1. Introduction
Glow discharge plasma nitriding (GDPN) is a surface treatment widely used to improve the tribo-mechanical behavior of stainless-steels. Then, after the GDPN treatment, steel surfaces are expected to increase in hardness and wear resistance. Furthermore, depending on the microstructure of the formed layer, the corrosion resistance should be maintained or even improved after nitriding. Keeping the corrosion resistance at the same level as stainless-steel depends on the control of the formation of chromium and iron nitrides, by performing treatments at low temperatures and suitable times [
1]. The modified layers are generally desirable to be rich in nitrogen-expanded austenite (γ
N) phase, promoting excellent characteristics in stainless-steels [
2,
3].
There are some limitations associated with the use of GDPN treatment, such as the difficulty in treating samples with complex geometries, and the formation of non-uniform layers or edge effects. Both phenomena are associated with variations of the electric field around the sample in the plasma [
4,
5,
6]. Furthermore, after GDPN treatments, surfaces undergo micrometric changes in the surface morphology due to sputtering/deposition and orientation-dependent anisotropic swelling. A relatively new technique that avoids these undesirable effects on the surface morphology is the cathodic cage plasma nitriding (CCPN) [
5,
6,
7,
8].
In the CCPN, a type of Physical Vapor Deposition (PVD) process, the sample is electrically isolated from the cathode and immersed in a floating electrical potential due to the presence of the surrounding and biased cathodic cage. Scientific evidence shows that a Kölbel’s-like “sputtering and re-condensation” model, supposedly valid to the GDPN technique, is also applicable in a similar way to CCPN by considering the interaction among species from the atmosphere and adsorption on the surface [
9]. In this process, electrons electrically trapped in the holes of the cage successively collide with atoms in the atmosphere, increasing the ion density. In this region, the ions, in turn, bombard the inner walls of the holes, sputtering atoms from the cage that will eventually be deposited on the surface of the sample. Thus, the plasma atmosphere, chemical composition of the cathodic cage, and arrangement of holes are parameters that govern the characteristics of the deposited film. In general, even carbon steel cages result in the deposition of iron nitrides on the electrically isolated substrates [
5,
6,
7]. Another relevant feature of this technique is forming films that are brittle and thinner than the modified layers obtained by GDPN treatments, when employing the same treatment times [
8,
10]. However, the CCPN also allows the formation of a modified layer below the film due to nitrogen diffusion in the bulk, which is heated by thermal radiation. To our knowledge, it is not common to evaluate the modified diffusion layer formed below the film in CCPN treatments.
The wear performance of nitride layers depends on two aspects involving deformation. The first one is the level of the strain of the substrate, which can deform during contact between two bodies and correlates with hardness. Pintaude et al. [
11] applied a method similar to CCPN, named active screen plasma nitriding (ASPN), for two duplex stainless-steels. They verified, after scratch tests, an occurrence of microcracks much more significant on the softer substrate than the harder one, featuring an eggshell effect. The other aspect is the relation between the load conditions and the layer thickness. For thicker nitrided layers processed onto martensitic stainless by ASPN, Rovani et al. [
12] described less occurrence of cracks, as the thickness of the layers increased. In the same work, when the load in the scratch tests was reduced from 15 N to 8 N, cracks were not observed on the worn surfaces of the nitrided layers, emphasizing that the treated surfaces have a critical loading value that is important for the application of these layers [
12]. These characteristics are important for films processed by CCPN and will be explored in the present study.
The surface mechanical strengthening of the AISI 316 stainless-steel finds applications in several areas, such as in biomedical devices, making it a good reason to investigate varied methods of nitriding for this alloy. Samanta et al. [
13] carried out a detailed study of the mechanical and tribological behavior of nitrided AISI 316L surfaces, however they employed only one technique, the GDPN. Herein, the tribo-mechanical performance of 316 surfaces modified by GDPN, analyzed in micro/nanoscale, were contrasted with those subjected to CCPN. The study aims to contribute to the understanding of how the structure and microstructure of the layers affect the wear performance, as well as to the proper selection of the nitriding method.
