Microstructure Evolution and Competitive Reactions during Quenching and Partitioning of a Model Fe–C–Mn–Si Alloy
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. In Situ Investigation by High-Energy X-ray Diffraction Experiments
- (1)
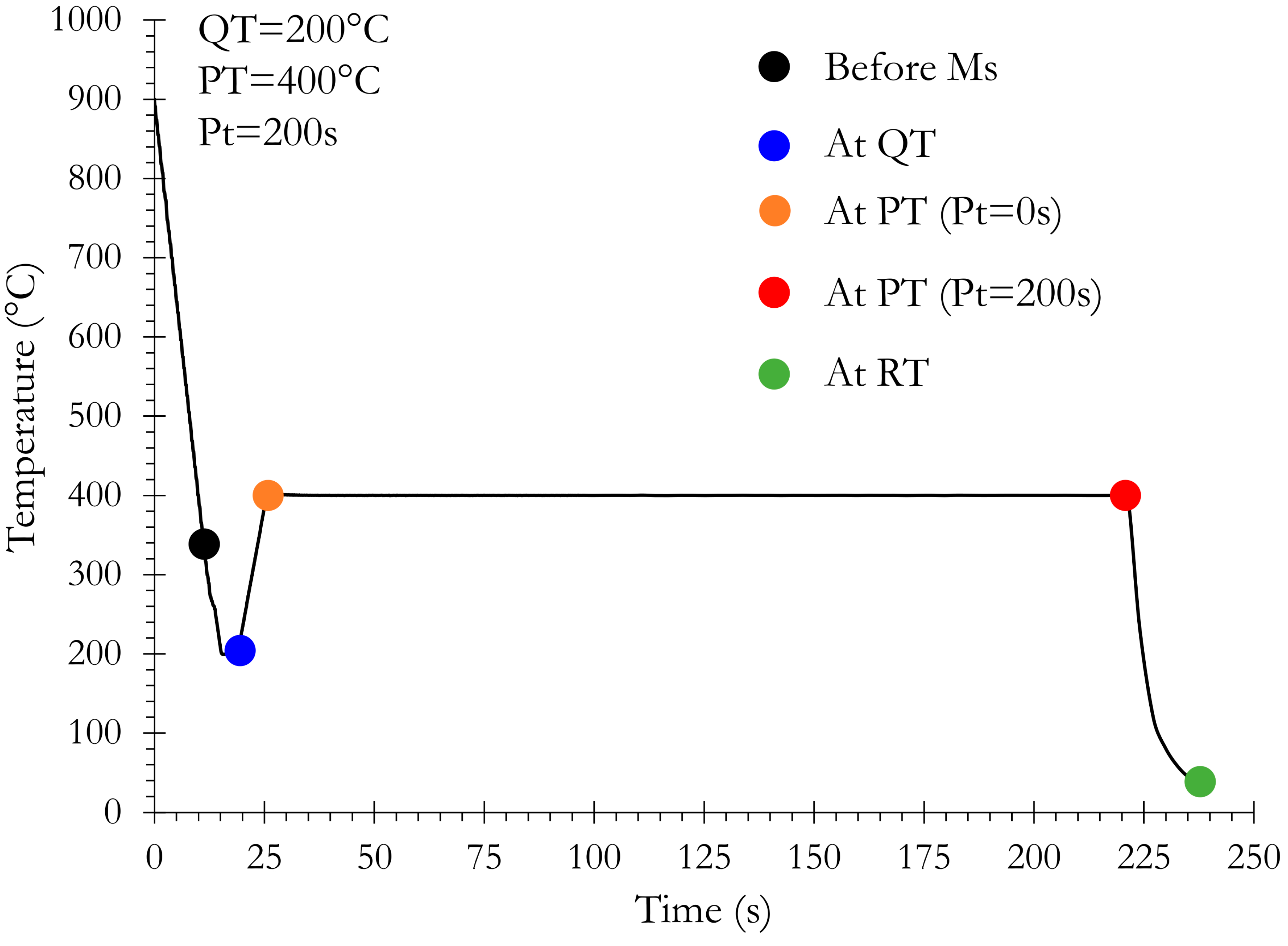
- A quenching from the fully austenitic domain (900 °C) to a temperature Ms during which no phase transformation occurs,
- (2)
- A temperature from Ms to QT (260, 230, and 200 °C) during which a significant increase in the BCT phase at the expense of austenite occurs. Martensite starts to form during the initial quench to QT at 324 °C, 301 °C, and 315 °C for respectively QT200, QT230, and QT260. At the end of the QT step, once the temperature of the sample is homogenized, the initial volume fraction of martensite depends on the following QTs: 85% at QT200, 76% at QT230, and 65% at QT260. The rate of this transformation is very fast in the first stages and becomes more sluggish at the final stage. This behavior has already been observed by [15] and was attributed to martensite transformation. Furthermore, the measured martensite start temperature (Ms) corresponds perfectly to the Ms temperature measured by dilatometry for the same alloy and in the same conditions of cooling [16] as calculated from empirical equations proposed by [17].
