge generated in removal of heavy metal by electrocoagulation. Chem. Eng. J. 2006, 122, 107–115. [Google Scholar] [CrossRef]
- Mollah, M.Y.A.; Schennach, R.; Parga, J.R.; Cocke, D.L. Electrocoagulation (EC)-science and applications. J. Hazard. Mater. 2001, 84, 29–41. [Google Scholar] [CrossRef]
- Thomson, J.C.; Azariah, J.; Viji, A.G.R. Impact of textile industries on river Noyyal and riverine groundwater quality in Tirupur, India. Environ. Monit. Assess. 1999, 18, 359–368. [Google Scholar]
- Lin, D.F.; Weng, C.H. Use of sewage sludge ash as brick material. J. Environ. Eng. 2001, 127, 922–727. [Google Scholar] [CrossRef]
- Weng, C.H.; Lin, D.F.; Chiang, P.C. Utilization of sludge as brick materials. Adv. Environ. Res. 2003, 7, 679–685. [Google Scholar]
- Chen, Y.; Wang, C.; Wang, Z. Residues and source identification of persistent organic pollutants in farmland soils irrigated by effluents from biological treatment plants. Environ. Int. 2005, 31, 778–783. [Google Scholar] [CrossRef]
- Manahan, S.E. Environmental Chemistry, 8th ed; Lewis Publisher: Boca Raton, Florida, FL, USA, 2005; pp. 110–117. [Google Scholar]
- Balasubramania, J.; Sabumon, P.C.; Lazar, J.U.; Ilangovan, R. Reuse of textile effluent treatment plant sludge in building materials. Waste Manag. 2006, 26, 22–28. [Google Scholar] [CrossRef]
- Baskar, R.; Begum, K.M.M.S.; Sundaram, S. Characterization and reuse of textile effluent treatment plant waste sludge in clay bricks. J. Univ. Chem. Technol. Metall. 2006, 41, 473–478. [Google Scholar]
- Patel, H.; Pandey, S. Exploring the reuse potential of chemical sludge from textile wastewater treatment plants in India—A hazardous waste. Am. J. Environ. Sci. 2009, 5, 106–110. [Google Scholar]
- Wilson, B.; Pyatt, F.B. Heavy metal dispersion, persistence, and bioaccumulation around an ancient copper mine situated in Anglesey, UK. Ecotoxicol. Environ. Saf. 2007, 66, 224–231. [Google Scholar] [CrossRef]
- Singh, K.P.; Mohan, D.; Sinha, S.; Dalwani, R. Impact assessment of treated/untreated wastewater toxicants discharged by sewage treatment plants on health, agricultural and environmental quality in the wastewater disposal area. Chemosphere 2004, 55, 227–255. [Google Scholar] [CrossRef]
- Muchuweti, M.; Birkett, J.W.; Chinyanga, E.; Zvauya, R.; Scrimshaw, M.D.; Lester, J.N. Heavy metal content of vegetables irrigated with mixture of wastewater and sewage sludge in Zimbabwe: Implications for human health. Agr. Ecosyst. Environ. 2006, 112, 41–48. [Google Scholar] [CrossRef]
- Senesi, G.S.; Baldassarre, G.; Senesi, N.; Redina, B. Trace element inputs into soils by anthropogenic activities and implications for human health. Chemosphere 1999, 39, 343–377. [Google Scholar] [CrossRef]
- Turkdogan, M.K.; Kilicel, F.; Kara, K.; Tuncer, I. Heavy metals in soil, vegetables and fruits in the endemic upper gastrointestinal cancer region of Turkey. Environ. Toxicol. Pharmacol. 2002, 13, 175–179. [Google Scholar]
- Kocasoy, G.; Sahin, V. Heavy metal removal from industrial wastewater by clinoptilolite. J. Environ. Sci. Health 2007, 42, 2139–2146. [Google Scholar]
- Santra, S.; Mitra, D.; Sarkar, M.; Bhattacharya, D.; Denker, A.; Opitz-Coutureau, J.; Rauschenberg, J. Analysis of some coins by energy dispersive X-ray fluorescence (EDXRF) and high energy particle induced X-ray emission (PIXE) techniques. Nucl. Instrum. Methods Phys. Res. Sect. B 2005, 229, 465–470. [Google Scholar] [CrossRef]
- Singh, V.; Agrawal, H.M.; Joshi, G.C.; Sudershan, M.; Sinha, A.K. Elemental profile of agricultural soil by the EDXRF technique and use of the Principal Component Analysis (PCA) method to interpret the complex data. Appl. Radiat. Isot. 2011, 69, 969–974. [Google Scholar] [CrossRef]
- Polat, R.; Gürol, A.; Karabulut, A.; Ertuğrul, M. Elemental composition of cement Kiln dust, raw material and cement from coal-fried cement factory using energy dispersive X-ray fluorescence spectroscopy. J. Quant. Spectrosc. Radiat. Transfer 2004, 83, 377–385. [Google Scholar] [CrossRef]
- Buduk, G.; Karabulut, A. X ray flurocense analysis of malachite ore concentrates in the Naruma Region, Spain. Spectrochim. Acta B 1999, 203, 119–124. [Google Scholar]
- Yu, K.N.; Yeung, Z.; Leea, L.; Stokes, M.J.; Kwok, R.C.W. Determination of multi-element profiles of soil using energy-dispersive X-ray fluorescenc (EDXRF). Appl. Radiat. Isot. 2002, 57, 279–284. [Google Scholar]
- Vijayan, V.; Rautray, T.R.; Basa, D.K. EDXRF study of Indian punch-marked silver coins. Nucl. Instr. Meth. 2004, 225, 353–356. [Google Scholar] [CrossRef]