2. Materials and Methods
Commercial samples of AISI 316 austenitic stainless-steel (15.7% Cr, 11.6% Ni, 1.7% Mn, 1.9% Mo, 0.3% Si, 0.1% C, 0.03% S, and 0.03% P, with Fe in balance) supplied by the company Villares Metals (São Paulo, SP, Brazil) were mechanically polished with sandpapers (SiC) and diamond pastes up to a 1 μm particle size, and the final polishing was carried out in a 15% volume solution of colloidal silica in H2O2. Before nitriding, the samples were cleaned with two ultrasound baths in acetone.
The equipment used for GDPN and CCPN is a custom-made equipament consisting of a vacuum chamber, an exhaust and a gas supply system, a voltage source, a K-type thermocouple and electronic sensors, described in detail in previous reports [
6,
14,
15]. The treatment atmosphere was controlled by a flowmeter (model MKS 1179A, MKS Instruments, Andover, MA, USA), and the temperature by a thermocouple connected to the center of the sample holder (cathode). Voltage and current were monitored by a voltmeter and ammeter , both supplied by the company Minipa (São Paulo, SP, Brazil) and integrated into the voltage source. The chamber’s internal pressure was measured by an Active Gauge controller RS 232 Edwards pressure gauge (Edwards Ltd., Burgess Hill, UK).
The samples were subjected to sputtering with H2 at (150 ± 10) °C and 300 Pa for one hour to remove the oxide surface layer and then nitrided by GDPN. An atmosphere of 1:4 (N2:H2) (in volume), resulting in a total pressure of 300 Pa and a total flux of 20 sccm, was used. Both nitriding treatments by CCPN and GDPN were carried out at temperatures of 350 °C, 400 °C, and 450 °C for 6 h.
For CCPN treatments, a cathodic cage made of AISI 1008 steel was placed into the same GDPN equipment. Schematic diagrams for the GDPN and CCPN are shown in
Figure 1. The material´s chemical composition was 0.1% C, 0.5% Mn, 0.04% P, and 0.05% S (wt.%), in balance with Fe. The cage dimensions were 112 mm × 25 mm × 0.8 mm (diameter × height × thickness), the diameter of the cage holes was 8 mm, and the distance between the center of adjacent holes was 9.2 mm. The samples were placed on an alumina insulator with a diameter of 55.8 mm. The cage was positioned above the cathode to enclose the alumina insulator assembly and the samples. The samples remained electrically isolated in this arrangement, and the cage was in electrical contact with the cathode. Initially, the cage was sputtered with H
2 at 200 ± 5 °C for one hour at 30 Pa. After sputtering, the samples were nitrided by CCPN in an N
2:H
2 atmosphere of 4:1 (in volume), resulting in a total pressure of the order of 80 Pa and an N
2/H
2 gas flow of 16/4 sccm. These flow and pressure values in CCPN treatments were defined based on optimized sample properties in previous work [
6,
14,
15]. The pressure value was defined as being the hollow cathode pressure the cage uses for that gaseous mixture, which is necessary to maximize the film thickness. The voltage varied between 464 V and 588 V during nitriding, according to the treatment temperature. A temperature of 450 °C was chosen to evaluate the formation of chromium nitrides in the different techniques. The nitriding of AISI 316 steel for this work by GDPN and CCPN was carried out concomitantly with other martensitic steels, whose research was previously published [
8,
10].
The cross-sections of the samples were mechanically polished and analyzed by field-emission gun scanning electron microscopy (FEG-SEM) model Mira3) (TESCAN, Kohoutovice, Czech Republic) equipped with energy dispersive spectroscopy (EDS) model XMaxN SDD (Oxford, Abingdon, UK) to evaluate the nitrogen uptake in the modified layers. Then, the chemical etching was carried out with Murakami’s reagent composed by 10 g KOH and 10 g K3Fe(CN)6 from the brand Sigma Aldrich (San Luis, MO, USA) and 50 ml H2O, allowing the thickness of the modified layers to be measured through FEG-SEM analysis. In the samples treated by the CCPN technique, the thickness of the films was determined by optical interferometry (OI) model Talysurf CCI—Lite 3D (Taylor Hobson, Leicester, UK) on the surfaces of the samples, analyzing the height difference between the film surface and the interface with the modified layer in regions where the nitrides film was detached. OI estimated the roughness of all surfaces.