- (3)
- A stagnant stage during reheating from the QT in which the microstructural state remains globally unchanged. The duration of this stage, clearly visible on the evolution of austenite fraction, is longer with the decreasing QT.
- (4)
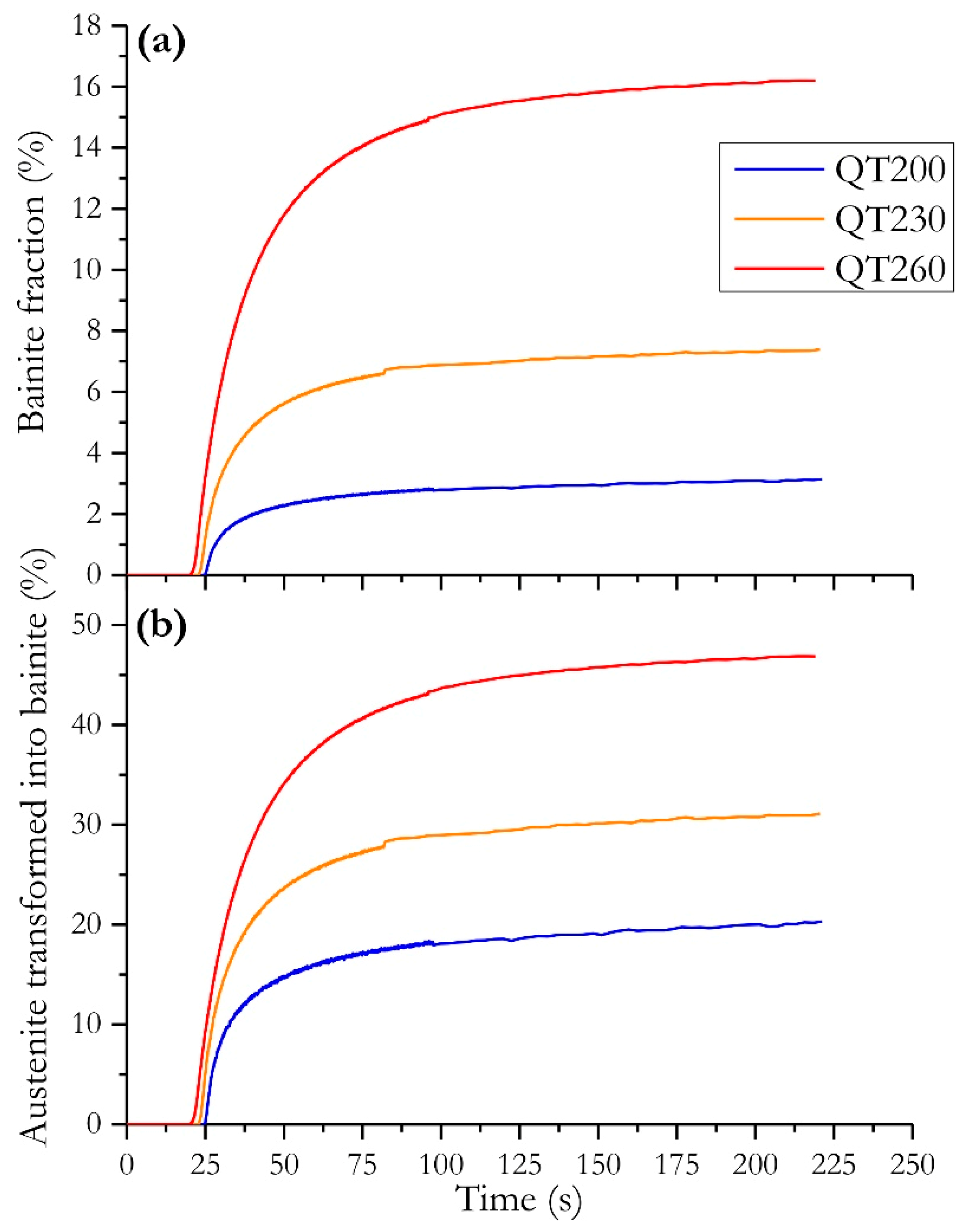
- A significant and slow increase in a new BCT phase fraction at the expense of austenite occurs during both reheating and the partitioning step. The nature of this new BCT phase is subject to much debate as it can be either bainite, isothermal martensite, or the marker of the motion of the α’/γ interface. We shall return more deeply to this point later.
