Silicon Poisoning and Effects of Tantalum on AlSi Alloys
Abstract
:1. Introduction
- heterogeneous nucleations;
2. Materials and Methods
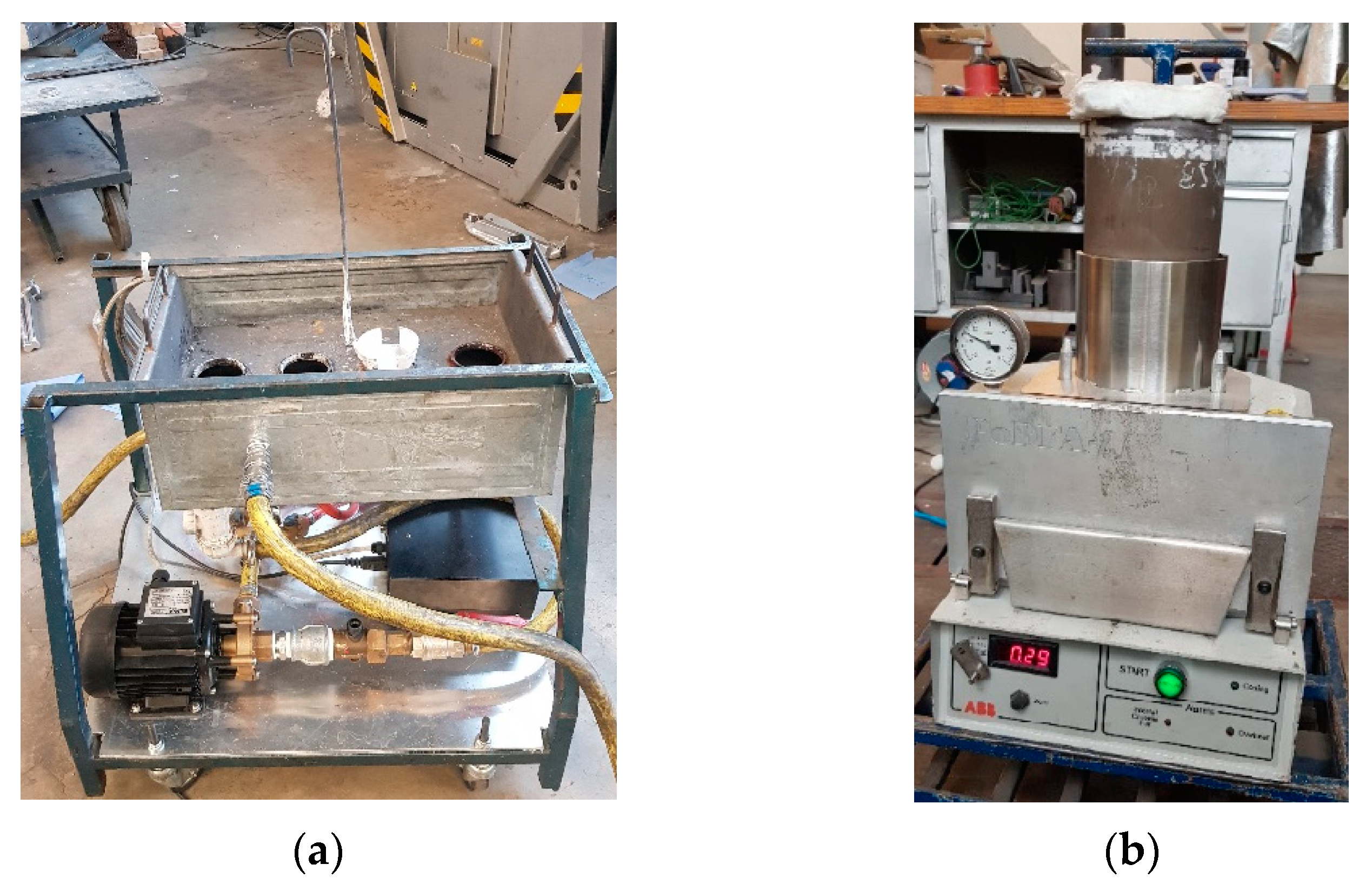
2.1. Material Preparation
- Addition of stoichiometric grain refiner (Al-2.2Ti-1B) in the ratio of 2 kg per tonne of melt;
- Adjustment of the free Ti content to 0.03 wt.% via the addition of Al-10Ti;
- Adjustment of the free Ti content to 0.06 wt.% via the addition of Al-10Ti;
- Increasing the free Ti content to 0.12 wt.% via the addition of Al-10Ti.