- Obiajunwa, E.I.; Pelemo, D.A.; Owolabi, S.A.; Fasasi, M.K.; Johnson-Fatokun, F.O. Characterisation of heavy metal pollutants of soils and sediments around a crude-oil production terminal using EDXRF. Nucl. Instrum. Methods Phys. Res. Sect. B 2002, 194, 61–64. [Google Scholar] [CrossRef]
- Obiajunwa, E.I.; Johnson-Fatokun, F.O.; Olaniyi, H.B.; Olowole, A.F. Determination of the elemental composition of aerosol samples in the working environment of a secondary lead smelting company in Nigeria using EDXRF technique. Nucl. Instrum. Methods Phys. Res. Sect. B 2002, 194, 65–68. [Google Scholar] [CrossRef]
- Joshi, G.C.; Agrawal, H.M.; Mohanta, B.; Sudarshan, M.; Sinha, A.K. Elemental study of Nainital Lake water by EDXRF. Nucl. Instrum. Methods Phys. Res. Sect. B 2006, 251, 223–226. [Google Scholar] [CrossRef]
- Pitarch, A.; Queralt, I. Energy dispersive X-ray fluorescence analysis of ancient coins: The case of Greek silver drachmae from the Emporion site in Spain. Nucl. Instrum. Methods Phys. Res. Sect. B 2010, 268, 1682–1685. [Google Scholar] [CrossRef]
- Elossaief, M.; Kallel, N.; Yaacoubi, A.; Benzina, M. Mineralogical identification, spectostoscopic characterization, and potential environmental uses of natural clay minerals on chromate removal from aquous solutions. Chem. Eng. J. 2011, 168, 1024–1031. [Google Scholar] [CrossRef]
- Akpan, I.O.; Amodu, A.E.; Akpan, A.E. Elemental analysis of limestone samples from Obajana and Mfamosing limestone deposits, Nigeria using nuclear technique. Appl. Radiat. Isot. 2011, 69, 1355–1358. [Google Scholar] [CrossRef]
- Awashthi, S.K. Prevention of Food Adulteration Act no 37 of 1954 Central and State Rules as Amended for 1999, 3rd ed; Ashoka Law House: New Delhi, India, 2000; pp. 1–71. [Google Scholar]
- CCME (Canadian Council of Ministers of the Environment), Canadian Soil Quality Guidelines for the Protection of the Environment and Human Health; Canadian Council of Ministers of the Environment: Winnipeg, Canada, 2003; pp. 1–175.
- SEPAC (State Environmental Protection Administration of China), Chinese Environmental Quality Standard for Soils; State of Environment Protection Bureau: Beijing, China, 1995; pp. 1–4.
- Su, D.C.; Wong, J.W.C. Chemical speciation and phytoavailability of Zn, Cu, Ni and Cd in soil amended with fly ash-stabilized sewage sludge. Environ. Int. 2003, 29, 895–900. [Google Scholar]
- Udom, B.E.; Mbagwu, J.S.C.; Adesodun, J.K.; Agbim, N.N. Distributions of zinc, copper, cadmium and lead in a tropical ultisol after long-term disposal of sewage. Environ. Int. 2004, 30, 467–470. [Google Scholar] [CrossRef]
- Dai, J.Y.; Xu, M.Q.; Chen, J.P.; Yang, X.P.; Ke, Z.S. PCDD/F, PAH and heavy metals in the sewage sludge from six wastewater treatment plants in Beijing China. Chemosphere 2007, 66, 353–361. [Google Scholar] [CrossRef]
- Gonzalez-Fernandez, O.; Aueralt, I.; Carvalho, M.L.; Garcia, G. Elemetal analysis of mining wastes by energy dispersive X ray fluorescence (EDXRF). Nucl. Instrum. Methods Phys. Res. Sect. B 2007, 262, 81–86. [Google Scholar] [CrossRef]
- Rahmat, M.N. Development of environmentally friendly building material: Analysis of the use of solidified industrial waste. Available online: http://130.203.133.150/showciting?cid=13498366 (accessed on 8 October,2012).
- Tay, J.H.; Show, K.Y. Constructive Sludge Disposal Option Converting Sludge into Innovative Civil Engineering Materials. In Proceedings of 7th International Association on Water Quality (IAWQ). Asia-Pacific Regional Conference, Taipei, Taiwan, 1999; pp. 1023–1028.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Adyel, T.M.; Rahman, S.H.; Khan, M.; Islam, S.M.N. Analysis of Heavy Metal in Electrocoagulated Metal Hydroxide Sludge (EMHS) from the Textile Industry by Energy Dispersive X-Ray Fluorescence (EDXRF). Metals 2012, 2, 478-487. https://doi.org/10.3390/met2040478
Adyel TM, Rahman SH, Khan M, Islam SMN. Analysis of Heavy Metal in Electrocoagulated Metal Hydroxide Sludge (EMHS) from the Textile Industry by Energy Dispersive X-Ray Fluorescence (EDXRF). Metals. 2012; 2(4):478-487. https://doi.org/10.3390/met2040478
Chicago/Turabian StyleAdyel, Tanveer Mehedi, Syed Hafizur Rahman, Mala Khan, and S.M. Nazrul Islam. 2012. "Analysis of Heavy Metal in Electrocoagulated Metal Hydroxide Sludge (EMHS) from the Textile Industry by Energy Dispersive X-Ray Fluorescence (EDXRF)" Metals 2, no. 4: 478-487. https://doi.org/10.3390/met2040478