X-ray diffraction (XRD) data were collected by the X-ray diffractometer model Ultima IV (Rigaku, Tokyo, Japan.), with CuKα radiation (λ = 0.15406 nm), Bragg–Brentano geometry (θ–2θ), a 0.02° step, and a counting time of 4 s. The diffraction peaks were identified using powder crystallographic data sheets and literature data to γ
N phase peaks [
3,
16].
The nanoindentation technique was applied according to the ISO 14577-1 (2002) standard to measure the hardness and elastic modulus. The equipment used in the tests was an Nanoindenter XP (MTS Systems Corporation, Eden Prairie, MN, USA). Here, 25 indentations were performed with a Berkovich indenter arranged in a matrix with 100 μm between the indentations. To obtain profiles of hardness and modulus of elasticity by depth, multiple-loading cyclic nanoindentation was used. The tests were carried out in the central regions of the samples with a maximum load of 400 mN and 8 loading–unloading cycles. For the analysis of the loading curves, the contact stiffness technique was used to minimize the roughness effect [
17].
The nanoscratch tests were performed on the same equipment used in the nanoindentation tests. They were carried out with the Berkovich tip, with displacement towards one of the vertices, ramp loading (linear from zero), and a maximum load of 400 mN. The sliding speed was 10 μm/s, and the scratch length was 600 μm. The images of the nanoindentations and scratches were performed with a SEM model JSM-6360LV (JEOL, Peabody, MA, USA).
Author Contributions
Conceptualization, B.C.E.S.K., G.B.D.S. and S.L.R.D.S.; methodology, B.C.E.S.K., G.B.D.S. and S.L.R.D.S.; formal analysis, B.C.E.S.K., G.B.D.S., S.L.R.D.S., C.M.L., C.A.J., R.F.C. and G.P.; investigation, B.C.E.S.K. and R.F.C.; data curation, B.C.E.S.K.; writing—original draft preparation, B.C.E.S.K.; writing—review and editing, G.B.D.S., S.L.R.D.S., C.M.L., C.A.J., R.F.C. and G.P. All authors have read and agreed to the published version of the manuscript.
Funding
Bruna Kurelo was supported by a postdoctoral research scholarship PNPD granted by the Coordination for the Improvement of Higher Education Personnel during the completion of this research.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data used in this research are available in the article.
Acknowledgments
The authors thank the C-LABMU/UEPG, CMCM/UTFPR, LabNano/UFPR and CME/UFPR for the use of characterization facilities.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Olzon-Dionysio, M.; de Souza, S.D.; Basso, R.L.O.; de Souza, S. Application of Mössbauer Spectroscopy to the Study of Corrosion Resistance in NaCl Solution of Plasma Nitrided AISI 316L Stainless Steel. Surf. Coat. Technol. 2008, 202, 3607–3614. [Google Scholar] [CrossRef]
- Nascimento, F.C.; Foerster, C.E.; Da Silva, S.L.R.; Lepienski, C.M.; Siqueira, C.J.D.M.; Junior, C.A. A Comparative Study of Mechanical and Tribological Properties of AISI-304 and AISI-316 Submitted to Glow Discharge Nitriding. Mater. Res. 2009, 12, 173–180. [Google Scholar] [CrossRef] [Green Version]
- Dong, H. S-Phase Surface Engineering of Fe-Cr, Co-Cr and Ni-Cr Alloys. Int. Mater. Rev. 2010, 55, 65–98. [Google Scholar] [CrossRef]
- Olzon-Dionysio, M.; Campos, M.; Kapp, M.; de Souza, S.; de Souza, S.D. Influences of Plasma Nitriding Edge Effect on Properties of 316L Stainless Steel. Surf. Coat. Technol. 2010, 204, 3623–3628. [Google Scholar] [CrossRef]