2.2. Characterization Techniques
3. Results
3.1. Investigation of the Silicon Poisoning Effect
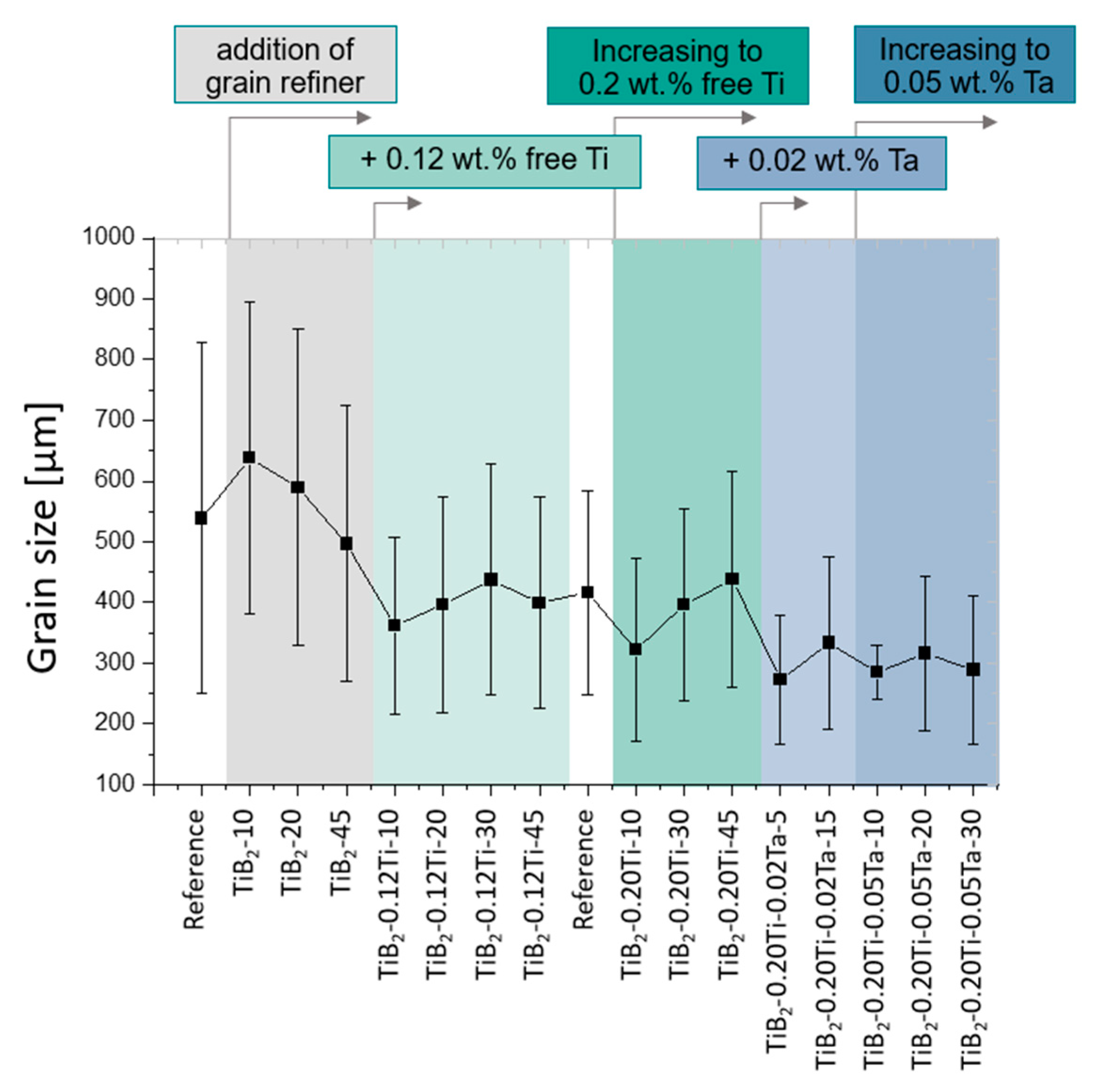
3.1.1. TP1 Grain Size Evaluation
3.1.2. PoDFA Evaluation of the τ1 Phase
3.2. Influence of Tantalum on τ1 Phase
3.2.1. TP1 Grain Size Evaluation
3.2.2. PoDFA Analysis of τ1 Phase with Tantalum
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kori, S.A.; Murty, B.S.; Chakraborty, M. Development of an efficient grain refiner for Al–7Si alloy and its modification with strontium. Mater. Sci. Eng. A 2000, 283, 94–104. [Google Scholar] [CrossRef]
- Ghosh, M.; Ghosh, A.; Roy, A. Renewable and Sustainable Materials in Automotive Industry. In Encyclopedia of Renewable and Sustainable Materials; Elsevier: Amsterdam, The Netherlands, 2020; Volume 3, pp. 162–179. [Google Scholar]
- Murray, J.L.; McAlister, A.J. The Al-Si (Aluminum-Silicon) System. Bull. Alloy Phase Diagr. 1984, 5, 74–84. [Google Scholar] [CrossRef]
- Basak, S.; Biswas, P.; Patra, S.; Roy, H.; Mondal, M.K. Effect of TiB2 and Al3Ti on the microstructure, mechanical properties and fracture behaviour of near eutectic Al-12.6Si alloy. Int. J. Miner. Metall. Mater. 2021, 28, 1174–1185. [Google Scholar] [CrossRef]
- Fan, Z.; Gao, F.; Wang, Y.; Men, H.; Zhou, L. Effect of solutes on grain refinement. Prog. Mater. Sci. 2022, 123, 100809. [Google Scholar] [CrossRef]
- Guan, R.G.; Tie, D. A Review on Grain Refinement of Aluminum Alloys: Progresses, Challenges and Prospects. Acta Metall. Sin. (Engl. Lett.) 2017, 30, 409–432. [Google Scholar] [CrossRef]
- Sunitha, K.; Gurusami, K. Study of Al-Si alloys grain refinement by inoculation. Mater. Today Proc. 2021, 43, 1825–1829. [Google Scholar] [CrossRef]
- Carlsson, S. Grain refinement of aluminium by titanium diboride particles: The importance of nucleation, growth restriction, and cooling rate. Master’s Thesis, KTH, Stockholm, Sweden, 2019. [Google Scholar]
