Electronic Structure and Hardness of Mn3N2 Synthesized under High Temperature and High Pressure
Abstract
:1. Introduction
2. Experiment and Simulation Details
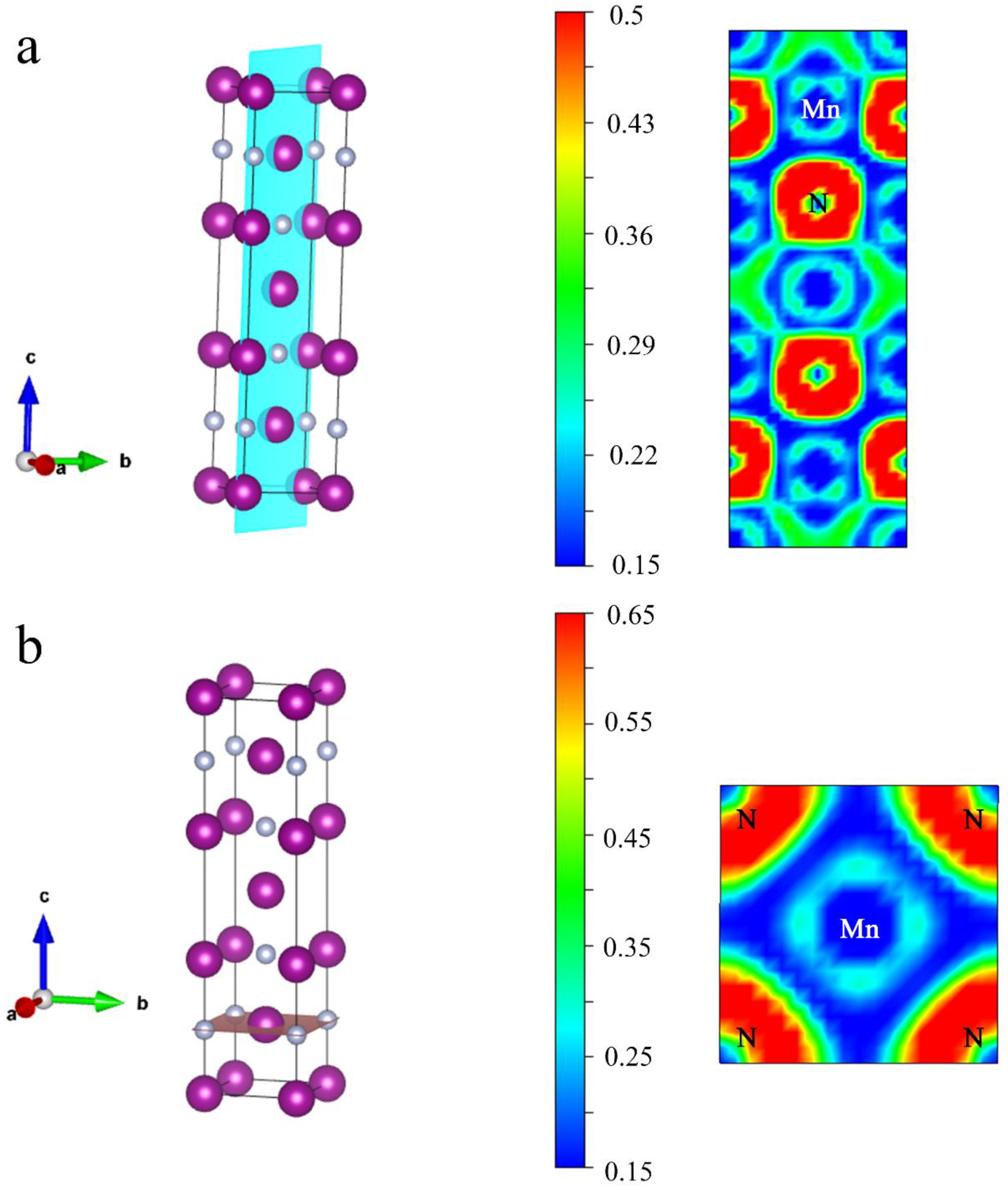
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, X.Q.; Niu, H.; Li, D.; Li, Y. Modeling hardness of polycrystalline materials and bulk metallic glasses. Intermetallics 2011, 19, 1275–1281. [Google Scholar] [CrossRef] [Green Version]
- Gao, F.M.; He, J.; Wu, E.; Liu, S.; Yu, D.; Li, D.; Zhang, S.; Tian, Y. Hardness of covalent crystals. Phys. Rev. Lett. 2003, 91, 015502. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.J.; Li, L.; Liu, Z.; Yu, D.; He, J.; Liu, R.; Xu, B.; Tian, Y.; Wang, H.-T. Hardness of covalent compounds: Roles of metallic component and d valence electrons. J. Appl. Phys. 2008, 104, 023503. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, D.; Zheng, W.; Ma, Y.; Chen, C. Anomalous Stress Response of Ultrahard WBn Compounds. Phys. Rev. Lett. 2015, 115, 185502. [Google Scholar] [CrossRef] [Green Version]
