Corrosion Protection of Electrically Conductive Surfaces
Abstract
:1. Introduction
1.1. Corrosion
| Material | H2S | SO2 | Cl2/4 d | NO2 | NH3 | air/120 °C | Salt fog |
|---|---|---|---|---|---|---|---|
| Au, 3 µm | 1 | 1 | 2 | 1 | 1 | 1 | 1 |
| AuCo1, 3 µm | 1 | 1 | 3 | 1 | 1 | 3 | 1 |
| AuNi10, 3 µm | 2 | 2 | 1 | 1 | 1 | 2 | 1 |
| Au, 0.2 µm | 1 | 3 | 3 | 3 | 1 | 3 | 2 |
| Ni, 3 µm/Au, 1 µm | 3 | 1 | 3 | 1 | 1 | 1 | 1 |
| Ni, 3 µm/Au, 3 µm | 1 | 1 | 3 | 1 | 1 | 1 | 1 |
| Ag, 10 µm | 2 | 1 | 2 | 3 | 1 | 1 | 2 |
| Ni/Pd, 3 µm | 2 | 2 | 3 | 1 | 1 | 1 | 1 |
| Chem. Sn, 0.5 µm | 2 | 2 | 3 | 3 | 1 | 3 | 2 |
| electroplated Sn, dull, 15 µm | 1 | 3 | 1 | 1 | 1 | 1 | 2 |
| electroplated Sn, bright, 15 µm | 1 | 2 | 3 | 1 | 1 | 1 | 2 |
| PbSn 40/60, 6 µm | 1 | 1 | 2 | 1 | 1 | 1 | 2 |