- Kashyap, K.T.; Chandrashekar, T. Effects and mechanisms of grain refinement in aluminium alloys. Bull. Mater. Sci. 2001, 24, 345–353. [Google Scholar] [CrossRef]
- Quested, T.E.; Dinsdale, A.T.; Greer, A.L. Thermodynamic modelling of growth-restriction effects in aluminium alloys. Acta Mater. 2005, 53, 1323–1334. [Google Scholar] [CrossRef]
- Jin, D.; Li, H.; Yang, C.; Han, Y.; Zhu, Z.; Miao, Y.; Xu, C.; Chen, B. The Effects of Mg and Si Contents on the Microstructure and Solidification Behavior of Dilute Al-Mg-Si-Fe Alloys. JOM 2023, 75, 4845–4857. [Google Scholar] [CrossRef]
- Mitrašinović, A.M.; Hernández, F.C. Determination of the growth restriction factor and grain size for aluminum alloys by a quasi-binary equivalent method. Mater. Sci. Eng. A 2012, 540, 63–69. [Google Scholar] [CrossRef]
- Watanabe, Y.; Mihara-Narita, M.; Sato, H. Grain Refinement of Cast Aluminum by Heterogeneous Nucleation Site Particles with High Lattice Matching. Mater. Trans. 2023, 64, 1083–1097. [Google Scholar] [CrossRef]
- Minagawa, A. Light Metals 2020; Tomsett, A., Ed.; Springer International Publishing: Cham, Switzerland, 2000; pp. 988–993. [Google Scholar]
- Zhang, L.L.; Jiang, H.X.; Jie, H.E.; Zhao, J.Z. Kinetic behaviour of TiB2 particles in Al melt and their effect on grain refinement of aluminium alloys. Trans. Nonferrous Met. Soc. China 2020, 30, 2035–2044. [Google Scholar] [CrossRef]
- Furuta, S.; Minagawa, A.; Matsui, I.; Murakami, Y.; Omura, N.; Takeuchi, A.; Uesugi, K.; Kobayashi, M. Direct observations of nucleant TiB2 particles in cast aluminum by synchrotron radiation multiscale tomography. Materialia 2020, 10, 100663. [Google Scholar] [CrossRef]
- Li, J.; Hage, F.S.; Ramasse, Q.M.; Schumacher, P. The nucleation sequence of α-Al on TiB2 particles in Al-Cu alloys. Acta Mater. 2021, 206, 116652. [Google Scholar] [CrossRef]