- Chung, H.Y.; Weinberger, M.B.; Yang, J.M.; Tolbert, S.H.; Kaner, R.B. Correlation between hardness and elastic moduli of the ultraincompressible transition metal diborides RuB2, OsB2, and ReB2. Appl. Phys. Lett. 2008, 92, 261904. [Google Scholar] [CrossRef] [Green Version]
- Weinberger, M.B.; Levine, J.B.; Chung, H.; Cumberland, R.W.; Rasool, H.I.; Yang, J.-M.; Kaner, R.B.; Tolbert, S.H. Incompressibility and Hardness of Solid Solution Transition Metal Diborides: Os1-xRuxB2. Chem. Mater. 2009, 21, 1915–1921. [Google Scholar] [CrossRef]
- Liang, Y.; Zhang, B. Mechanical and electronic properties of superhard ReB2. Phys. Rev. B 2007, 76, 132101. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, D.Q.; Pan, M.X.; Wang, W.H. Glass forming properties of Zr-based bulk metallic alloys. J. Non-Cryst. Solids 2003, 315, 206–210. [Google Scholar] [CrossRef]
- Gou, H.Y.; Hou, L.; Zhang, J.; Gao, F. Pressure-induced incompressibility of ReC and effect of metallic bonding on its hardness. Appl. Phys. Lett. 2008, 92, 241901. [Google Scholar] [CrossRef]
- Fu, H.Z.; Peng, W.M.; Gao, T. Structural and elastic properties of ZrC under high pressure. Mater. Chem. Phys. 2009, 115, 789–794. [Google Scholar] [CrossRef]
- Simunek, A. Vickers hardness of Ru-, Ta-, W-, Re-, Os-, and Ir carbides and nitrides calculated by the bond strength method. MRS Commun. 2022, 12, 759–761. [Google Scholar] [CrossRef]