| hot dip tin, 6 µm | 1 | 1 | 3 | 1 | 1 | 1 | 2 |
| Ni, 3 µm | 3 | 3 | 3 | 2 | 2 | 2 | 2 |
| Cu | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
1.2. Fretting Corrosion
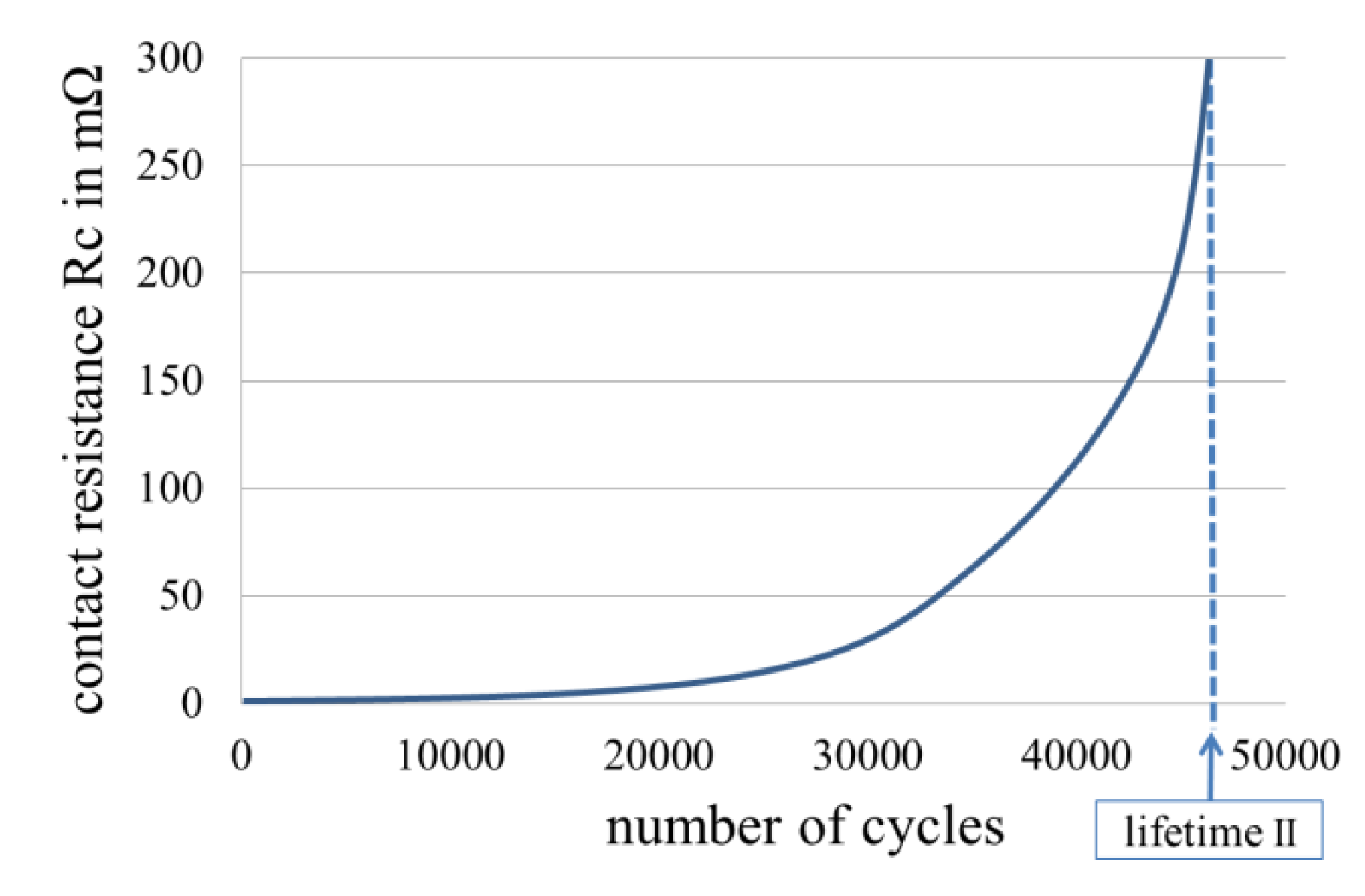
1.3. Development of Fretting Corrosion
1.4. Fretting Wear
1.5. Wear Patterns of Electrical Contacts



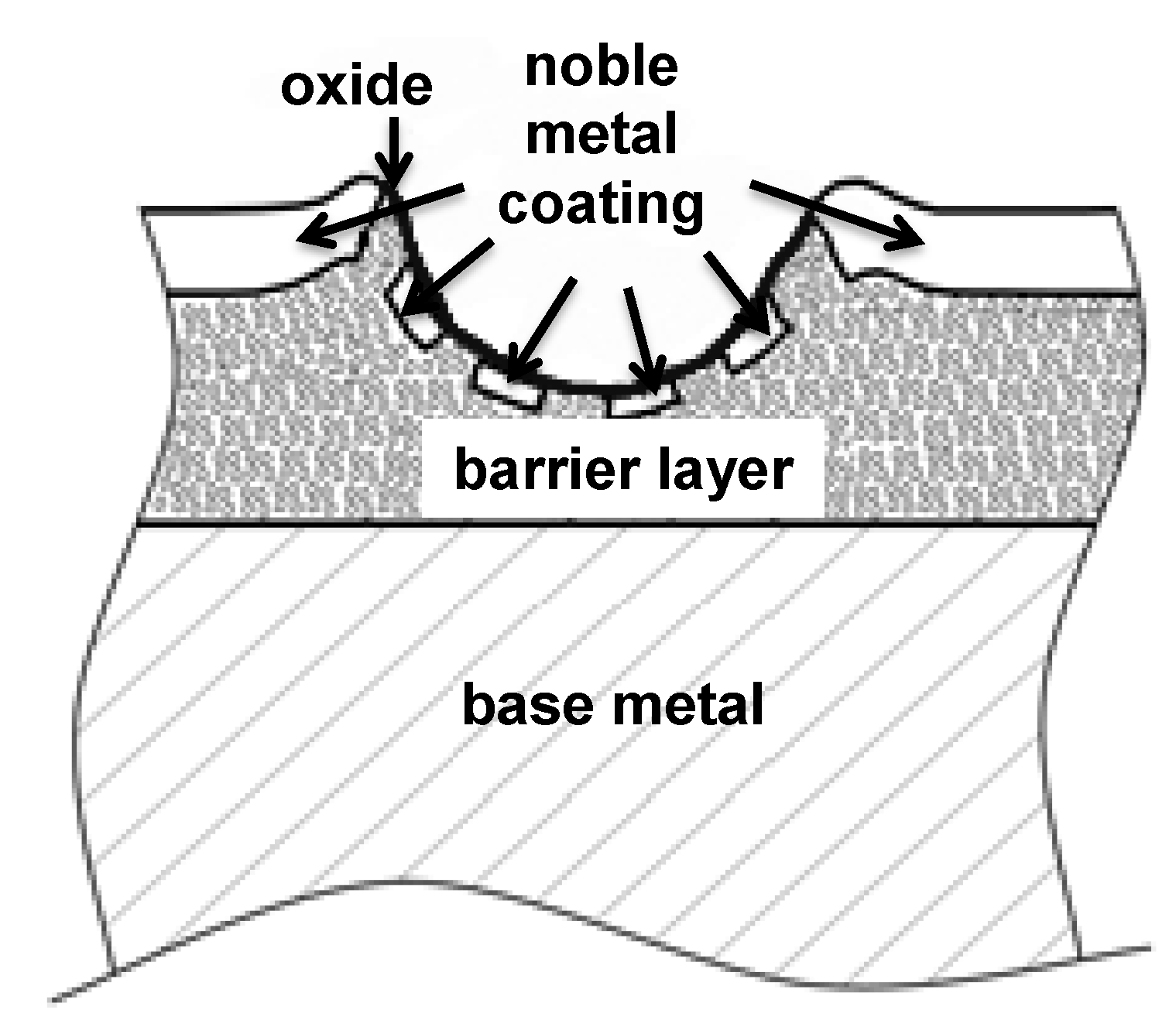
1.6. The Main Scope—Solutions for Combined Protection against Corrosion, Fretting Wear and Fretting Corrosion
- Increasing the hardness by means of:
- Optimized proportion of alloy elements and;
- Hard nanoparticles;
- Improved wear resistance and wear pattern of coatings;
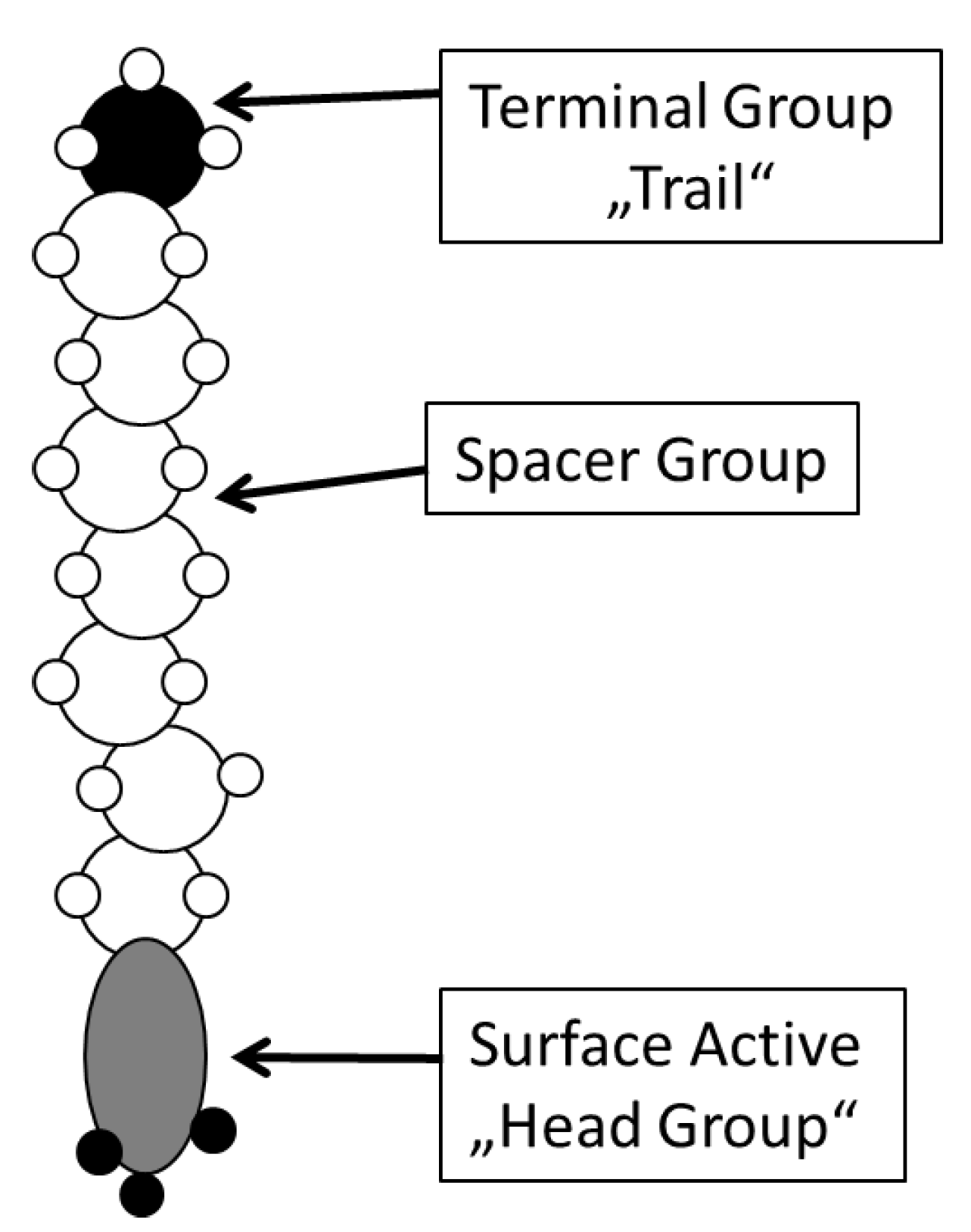
- Improved surface perfection with thin coatings by employing SAM (self assembled monolayer).