- Quested, T.E.; Greer, A.L. The effect of the size distribution of inoculant particles on as-cast grain size in aluminium alloys. Acta Mater. 2004, 52, 3859–3868. [Google Scholar] [CrossRef]
- Hu, S.; Li, S.; Li, H.; Zhou, Y. Large-scale growth of TiB2 hexagonal platelets. J. Alloys Compd. 2017, 690, 930–935. [Google Scholar] [CrossRef]
- Bunn, A.M.; Schumacher, P.; Kearns, M.A.; Boothroyd, C.B.; Greer, A.L. Grain refinement by Al–Ti–B alloys in aluminium melts: A study of the mechanisms of poisoning by zirconium. Mater. Sci. Technol. 1999, 15, 1115–1123. [Google Scholar] [CrossRef]
- Dantzig, J.A.; Rappaz, M. Solidification; Presses Polytechniques et Universitaires Romandes: Lausanne, Switzerland, 2009. [Google Scholar]
- Gröbner, J.; Mirković, D.; Schmid-Fetzer, R. Thermodynamic aspects of grain refinement of Al–Si alloys using Ti and B. Mater. Sci. Eng. A 2005, 395, 10–21. [Google Scholar] [CrossRef]
- Greer, A.L.; Bunn, A.M.; Tronche, A.; Evans, P.V.; Bristow, D.J. Modelling of inoculation of metallic melts: Application to grain refinement of aluminium by Al–Ti–B. Acta Mater. 2000, 48, 2823–2835. [Google Scholar] [CrossRef]
- Greer, A.L.; Cooper, P.S.; Meredith, M.W.; Schneider, W.; Schumacher, P.; Spittle, J.A.; Tronche, A. Grain refinement of aluminium alloys by inoculation. Adv. Eng. Mater. 2003, 5, 81–91. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, H.; He, J.; Zhao, J. Improved grain refinement in aluminium alloys by re-precipitated TiB2 particles. Mater. Lett. 2022, 312, 131657. [Google Scholar] [CrossRef]
- Kori, S.A.; Murty, B.S.; Chakraborty, M. Influence of silicon and magnesium on grain refinement in aluminium alloys. Mater. Sci. Technol. 1999, 15, 986–992. [Google Scholar] [CrossRef]
- Johnsson, M. Grain refinement of aluminium studied by use of a thermal analytical technique. Thermochim. Acta 1995, 256, 107–121. [Google Scholar] [CrossRef]
- Mohanty, P.S.; Gruzleski, J.E. Grain refinement mechanisms of hypoeutectic Al Si alloys. Acta Mater. 1996, 44, 3749–3760. [Google Scholar] [CrossRef]