- Zhang, R.F.; Legut, D.; Niewa, R.; Argon, A.S.; Veprek, S. Shear-induced structural transformation and plasticity in ultraincompressible ReB2 limit its hardness. Phys. Rev. B 2010, 82, 104104. [Google Scholar] [CrossRef]
- Chen, X.Q.; Fu, C.L.; Krčmar, M.; Painter, G.S. Electronic and structural origin of ultraincompressibility of 5d transition-metal diborides MB2 (M = W, re, os). Phys. Rev. Lett. 2008, 100, 196403. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.Y.; Weinberger, M.B.; Levine, J.B.; Kavner, A.; Yang, J.M.; Tolbert, S.H.; Kaner, R.B. Synthesis of ultra-incompressible superhard rhenium diboride at ambient pressure. Science 2007, 316, 436–439. [Google Scholar] [CrossRef] [PubMed]
- Haines, J.; Leger, J.M.; Bocquillon, G. Synthesis and design of superhard materials. Annu. Rev. Mater. Res. 2001, 31, 1–23. [Google Scholar] [CrossRef]
- Yao, H.Z.; Ouyang, L.Z.; Ching, W.Y. Ab initio calculation of elastic constants of ceramic crystals. J. Am. Ceram. Soc. 2007, 90, 3194–3204. [Google Scholar] [CrossRef]