- The passivation layer should not increase or destabilize the electrical resistance markedly and;
- For many applications the passivation layer must resist the operating temperature which is the sum of resistive heating and ambient temperature.

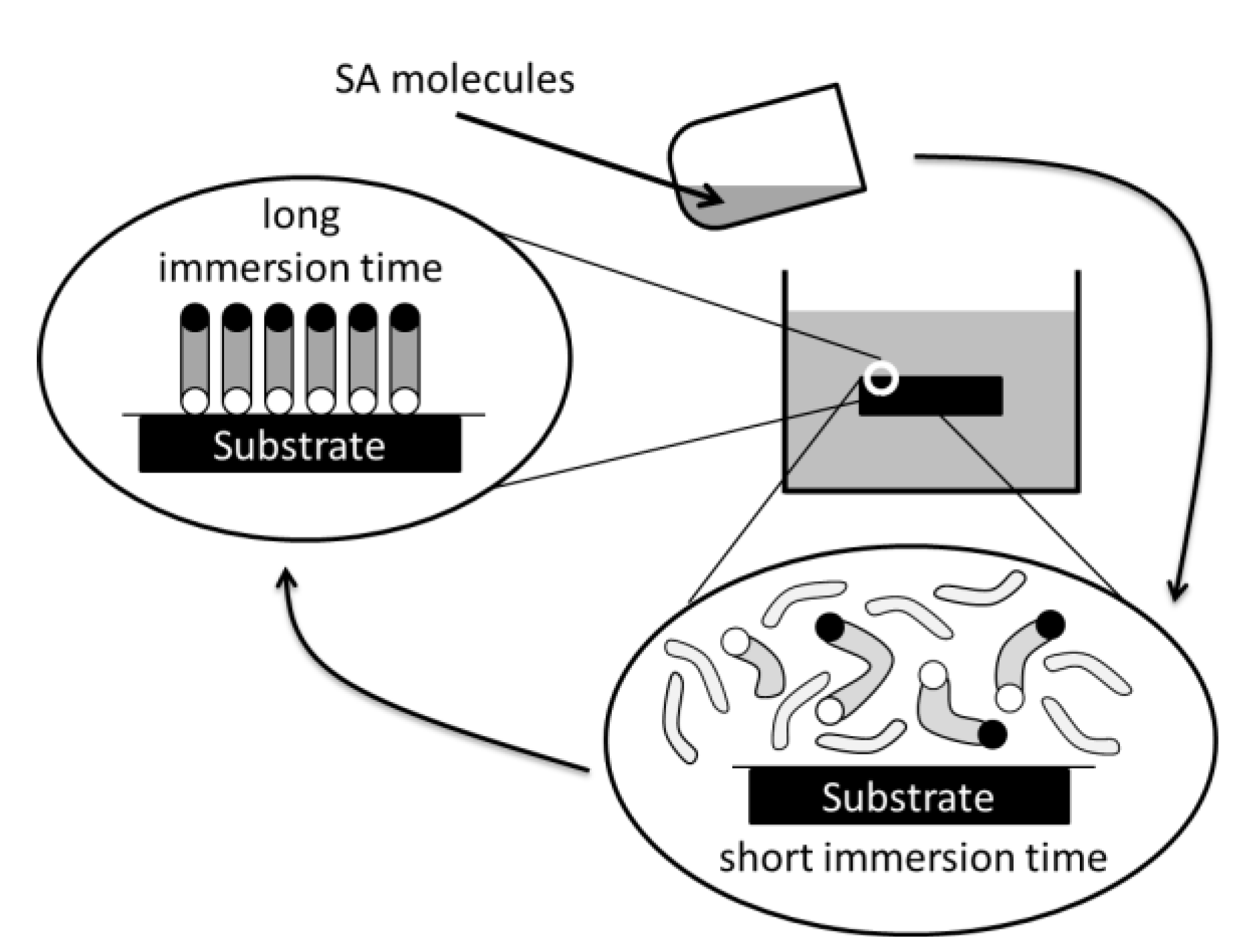
2. Experimental Section
2.1. Corrosion Tests
2.2. Measurement of Fretting Corrosion and Wear and Surface Analysis

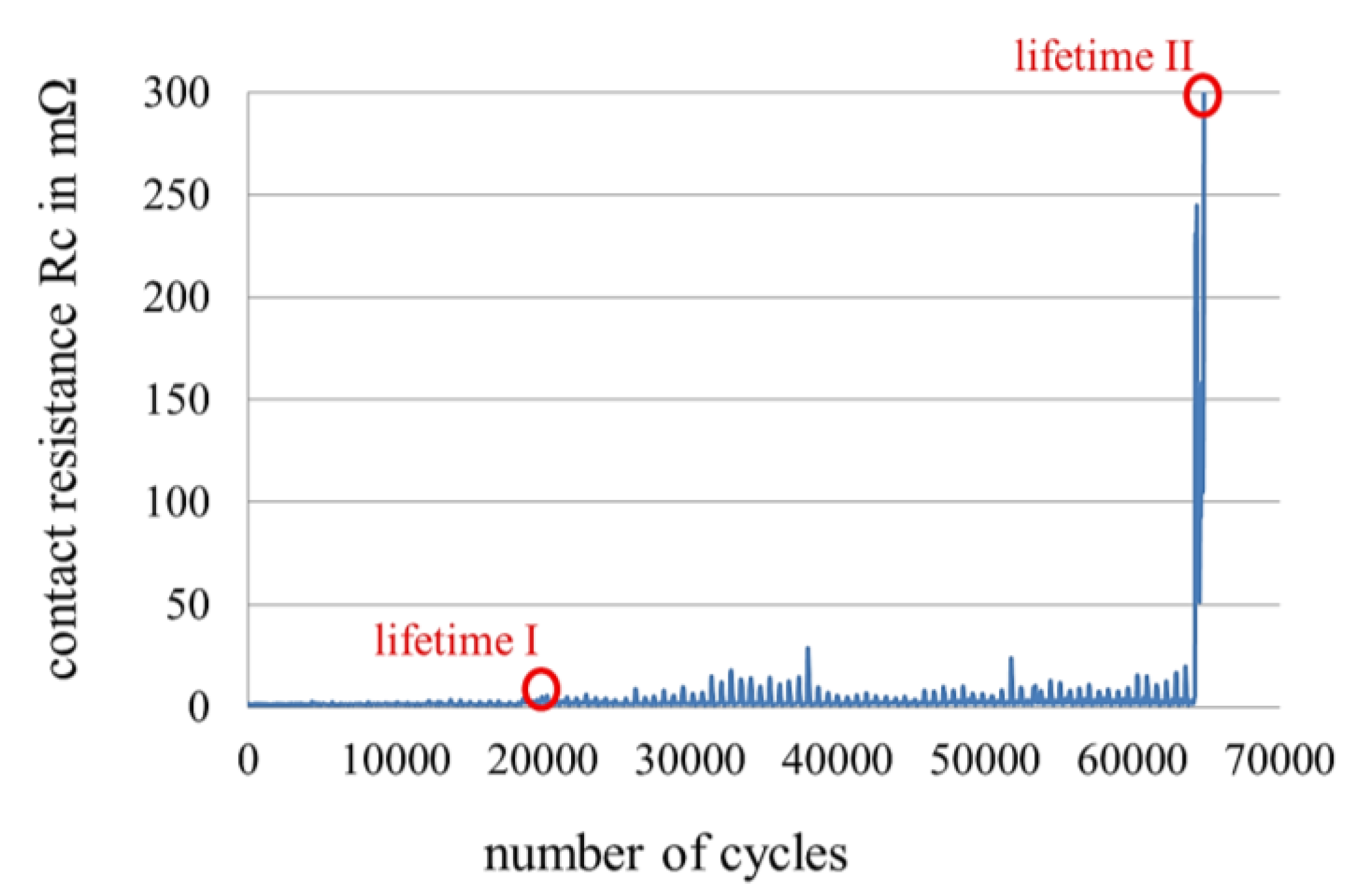
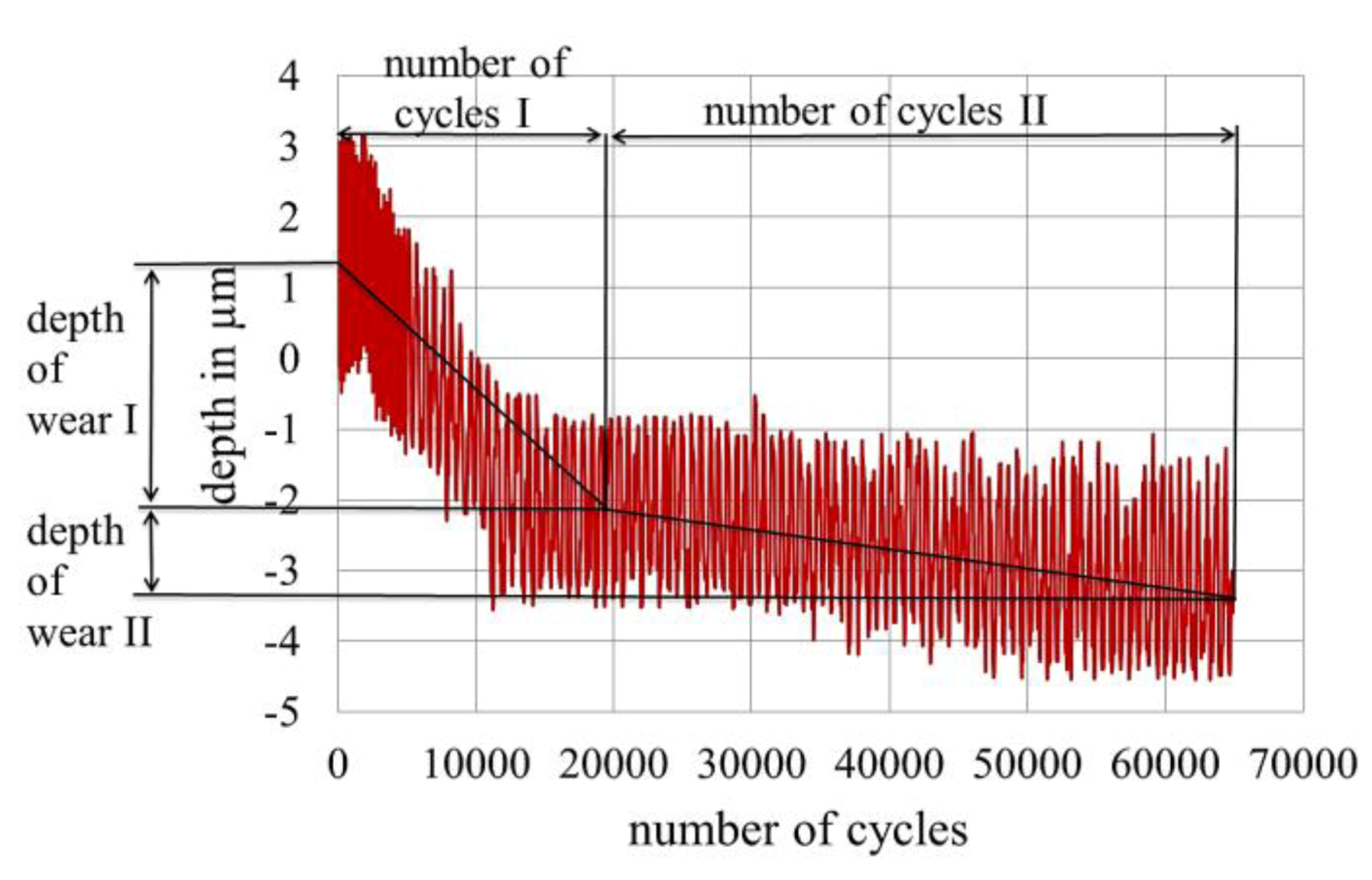
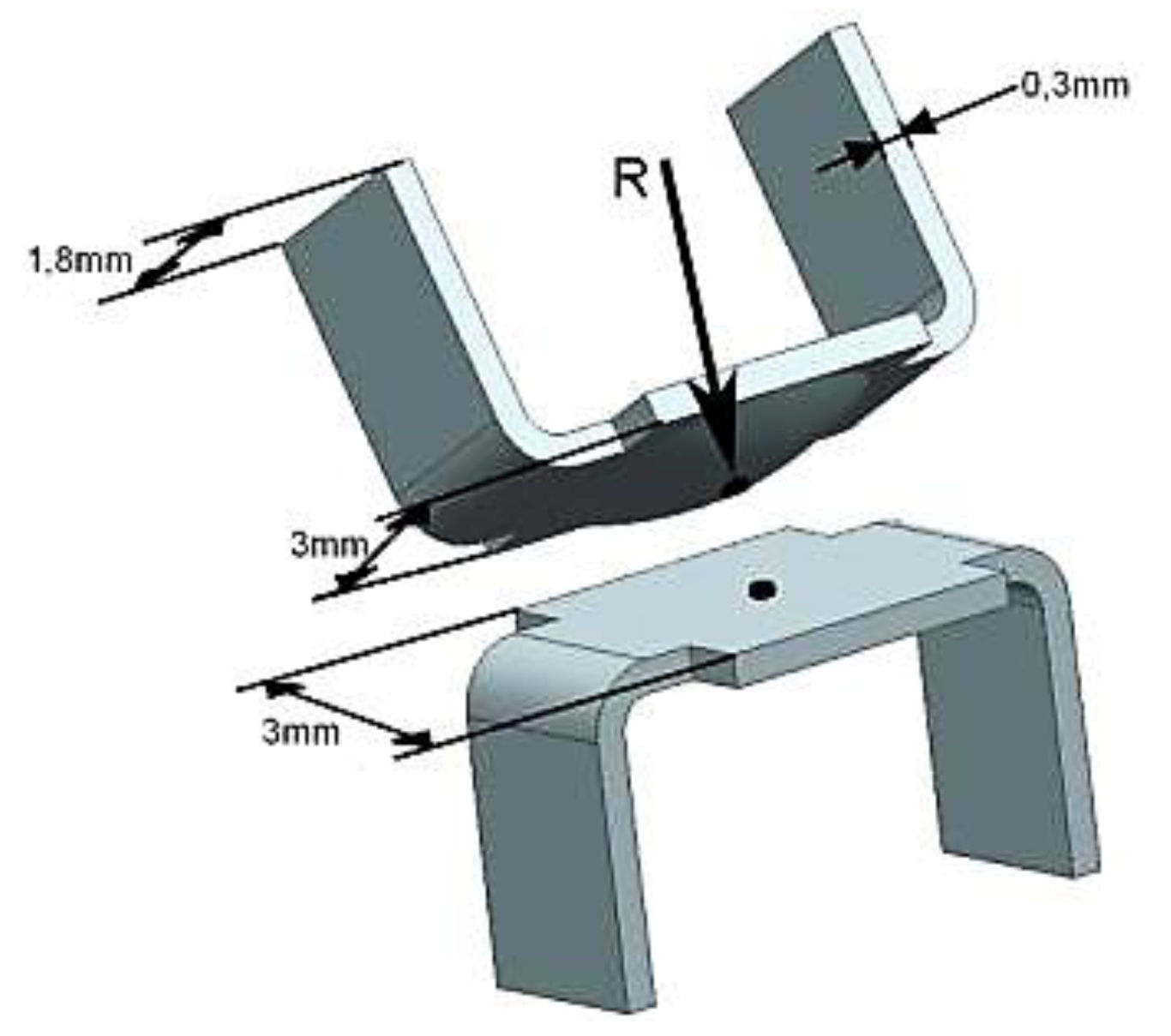
2.3. Sample Size, Base Materials and Plating Materials

3. Results and Discussion
3.1. Porosity and Corrosion Resistance
3.1.1. Thickness and Porosity of Gold Coating

3.1.2. SAM and Porosity
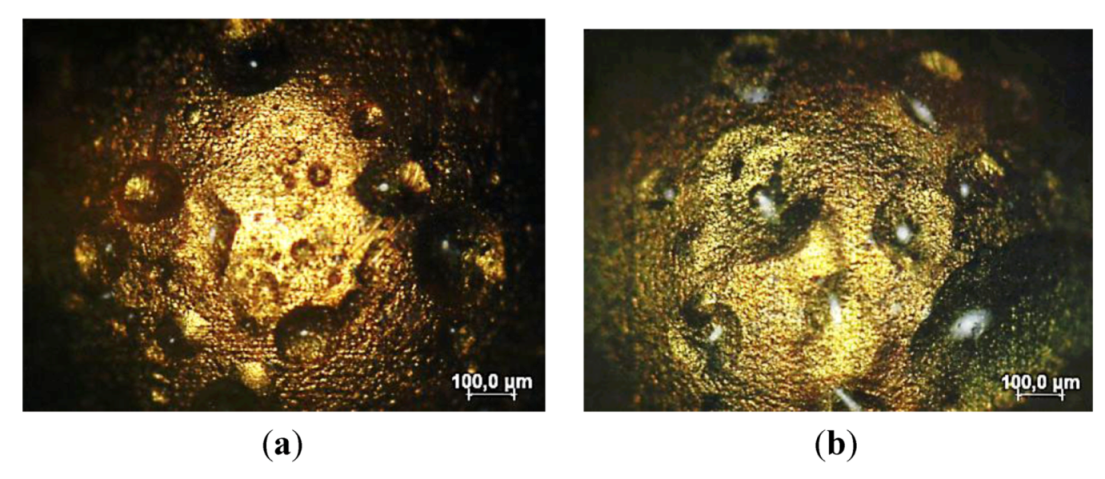
3.2. Corrosion Resistance of Other Coating Materials