- Junior, C.A.; Freitas, N.V.D.S.; De Morais, P.B.; Vitoriano, J.D.O. Changing the Characteristics of the Nitrided Layer Using Three Different Plasma Configurations. Rev. Mater. 2019, 24, 1–8. [Google Scholar] [CrossRef]
- De Sousa, R.R.M.; De Araújo, F.O.; Gontijo, L.C.; Da Costa, J.A.P.; Alves, C. Cathodic Cage Plasma Nitriding (CCPN) of Austenitic Stainless Steel (AISI 316): Influence of the Different Ratios of the (N2/H2) on the Nitrided Layers Properties. Vacuum 2012, 86, 2048–2053. [Google Scholar] [CrossRef]
- Alves, C.; de Araújo, F.O.; Ribeiro, K.J.B.; da Costa, J.A.P.; Sousa, R.R.M.; de Sousa, R.S. Use of Cathodic Cage in Plasma Nitriding. Surf. Coat. Technol. 2006, 201, 2450–2454. [Google Scholar] [CrossRef]
- Kurelo, B.C.; de Souza, G.B.; da Silva, S.L.R.; Daudt, N.D.F.; Alves, C.; Torres, R.D.; Serbena, F.C. Tribo-Mechanical Features of Nitride Coatings and Diffusion Layers Produced by Cathodic Cage Technique on Martensitic and Supermartensitic Stainless Steels. Surf. Coat. Technol. 2015, 275, 41–50. [Google Scholar] [CrossRef]
- Saeed, A.; Khan, A.W.; Jan, F.; Abrar, M.; Khalid, M.; Zakaullah, M. Validity of “Sputtering and Re-Condensation” Model in Active Screen Cage Plasma Nitriding Process. Appl. Surf. Sci. 2013, 273, 173–178. [Google Scholar] [CrossRef]
- Kurelo, B.C.E.S.; De Souza, G.B.; Da Silva, S.L.R.; Serbena, F.C.; Foerster, C.E.; Alves, C. Plasma Nitriding of HP13Cr Supermartensitic Stainless Steel. Appl. Surf. Sci. 2015, 349, 403–414. [Google Scholar] [CrossRef]
- Pintaude, G.; Rovani, A.C.; das Neves, J.C.K.; Lagoeiro, L.E.; Li, X.; Dong, H.S. Wear and Corrosion Resistances of Active Screen Plasma-Nitrided Duplex Stainless Steels. J. Mater. Eng. Perform. 2019, 28, 3673–3682. [Google Scholar] [CrossRef]
- Rovani, A.C.; Breganon, R.; de Souza, G.S.; Brunatto, S.F.; Pintaúde, G. Scratch Resistance of Low-Temperature Plasma Nitrided and Carburized Martensitic Stainless Steel. Wear 2017, 376–377, 70–76. [Google Scholar] [CrossRef]
- Samanta, A.; Chakraborty, H.; Bhattacharya, M.; Ghosh, J.; Sreemany, M.; Bysakh, S.; Rane, R.; Joseph, A.; Jhala, G.; Mukherjee, S.; et al. Nanotribological Response of a Plasma Nitrided Bio-Steel. J. Mech. Behav. Biomed. Mater. 2017, 65, 584–599. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, R.R.M.; de Araújo, F.O.; da Costa, J.A.P.; Brandim, A.D.S.; de Brito, R.A.; Alves, C. Cathodic Cage Plasma Nitriding: An Innovative Technique. J. Metall. 2012, 2012, 1–6. [Google Scholar] [CrossRef] [Green Version]
- de Sousa, R.R.M.; de Araújo, F.O.; Gontijo, L.C.; da Costa, J.A.P.; Nascimento, I.O.; Alves, C., Jr. Cathodic Cage Plasma Nitriding of Austenitic Stainless Steel (AISI 316): Influence of the Working Pressure on the Nitrided Layers Properties. Mater. Res. 2014, 17, 427–433. [Google Scholar] [CrossRef] [Green Version]
- Manova, D.; Mändl, S.; Neumann, H.; Rauschenbach, B. Formation of Metastable Diffusion Layers in Cr-Containing Iron, Cobalt and Nickel Alloys after Nitrogen Insertion. Surf. Coat. Technol. 2017, 312, 81–90. [Google Scholar] [CrossRef]
- Souza, G.; Foerster, C.; Silva, S.; Lipienski, C. Nanomechanical Properties of Rough Surfaces. Mater. Res. 2006, 9, 159–163. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, W.; Kurelo, B.C.E.S.; Ditzel, D.G.; Foerster, C.E.; Serbena, F.C.; de Souza, G.B. On the S-Phase Formation and the Balanced Plasma Nitriding of Austenitic-Ferritic Super Duplex Stainless Steel. Appl. Surf. Sci. 2018, 434, 1161–1174. [Google Scholar] [CrossRef]