- Akrami, S.; Edalati, P.; Fuji, M.; Edalati, K. High-entropy ceramics: Review of principles, production and applications. Mater. Sci. Eng. R-Rep. 2021, 146, 100644. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef] [Green Version]
- Ningthoujam, R.S.; Gajbhiye, N.S. Synthesis, electron transport properties of transition metal nitrides and applications. Prog. Mater. Sci. 2015, 70, 50–154. [Google Scholar] [CrossRef]
- Feng, X.; Bao, K.; Tao, Q.; Li, L.; Shao, Z.; Yu, H.; Xu, C.; Ma, S.; Lian, M.; Zhao, X.; et al. Role of TM-TM Connection Induced by Opposite d-Electron States on the Hardness of Transition-Metal (TM = Cr, W) Mononitrides. Inorg. Chem. 2019, 58, 15573–15579. [Google Scholar] [CrossRef]
- Yu, R.; Chong, X.; Jiang, Y.; Zhou, R.; Yuan, W.; Feng, J. The stability, electronic structure, elastic and metallic properties of manganese nitrides. RSC Adv. 2015, 5, 1620–1627. [Google Scholar] [CrossRef]
- Zhang, Z.; Mi, W. Progress in ferrimagnetic Mn4N films and its heterostructures for spintronics applications. J. Phys. D Appl. Phys. 2021, 55, 013001. [Google Scholar] [CrossRef]
- Lei, L.; Hong, X.; Fangfang, L.; Yongqiang, Z.; Bin, B.; Kezhao, L. Preparation and Characteristics of Uranium Nitride Thin Films. Rare Met. Mater. Eng. 2015, 44, 1975–1978. [Google Scholar]