| Coating material | Thickness in micron | Contact resistance in milliohm | Change of contact resistance | |
|---|---|---|---|---|
| initial | After 1st cycle | After 2nd cycle | ||
| Ni | 4.3 | 73.08 | 8200% | 40000000% |
| Sn dull | 4.3 | 2.91 | 70%–180% | 350% |
| Sn bright | 6 | 2.29 | 14% | 5450% |
| Ag | 6.1 | 2.64 | 50% | 335% |
| Pure Au | 0.4 | 14.33 | −50% | 60%–225% |

3.3. Fretting Corrosion
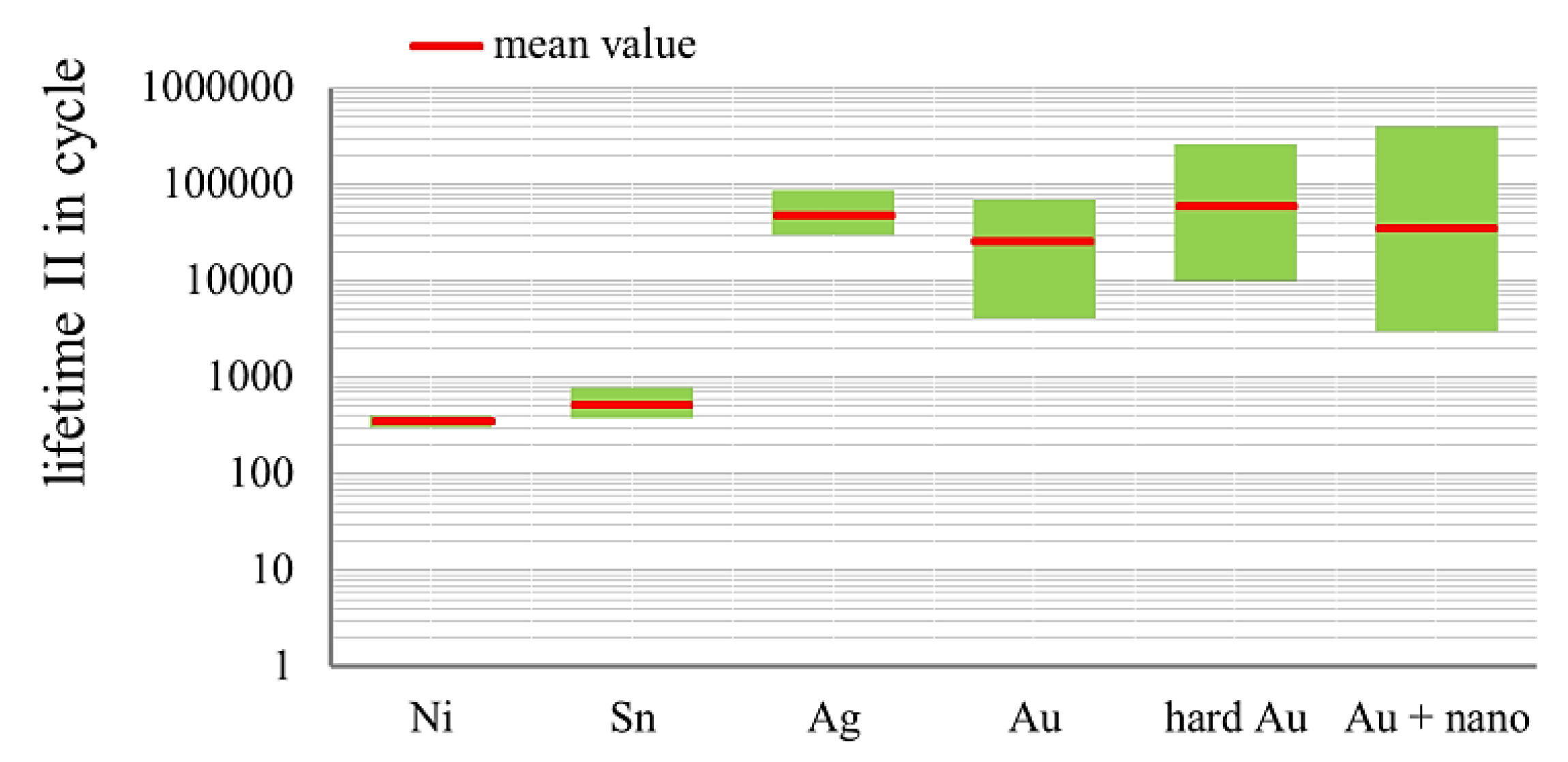
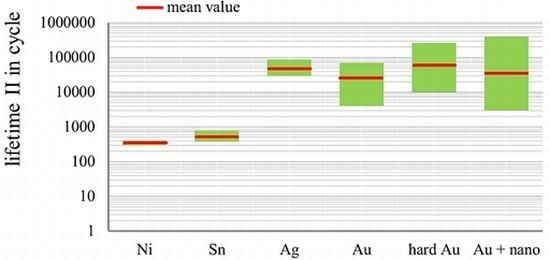
- There is a large potential in terms of improving the lifetime of gold layers with alloy elements and nanoparticles;
- Putting alloy elements or nanoscale particles alone in the layers would not be sufficient. Other basic conditions have to be taken into account in order to achieve the desired effects.