- Pastukh, I.M. Energy Model of Glow Discharge Nitriding. Tech. Phys. 2016, 61, 76–83. [Google Scholar] [CrossRef]
- Kovács, D.; Quintana, I.; Dobránszky, J. Effects of Different Variants of Plasma Nitriding on the Properties of the Nitrided Layer. J. Mater. Eng. Perform. 2019, 28, 5485–5493. [Google Scholar] [CrossRef] [Green Version]
- Tessier, F.; Navrotsky, A.; Niewa, R.; Leineweber, A.; Jacobs, H.; Kikkawa, S.; Takahashi, M.; Kanamaru, F.; DiSalvo, F.J. Energetics of Binary Iron Nitrides. Solid State Sci. 2000, 2, 457–462. [Google Scholar] [CrossRef]
- Nascimento, F.C.; Lepienski, C.M.; Foerster, C.E.; Assmann, A.; Da Silva, S.L.R.; Siqueira, C.J.D.M.; Chinelatto, A. Structural, Mechanical, and Tribological Properties of AISI 304 and AISI 316L Steels Submitted to Nitrogen-Carbon Glow Discharge. J. Mater. Sci. 2009, 44, 1045–1053. [Google Scholar] [CrossRef]
- Lepienski, C.; Nascimento, F.; Foerster, C.; da Silva, S.; Siqueira, C.D.M.; Alves, C. Glow Discharge Nitriding in AISI 304 at Different Nitrogen-Hydrogen Atmospheres: Structural, Mechanical and Tribological Properties. Mater. Sci. Eng. A 2008, 489, 201–206. [Google Scholar] [CrossRef]
- Chiu, L.H.; Su, Y.Y.; Chen, F.S.; Chang, H. Microstructure and Properties of Active Screen Plasma Nitrided Duplex Stainless Steel. Mater. Manuf. Process. 2010, 25, 316–323. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An Improved Technique for Determining Hardness and Elastic Modulus Using Load and Displacement Sensing Indentation Experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Borowski, T.; Adamczyk-Cieślak, B.; Brojanowska, A.; Kulikowski, K.; Wierzchoń, T. Surface Modification of Austenitic Steel by Various Glow-Discharge Nitriding Methods. Mater. Sci. 2015, 21, 376–381. [Google Scholar] [CrossRef]
- Sumiya, K.; Tokuyama, S.; Nishimoto, A.; Fukui, J.; Nishiyama, A. Application of Active-Screen Plasma Nitriding to an Austenitic Stainless Steel Small-Diameter Thin Pipe. Metals 2021, 11, 366. [Google Scholar] [CrossRef]
- Saha, R.; Nix, W.D. Effects of the Substrate on the Determination of Thin Film Mechanical Properties by Nanoindentation. Acta Mater. 2002, 50, 23–38. [Google Scholar] [CrossRef]
- Fraczek, T.; Prusak, R.; Ogórek, M.; Skuza, Z. Nitriding of 316L Steel in a Glow Discharge Plasma. Materials 2022, 15, 3081. [Google Scholar] [CrossRef]
- Borowski, T. Enhancing the Corrosion Resistance of Austenitic Steel Using Active Screen Plasma Nitriding and Nitrocarburising. Materials 2021, 14, 3320. [Google Scholar] [CrossRef]
- Corujeira Gallo, S.; Dong, H. Study of Active Screen Plasma Processing Conditions for Carburising and Nitriding Austenitic Stainless Steel. Surf. Coat. Technol. 2009, 203, 3669–3675. [Google Scholar] [CrossRef]
Figure 1.
Schematic diagrams for the GDPN and CCPN treatments.
Figure 2.
Surface microstructures of samples nitrided by CCPN and GDPN, obtained by OI.
Figure 3.
Cross-section SEM images of the plasma-nitrided samples by GDPN and CCPN at the indicated temperatures, etched with Murakami’s reagent. Regions indicated correspond to modified layers produced, respectively.
Figure 4.
The nitrogen concentration, in at.%, carried out in several points through the cross-section of the (a) GDPN- and (b) CCPN-treated samples.
Figure 5.
(a) Optical interferometry image of a region, on the CCPN 450 °C surface, showing the boundary between the nitride film and the hardened surface below it. (b–d) Analyses of the height differences between the film and modified layer surfaces.
Figure 6.