- Rognerud, E.G.; Rom, C.L.; Todd, P.K.; Singstock, N.R.; Bartel, C.J.; Holder, A.M.; Neilson, J.R. Kinetically Controlled Low-Temperature Solid-State Metathesis of Manganese Nitride Mn3N2. Chem. Mater. 2019, 31, 7248–7254. [Google Scholar] [CrossRef]
- Yin, W.W.; Lei, L.; Jiang, X.; Liu, P.; Liu, F.; Li, Y.; Peng, F.; He, D. High pressure synthesis and properties studies on spherical bulk epsilon-Fe3N. High Press. Res. 2014, 34, 317–326. [Google Scholar] [CrossRef]
- Lei, L.; Zhang, L.; Gao, S.; Hu, Q.; Fang, L.; Chen, X.; Xia, Y.; Wang, X.; Ohfuji, H.; Kojima, Y.; et al. Neutron diffraction study of the structural and magnetic properties of epsilon-Fe3N1.098 and epsilon-Fe2.322CO0.678N0.888. J. Alloy. Compd. 2018, 752, 99–105. [Google Scholar] [CrossRef]
- Wang, S.M.; Yu, X.; Zhang, J.; Wang, L.; Leinenweber, K.; He, D.; Zhao, Y. Synthesis, Hardness, and Electronic Properties of Stoichiometric VN and CrN. Cryst. Growth Des. 2016, 16, 351–358. [Google Scholar] [CrossRef]
- Chen, M.; Wang, S.; Zhang, J.; He, D.; Zhao, Y. Synthesis of Stoichiometric and Bulk CrN through a Solid-State Ion-Exchange Reaction. Chem. A Eur. J. 2012, 18, 15459–15463. [Google Scholar] [CrossRef]