3.3.1. Influence of Proportion of Alloy Element in Gold Coatings on the Lifetime in Fretting Corrosion Tests


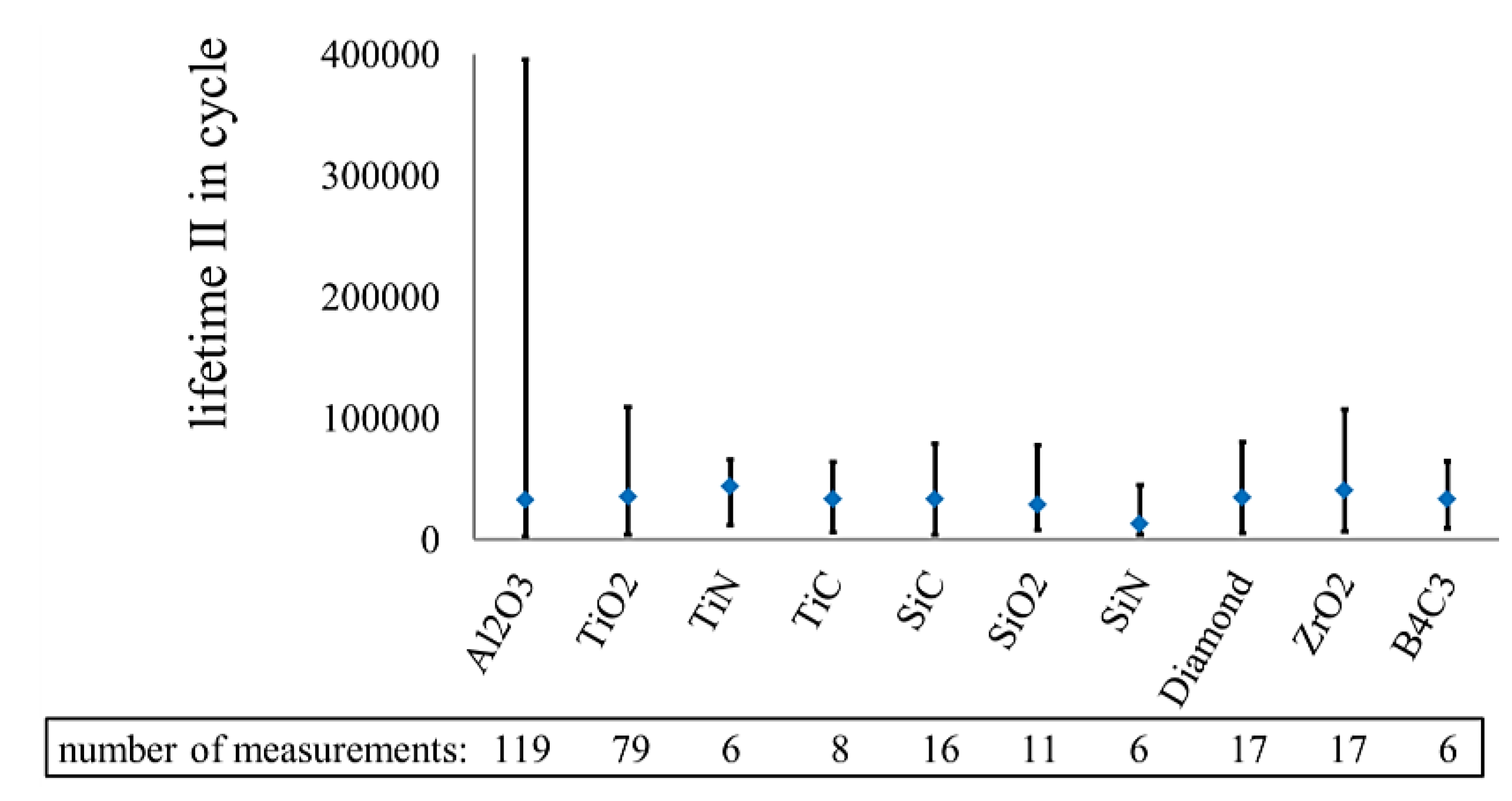
3.3.2. Influence of Nanoscale Particles in Electrolytes on the Lifetime in Fretting Corrosion Tests
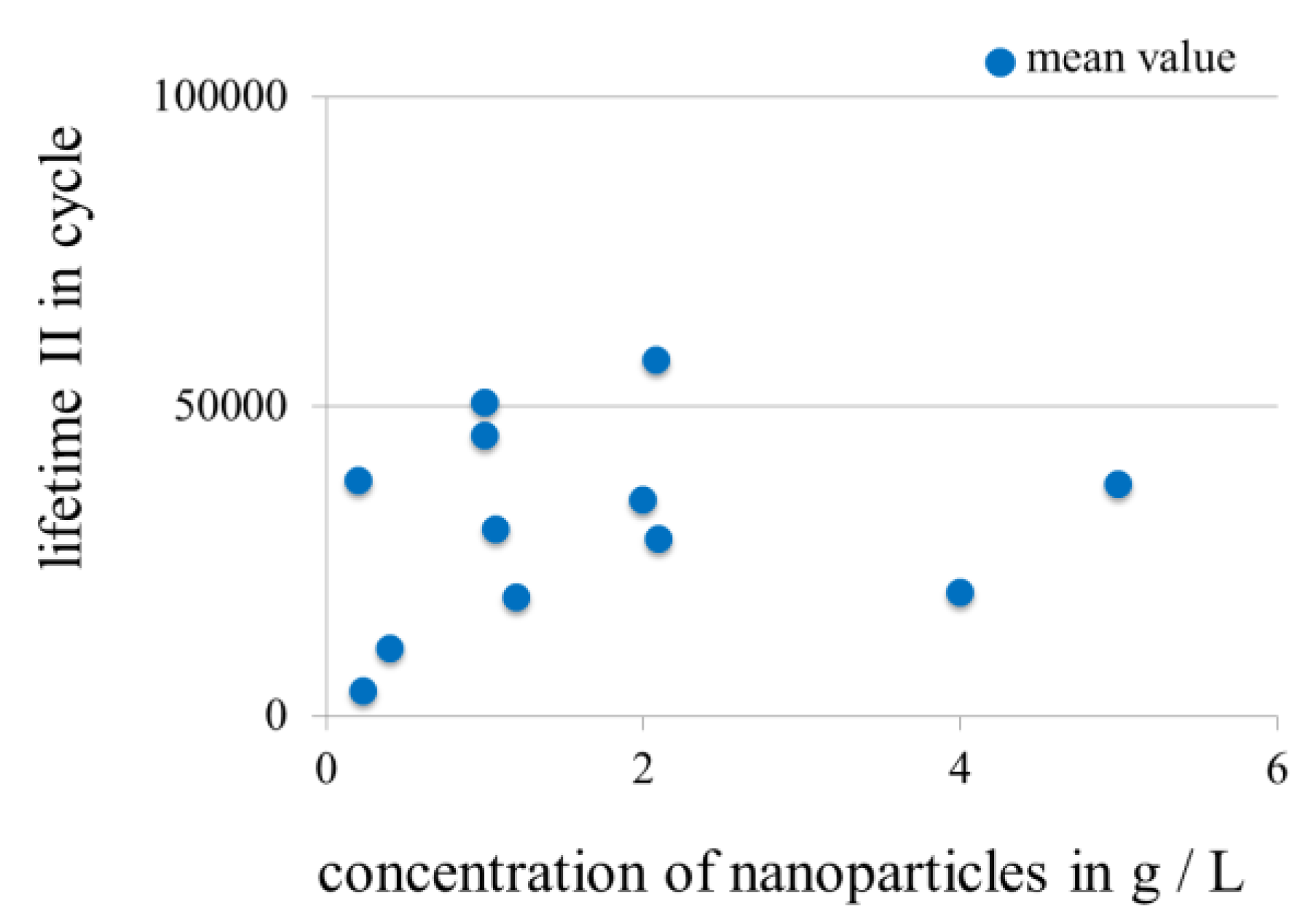
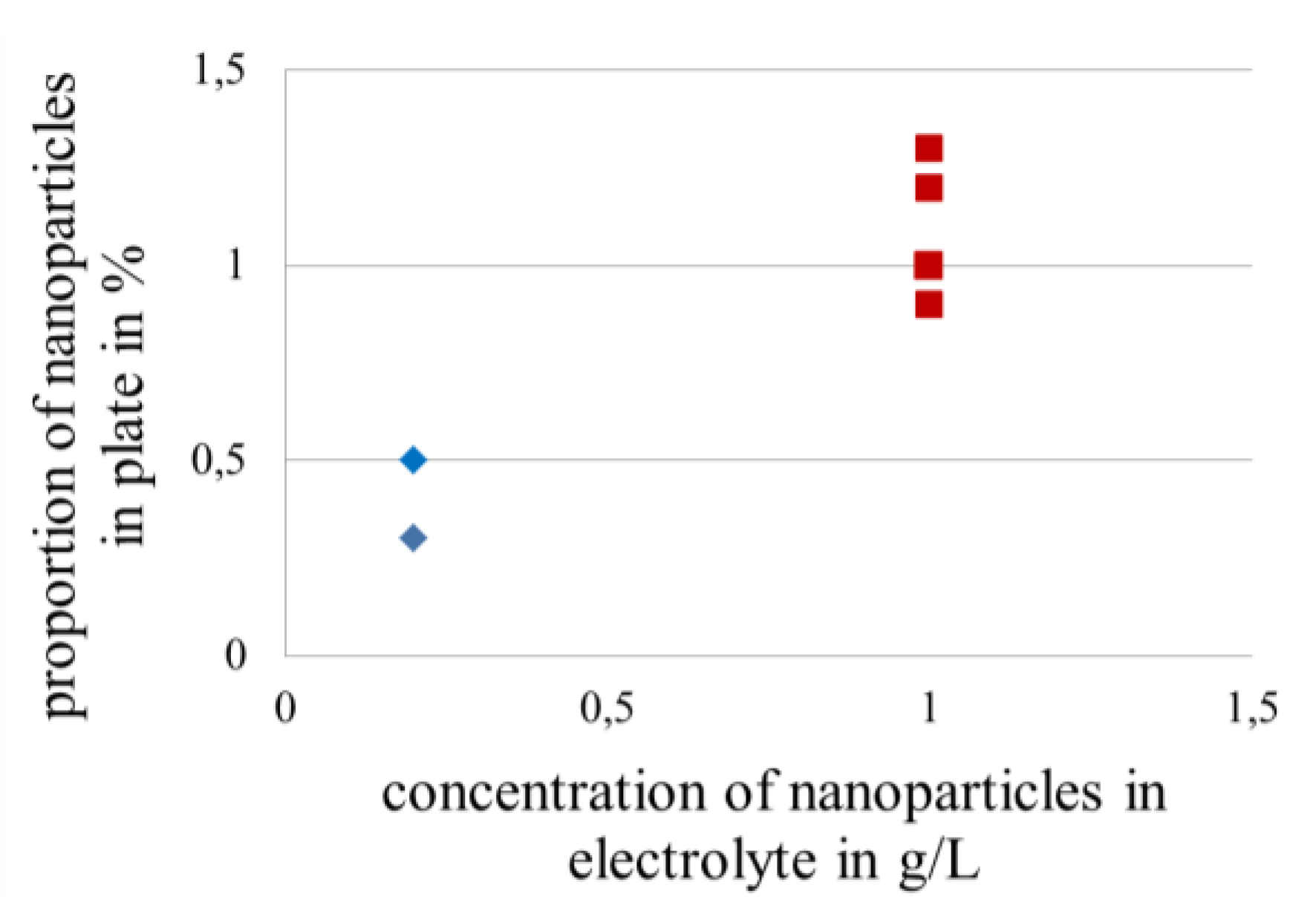
3.3.3. Influence of Concentration of Nanoscale Particles in Electrolytes on the Lifetime in Fretting Corrosion Tests
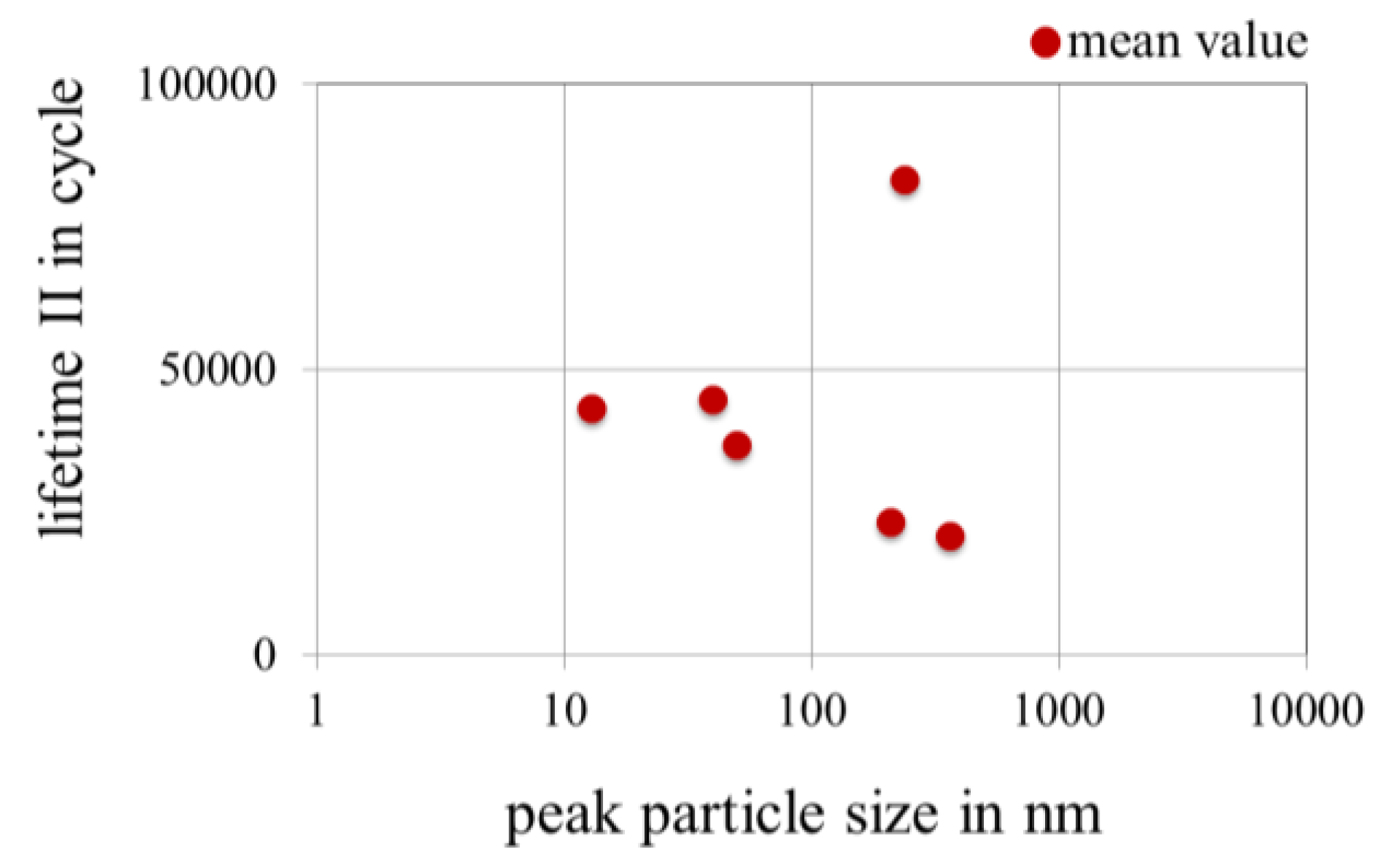
3.3.4. Effect of Peak Particle Size of Nanoparticles in Gold Coatings on the Lifetime in Fretting Corrosion Tests

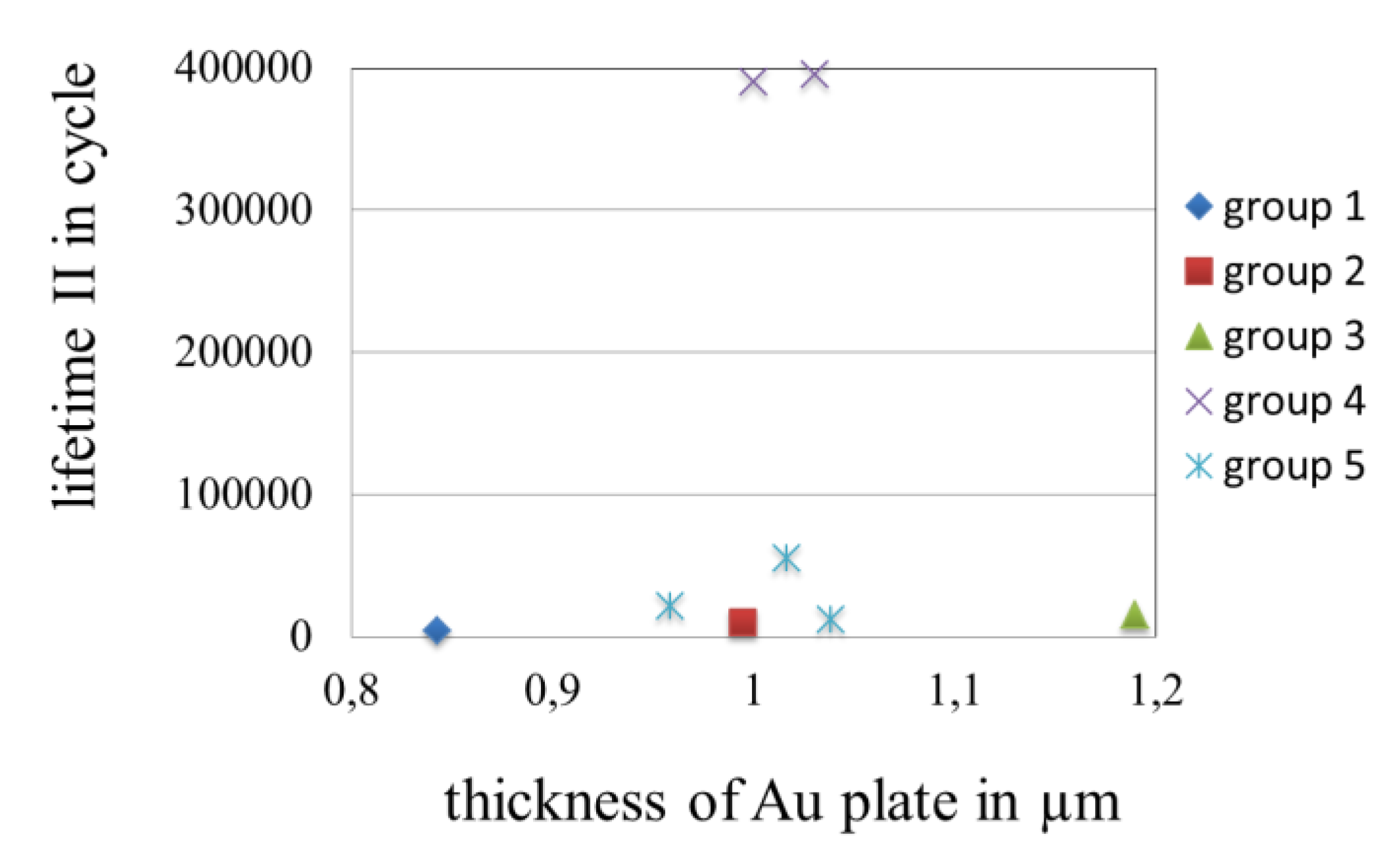
3.3.5. Effect of the Thickness of Gold Coatings on the Lifetime in Fretting Corrosion Tests


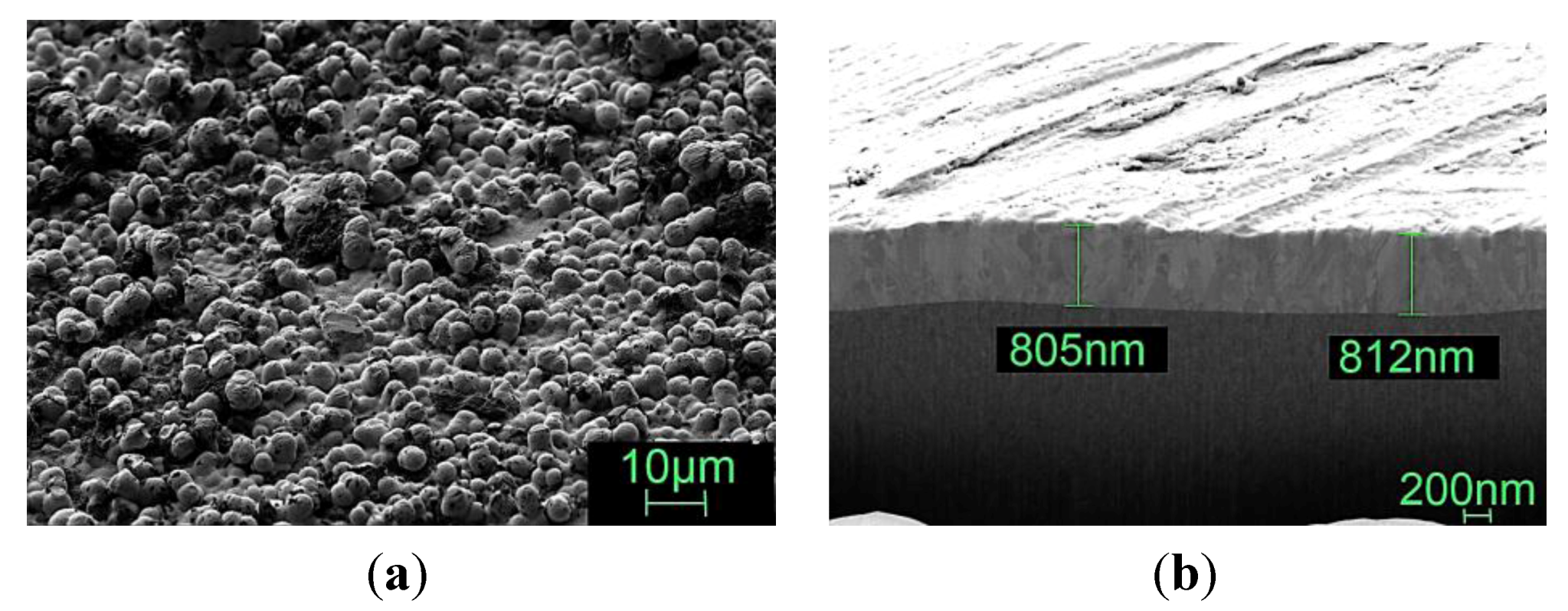
3.3.6. Topography of Gold Coatings
3.3.7. Wear Patterns
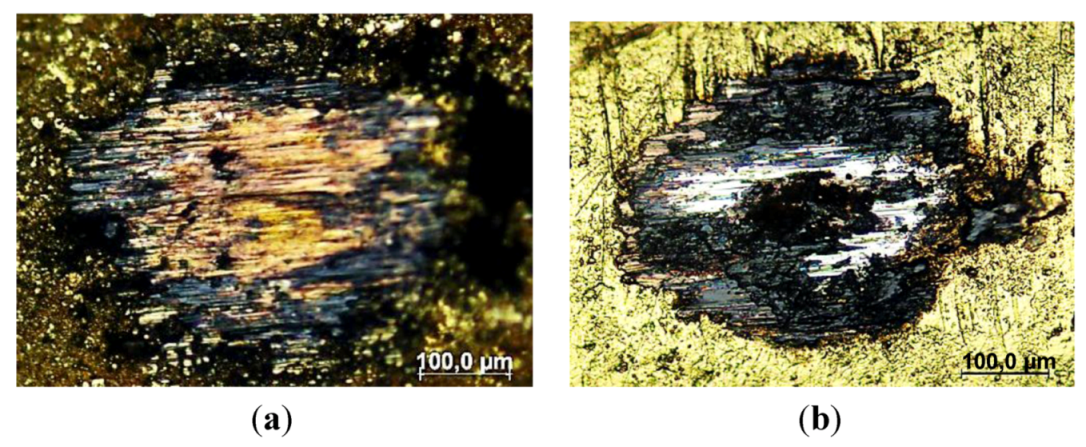
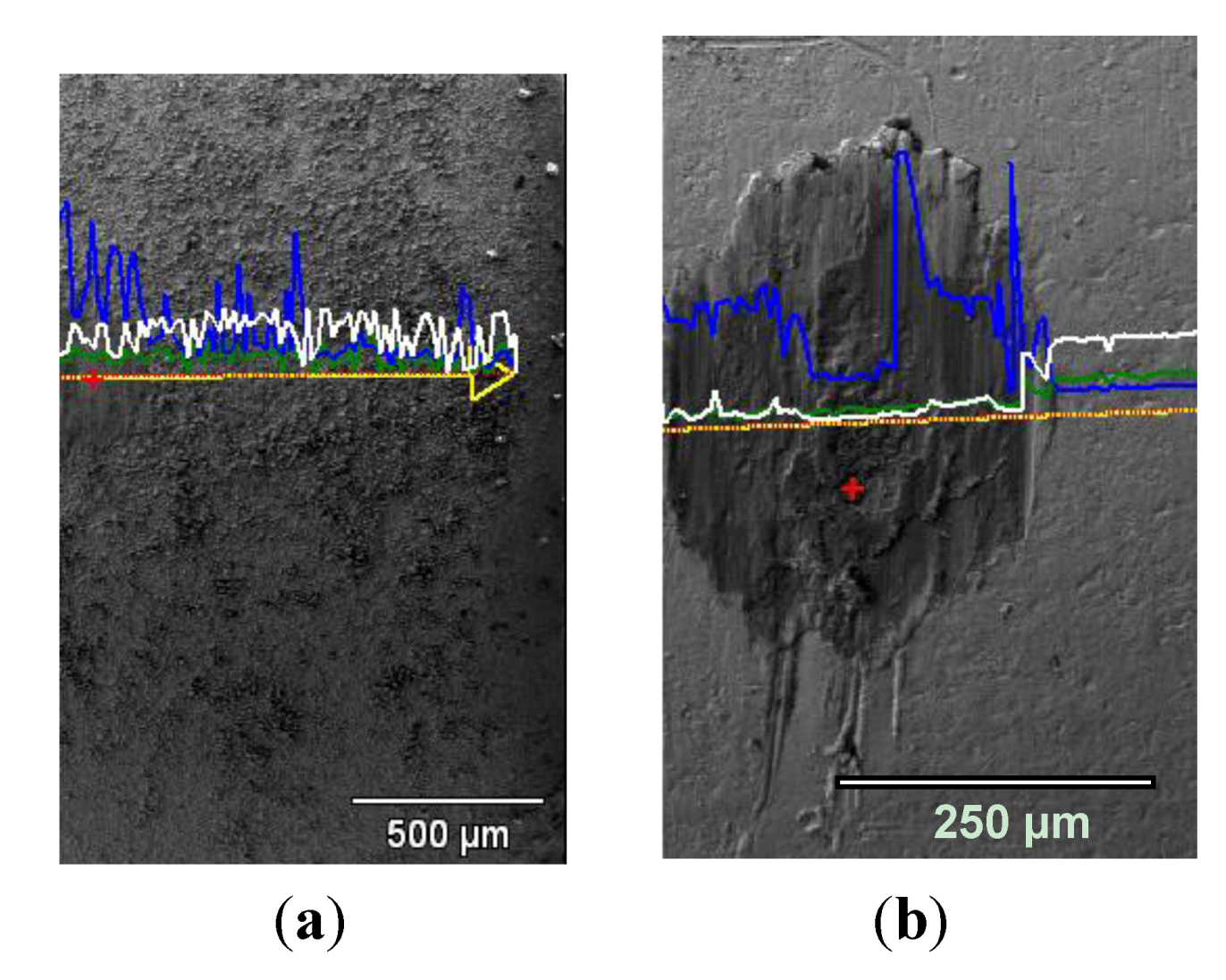
3.4. Wear of Corrosion Protecting Coatings
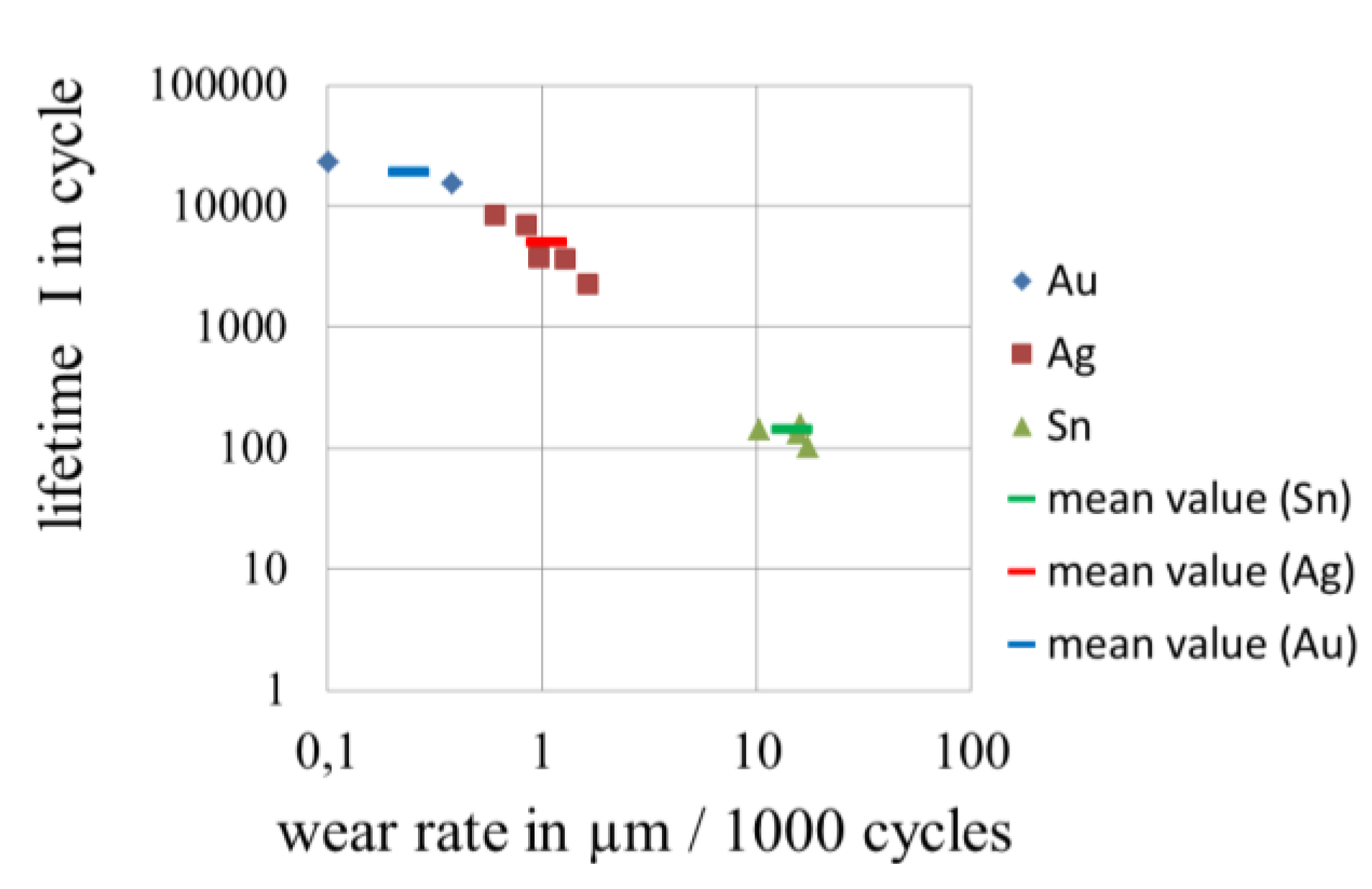



4. Conclusions
Acknowledgments
Conflict of Interest
References
- Landolt, D. Corrosion and Surface Chemistry of Metals; EPFL Press: Lausanne, Switzerland, 2007; pp. 227–274. [Google Scholar]
- Weast, R.C. CRC Handbook of Chemistry and Physics; CRC Press: Cleveland, OH, USA, 1978; pp. 141–146. [Google Scholar]
- Braunovic, M.; Konchits, V.V.; Myshkin, N.K. Electrical Contacts; CRC Press: Boca Raton, FL, USA, 2007; pp. 78–79. [Google Scholar]
- Jostan, J.L.; Mussinger, W.; Bogenschütz, A.F. Korrosionsschutzin der Elektronik (in German); Eugen G. Leuze Verlag: Saulgau, Germany, 1986; pp. 12–50. [Google Scholar]
- Braunovic, M. Fretting in electrical/electronic connections: A review. In IEICE Trans.; 2009; Volume 92-C, pp. 982–991. [Google Scholar]