X-ray diffractograms for samples untreated and nitrided by GDPN AISI 316 steel at different temperatures.
Figure 7.
X-ray diffractograms of samples untreated and nitrided by CCPN AISI 316 at different temperatures.
Figure 8.
X-ray diffractograms of the CCPN 450 °C sample for the nitrided layer and in the modified layer below the film deposited.
Figure 9.
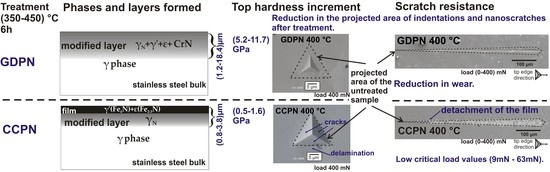
SEM micrographs of residual impressions produced by Berkovich tip indentation on untreated (a) and GDPN-nitrided (b–d) AISI 316 steel samples.
Figure 10.
SEM micrographs of residual impressions produced by Berkovich tip indentation on untreated (a) and CCPN-nitrided (b–d) AISI 316 steel samples.
Figure 11.
Hardness profiles of untreated and GDPN-nitrided samples.
Figure 12.
(a) Hardness profiles of untreated and CCPN-nitrided samples and (b) loading curves for all samples. The inset shows a pop-in event in the loading curve of the CCPN 450 °C sample.
Figure 13.
Hardness profiles of the CCPN 450 °C sample in the film and the region below the deposited film compared to the untreated sample.
Figure 14.
Nanoscratch profiles of untreated and GDPN-treated AISI 316 steel samples and micrographs obtained by backscattered SEM.
Figure 15.
Nanoscratch micrographs of untreated and CCPN-treated AISI 316 steel samples obtained by SEM.
Figure 16.
Profile graph and nanoscratch micrograph obtained by backscattered SEM of the AISI 316 steel sample treated at 400 °C for 6 h by CCPN.
Figure 17.
Cross-section topographies taken at the grooves’ middle regions, corresponding to 200 mN of applied load.
Table 1.
The thickness of modified layers and films, roughness, and hardness of the untreated and nitrided samples.
| Sample | Layer Thickness
Modified Layer (µm) | Layer Thickness Film (µm) | Roughness Ra (nm) | Roughness Rq (nm) | Top Hardness (GPa) |
|---|
| Untreated | - | - | 11.6 ± 1.7 | 14.7 ± 2.4 | 3.3 ± 0.3 |
| GDPN 350 °C | 1.2 ± 0.5 | - | 48.1 ± 6.5 | 37.8 ± 4.5 | 8.5 ± 2.9 |
| GDPN 400 °C | 4.3 ± 1.1 | - | 65.4 ± 7.5 | 83.6 ± 8.5 | 12 ± 0.7 |
| GDPN 450 °C | 18.4 ± 2.5 | - | 246.1 ± 30.1 | 319.1 ± 35.1 | 15.1 ± 1.6 |
| CCPN 350 °C | 0.5 ± 0.1 | 0.3 ± 0.1 | 8.7 ± 0.9 | 10.8 ± 1 | 3.8 ± 0.3 |
| CCPN 400 °C | 1.2 ± 0.1 | 0.5 ± 0.1 | 9.5 ± 0.9 | 11.7 ± 1.3 | 4.9 ± 0.4 |
| CCPN 450 °C | 3.0 ± 0.5 | 0.8 ± 0.1 | 18.7 ± 2.1 | 24.6 ± 2.9 | 3.6 ± 0.3 |
Table 2.
Critical load, maximum depth, and elastic recovery (under 200 mN load) of untreated and nitrided samples.
| Sample | Critical Load (mN) | Maximum Depth
(nm) | Elastic Recovery (%) |
|---|
| Untreated | - | 1375 ± 64 | 22 ± 8 |
| GDPN 350 °C | - | 1281 ± 66 | 33 ± 5 |
| GDPN 400 °C | - | 698 ± 67 | 55 ± 6 |
| GDPN 450 °C | - | 1254 ± 35 | 35 ± 3 |
| CCPN 350 °C | 9 ± 1 | 1434 ± 39 | - |
| CCPN 400 °C | 63 ± 13 | 1225 ± 24 | - |
| CCPN 450 °C | 24 ± 2 | 1575 ± 22 | - |
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).