- Blochl, P.E. Projector augmented-wave method. Phys. Rev. B Condens. Matter 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [Green Version]
- Kresse, G.; Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B Condens. Matter 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, S.; Dronskowski, R. The Crystal Orbital Hamilton Population (COHP) Method as a Tool to Visualize and Analyze Chemical Bonding in Intermetallic Compounds. Crystals 2018, 8, 225. [Google Scholar] [CrossRef] [Green Version]
- Mueller, P.C.; Ertural, C.; Hempelmann, J.; Dronskowski, R. Crystal Orbital Bond Index: Covalent Bond Orders in Solids. J. Phys. Chem. C 2021, 125, 7959–7970. [Google Scholar] [CrossRef]
- Toby, B.H. EXPGUI, a graphical user interface for GSAS. J. Appl. Crystallogr. 2001, 34, 210–213. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.J.; Wang, Y.; Lv, J.; Ma, Y. Materials discovery at high pressures. Nat. Rev. Mater. 2017, 2, 17005. [Google Scholar] [CrossRef]
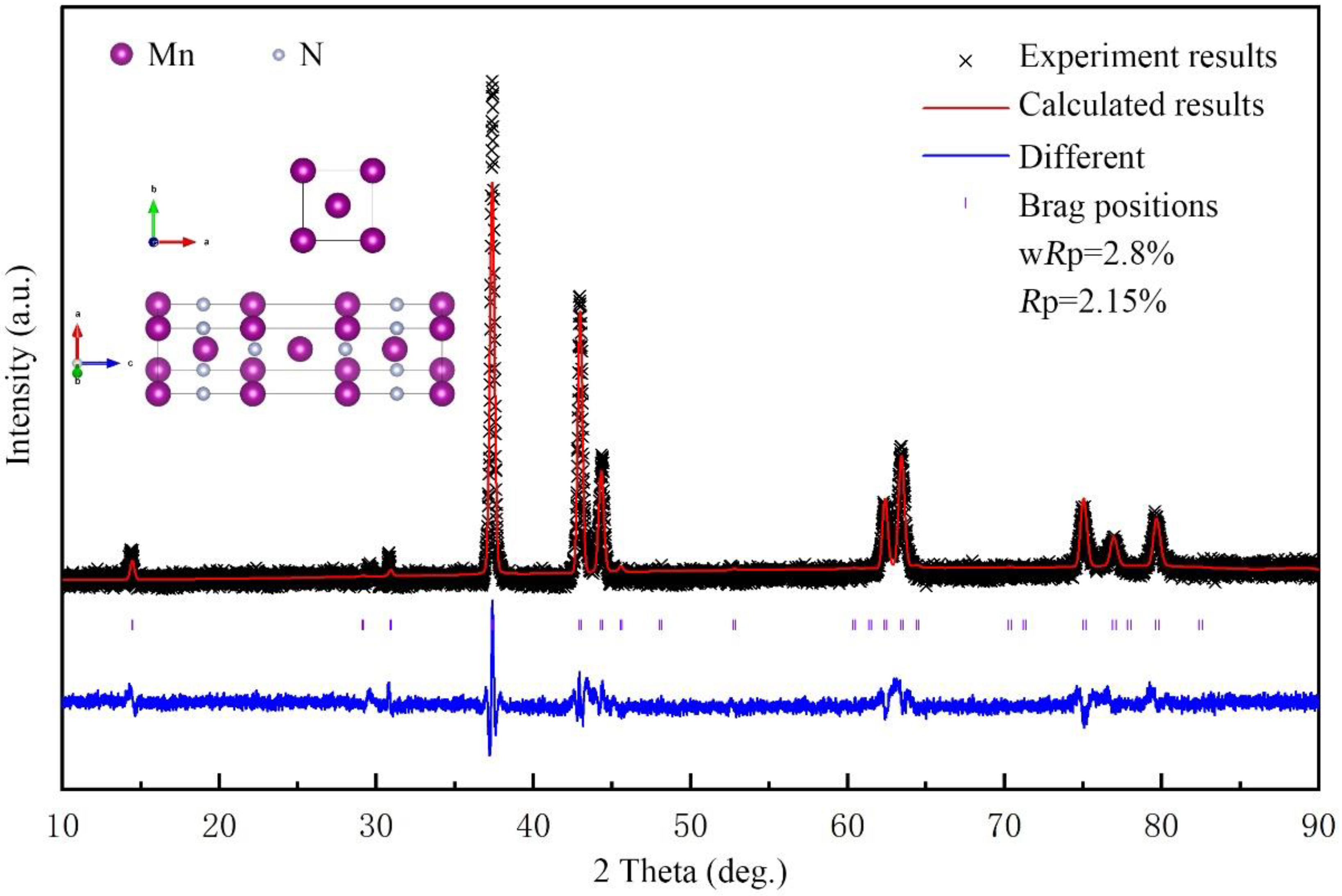
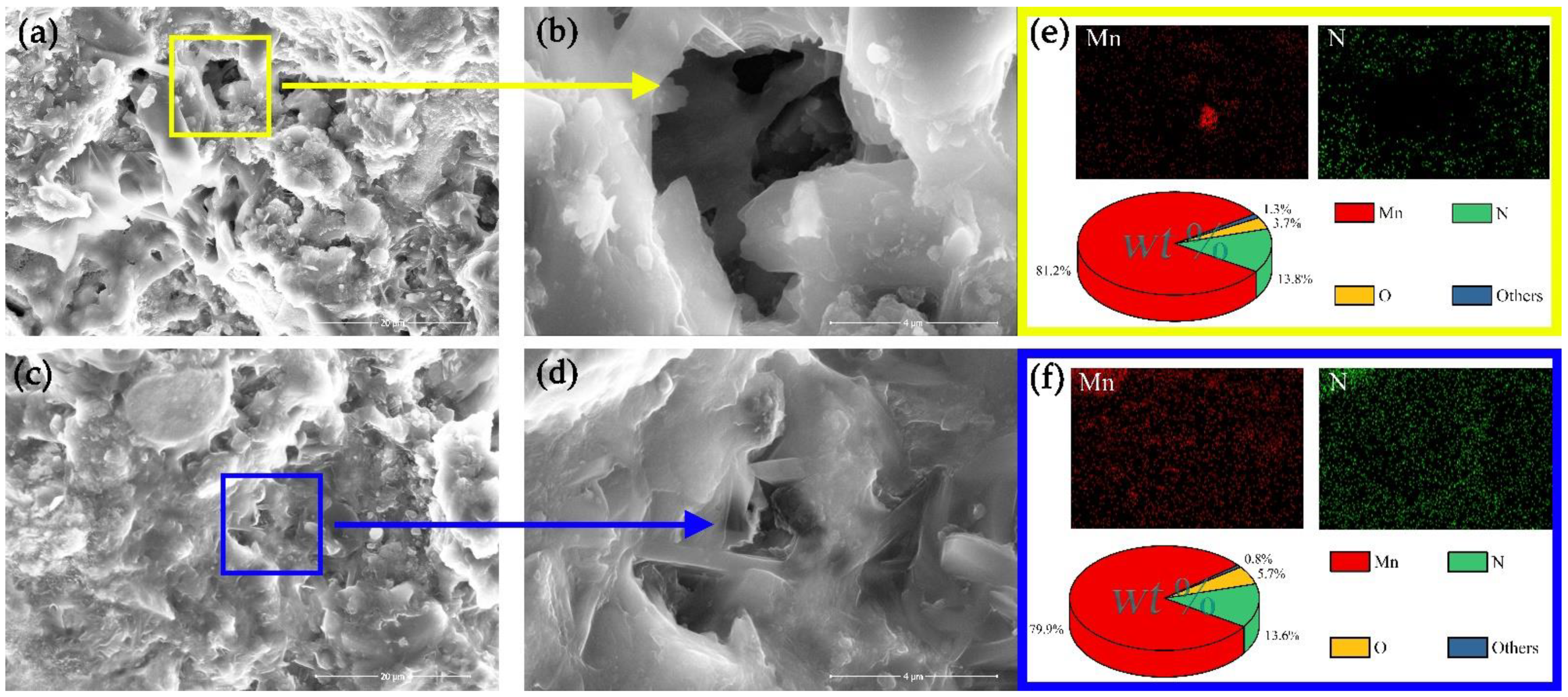
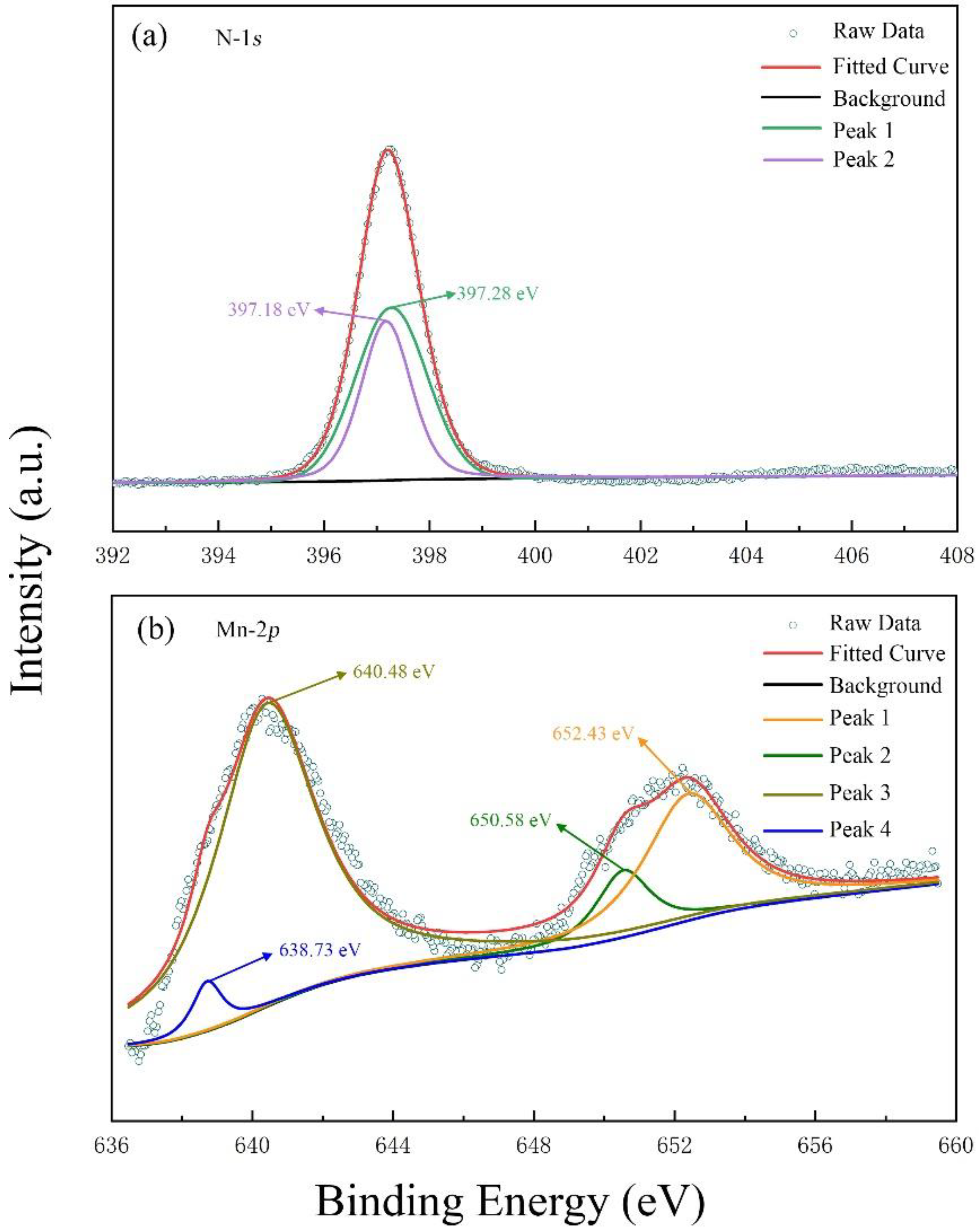
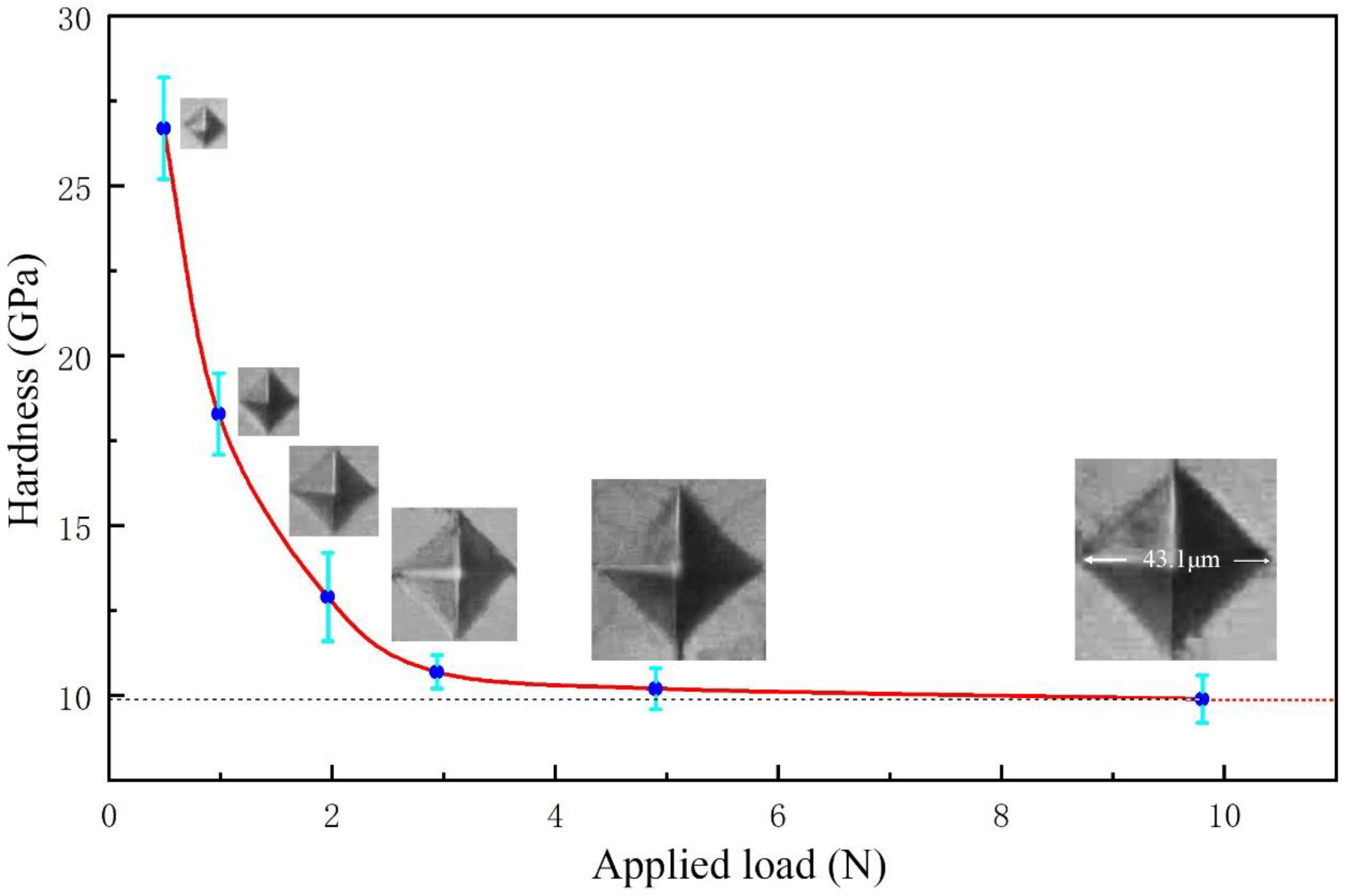