- Antler, M. Tribologyof Electronic Connectors, in Electrical Contacts—Principles and Applications; Marcell Dekker: New York, NY, USA, 1999; pp. 332–364. [Google Scholar]
- Song, J. Hochschule Ostwestfalen-Lippe University of Applied Sciences, Lemgo, Germany. Unpublished work, 2011.
- Kfz-Steckverbinder-Prüfvorschrift LV 214 (in German); Volkswagen AG: Wolfsburg, Germany, 2010.
- Ben Jemaa, N.; Swingler, J. Correlation between Wear and Electrical Behaviour of Contact Interfaces during Fretting Vibration. In Proceedings of ICEC, Fredericton, NB, Canada, August 13–16, 2006.
- McBride, J.W. On the Relationship between Surface Wear and Intermittency during Fretting in Electrical Contacts. In Proceedings of Holm Conference 2006, Montreal, QC, Canada, September 25–27, 2006.
- Song, J.; Koch, C. Wear Patterns and Lifetime of Electric Contacts. In Proceedings of Holm Conference 2008, Orlando, FL, USA, October 27–29, 2008.
- Gold Demand Trends. Available online: http://www.gold.org/investment/research/regular_reports/gold_demand_trends/ (accessed on 12 November 2012).
- Gold Survey 2010. Available online: www.gfms.co.uk (accessed on 12 November 2012).
- Christian, H.; Christopher, W.C. Recycling of gold from electronics: Cost-effective use through “Design for Recycling”. Gold Bull. 2010, 43, 209–220. [Google Scholar] [CrossRef]
- Glüsing, J.; Höges, C.; Jung, A.; Meyer, C.; Rao, P. Der Fluch des Goldes (in German). Der Spiegel. 17 March 2008. Available online: http://www.spiegel.de/spiegel/print/d-56240589.html (accessed on 12 November 2012).
- Endres, B. Edelmetallbeschichtungen für die Verbindungstechniken in derElektronik (in German). Galvanotechnik 2006, 97, 2636–2641. [Google Scholar]