- Wang, Y.; Que, Z.; Hashimoto, T.; Zhou, X.; Fan, Z. Mechanism for Si poisoning of Al-Ti-B grain refiners in Al alloys. Metall. Mater. Trans. 2020, 51, 5743–5757. [Google Scholar] [CrossRef]
- Bolzoni, L.; Babu, N.H. Towards industrial Al-Nb-B master alloys for grain refining Al-Si alloys. J. Mater. Res. Technol. 2019, 8, 5631–5638. [Google Scholar] [CrossRef]
- Doppelhofer, B. Kornfeinung in AlSi-Legierungen. Master’s Thesis, University of Leoben, Leoben, Austria, 2014. [Google Scholar]
- Pammer, M.; Jiehua, L.I.; Schumacher, P. Einfluss von Tantal auf die Kornfeinung einer Al10SiMg Legierung. Giess. Rundsch.-Proguss-Austria 2021, 2, 15–20. [Google Scholar]
- Haberl, K.; Geier, G.; Schumacher, P. Die Eignung des Unterdruckdichtetests zur Bestimmung der Schmelzereinheit von Aluminiumlegierungen. Giess. Rundsch. Proguss-Austria 2008, 55, 100–104. [Google Scholar]
- Quested, T.E. Understanding mechanisms of grain refinement of aluminium alloys by inoculation. Mater. Sci. Technol. 2004, 20, 1357–1369. [Google Scholar] [CrossRef]



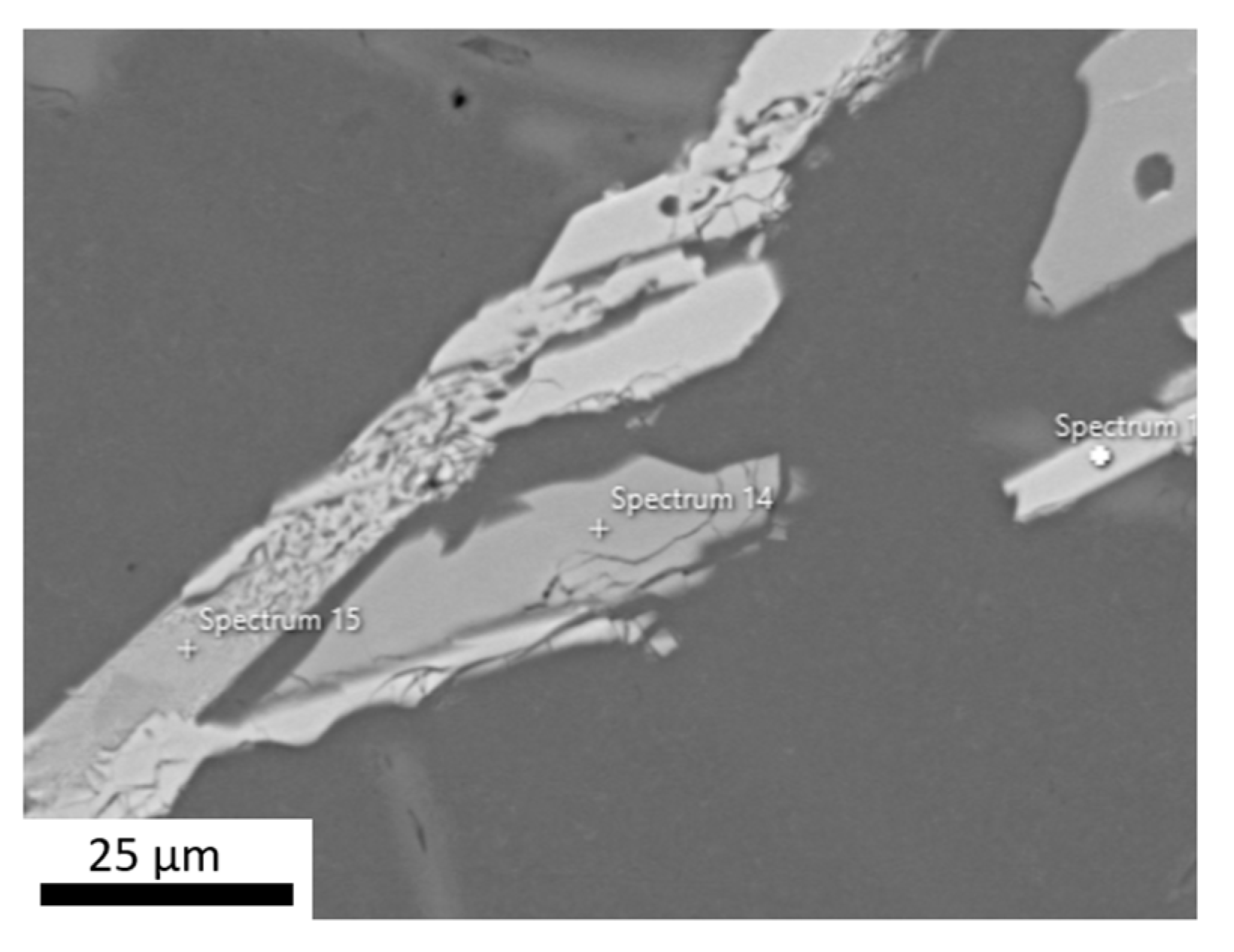




| Si | Ti | V | Al |
|---|---|---|---|
| 44.79 | 23.42 | 0.32 | Bal. |
| 48.43 | 29.03 | 0.69 | Bal. |
| 39.48 | 24.57 | 0.37 | Bal. |
| Si | Ti | Ta | Al | |
|---|---|---|---|---|
| (a) | 13.61 | 24.94 | 1.46 | Bal. |
| (b) | 12.92 | 25.01 | 0.51 | Bal. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pammer, M.; Pölzl, J.; Li, J. Silicon Poisoning and Effects of Tantalum on AlSi Alloys. Metals 2023, 13, 1917. https://doi.org/10.3390/met13121917
Pammer M, Pölzl J, Li J. Silicon Poisoning and Effects of Tantalum on AlSi Alloys. Metals. 2023; 13(12):1917. https://doi.org/10.3390/met13121917
Chicago/Turabian StylePammer, Maria, Johannes Pölzl, and Jiehua Li. 2023. "Silicon Poisoning and Effects of Tantalum on AlSi Alloys" Metals 13, no. 12: 1917. https://doi.org/10.3390/met13121917