| Identification Code | Mn3N2 |
|---|---|
| Space group | I4/mmm |
| Unit cell dimensions (Å) | a = 2.9769 Å, c = 12.2646 Å, a/b = 1 b/c = 0.2427 |
| Volume (Å3) | 108.69 |
| Mn-N | Mn-Mn Nearest Mn-Mn Next-Nearest | N-N | |
|---|---|---|---|
| −ICOHP (eV/pair) | 3.0303 | 0.7319 0.5650 | 0.0975 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Zhou, C.; Sun, G.; Wang, X.; Bao, K.; Zhu, P.; Zhu, J.; Wang, Z.; Zhao, X.; Tao, Q.; et al. Electronic Structure and Hardness of Mn3N2 Synthesized under High Temperature and High Pressure. Metals 2022, 12, 2164. https://doi.org/10.3390/met12122164
Zhang S, Zhou C, Sun G, Wang X, Bao K, Zhu P, Zhu J, Wang Z, Zhao X, Tao Q, et al. Electronic Structure and Hardness of Mn3N2 Synthesized under High Temperature and High Pressure. Metals. 2022; 12(12):2164. https://doi.org/10.3390/met12122164
Chicago/Turabian StyleZhang, Shoufeng, Chao Zhou, Guiqian Sun, Xin Wang, Kuo Bao, Pinwen Zhu, Jinming Zhu, Zhaoqing Wang, Xingbin Zhao, Qiang Tao, and et al. 2022. "Electronic Structure and Hardness of Mn3N2 Synthesized under High Temperature and High Pressure" Metals 12, no. 12: 2164. https://doi.org/10.3390/met12122164