- AMP, Golden Rules: Guidelines for the Use of Gold on Connector Contacts; AMP Incorporated: Harrisburg, PA, USA, 1996.
- Vinaricky, E. Elektrische Kontakte, Werkstoffe und Anwendungen (in German); Springer: Berlin, Germany, 2002; pp. 151–154. [Google Scholar]
- Hornyak, G.L.; Tibbals, H.F.; Dutta, J.; Moore, J.J. Introduction to Nanoscience & Nanotechnology; CRC Press: Boca Raton, FL, USA, 2009; pp. 1098–1099. [Google Scholar]
- Slocum, A.H. Precision Machine Design; SME: Dearborn, MI, USA, 1992; pp. 425–440. [Google Scholar]
- Abys, J.A. Self Assembled Monolayers—Application to Surface Finishing for Electronics; A Presentation of Cookson Electronics, Enthone Inc.: West Haven, CT, USA, 2011. [Google Scholar]
- Connectors for Electronic Equipment—Tests and Measurements; German Institute for Standardization: Berlin, Germany, 2003.
- ASTM B 845: Standard Guide for Mixed Flowing Gas (MFG) Tests for Electrical Contacts; ASTM: West Conshohocken, PA, USA, 1997.
- DIN 50018, Testing in a Saturated Atmosphere in the Presence of Sulfur Dioxide; German Institute for Standardization: Berlin, Germany, 1997.
- ISO 3231, Determination of Resistance to Humid Atmospheres Containing Sulfur Dioxide; ISO: Geneva, Switzerland, 1997.
- ASTM G87, Standard Practice for Conducting Moist SO2 Tests; ASTM: West Conshohocken, PA, USA, 2002.
- DIN EN 60068-2-52, Environmental Testing-Salt Mist, Cyclic (Sodium Chloride Solution); German Institute for Standardization: Berlin, Germany, 1996.
- ASTM B 735-06, Standard Test Method for Porositiy in Gold Coatings on Metal Substrates by Nitric Vapor; ASTM: West Conshohocken, PA, USA, 2011.
- IPC-TM-650, Test Methods Manual, Association Connecting Electronics Industries; IPC: Bannockburn, IL, USA, 1997.
- Sawitzki, F. Einflussparameter von Reibkorrosionsuntersuchungen (in German).
- Wang, L. Analyse der Einflussparameter und Wirkungsmechanismen von Nanopartikeln in der Oberflächenschutzschicht elektrischer Kontakte (in Germany).
- Antler, M. Materials, Coatings, and Platings, in Electrical Contacts—Principles and Applications; Marcell Dekker: New York, NY, USA, 1999; pp. 403–432. [Google Scholar]
- Song, J.; Koch, C.; Wang, L.; Stopic, S.; Bogovic, J.; Friedrich, B.; Möbius, A.; Fuhrmann, A. Nanotechnologie in der elektrischen Verbindungstechnik (in German). In Elektrische und Optische Verbindungstechnik; Labor für Feinsystemtechnik, Hochschule Ostwestfalen-Lippe University of Applied Sciences: Lemgo, Germany, 2011; pp. 61–66. [Google Scholar]
- Song, J.; Zibart, A.; Koch, C.; Shmidt, L.; Wang, L. Einfluss von Goldlegierungsarten und Anteilen auf die Lebensdauer von elektrischenKontakten (in German). In Elektrische und Optische Verbindungstechnik; Labor für Feinsystemtechnik, Hochschule Ostwestfalen-Lippe University of Applied Sciences: Lemgo, Germany, 2011; pp. 25–34. [Google Scholar]
- Shmidt, L. Optimierung der Oberflächen für elektrische Kontakte (in German).
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Song, J.; Wang, L.; Zibart, A.; Koch, C. Corrosion Protection of Electrically Conductive Surfaces. Metals 2012, 2, 450-477. https://doi.org/10.3390/met2040450
Song J, Wang L, Zibart A, Koch C. Corrosion Protection of Electrically Conductive Surfaces. Metals. 2012; 2(4):450-477. https://doi.org/10.3390/met2040450
Chicago/Turabian StyleSong, Jian, Liangliang Wang, Andre Zibart, and Christian Koch. 2012. "Corrosion Protection of Electrically Conductive Surfaces" Metals 2, no. 4: 450-477. https://doi.org/10.3390/met2040450