3.2. Metallographic Analysis
3.3. Carbon Trapping in Martensite
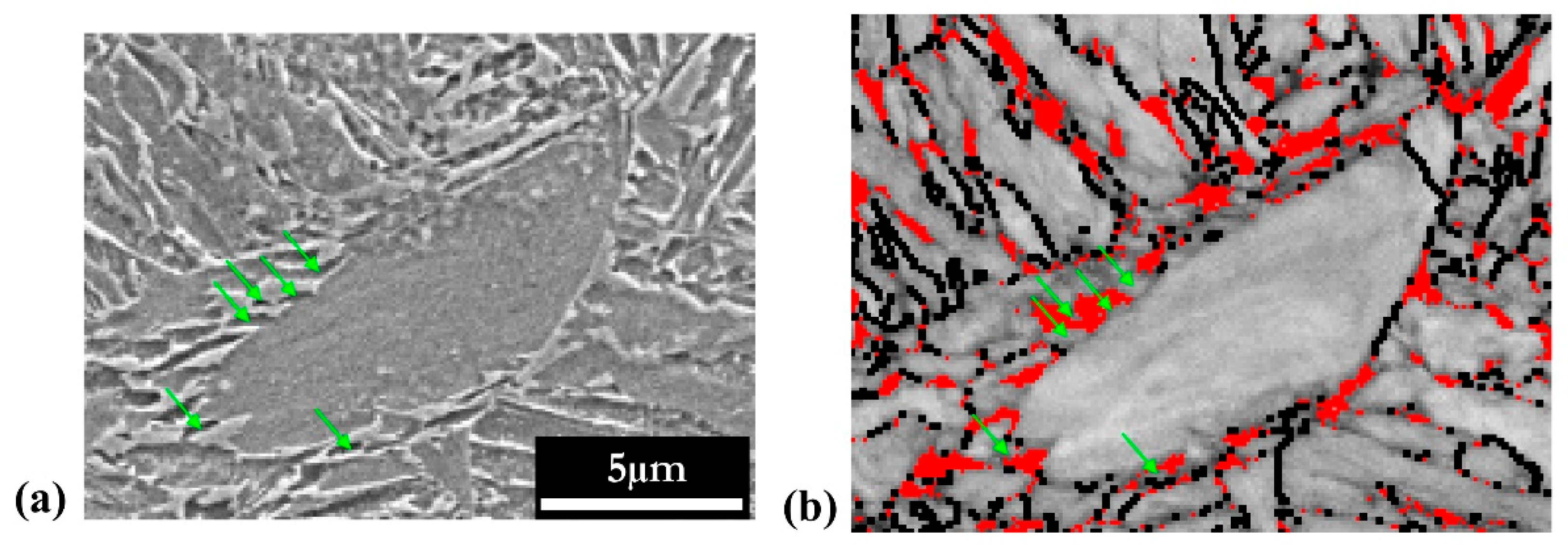
3.3.1. Carbide Precipitation in Martensite
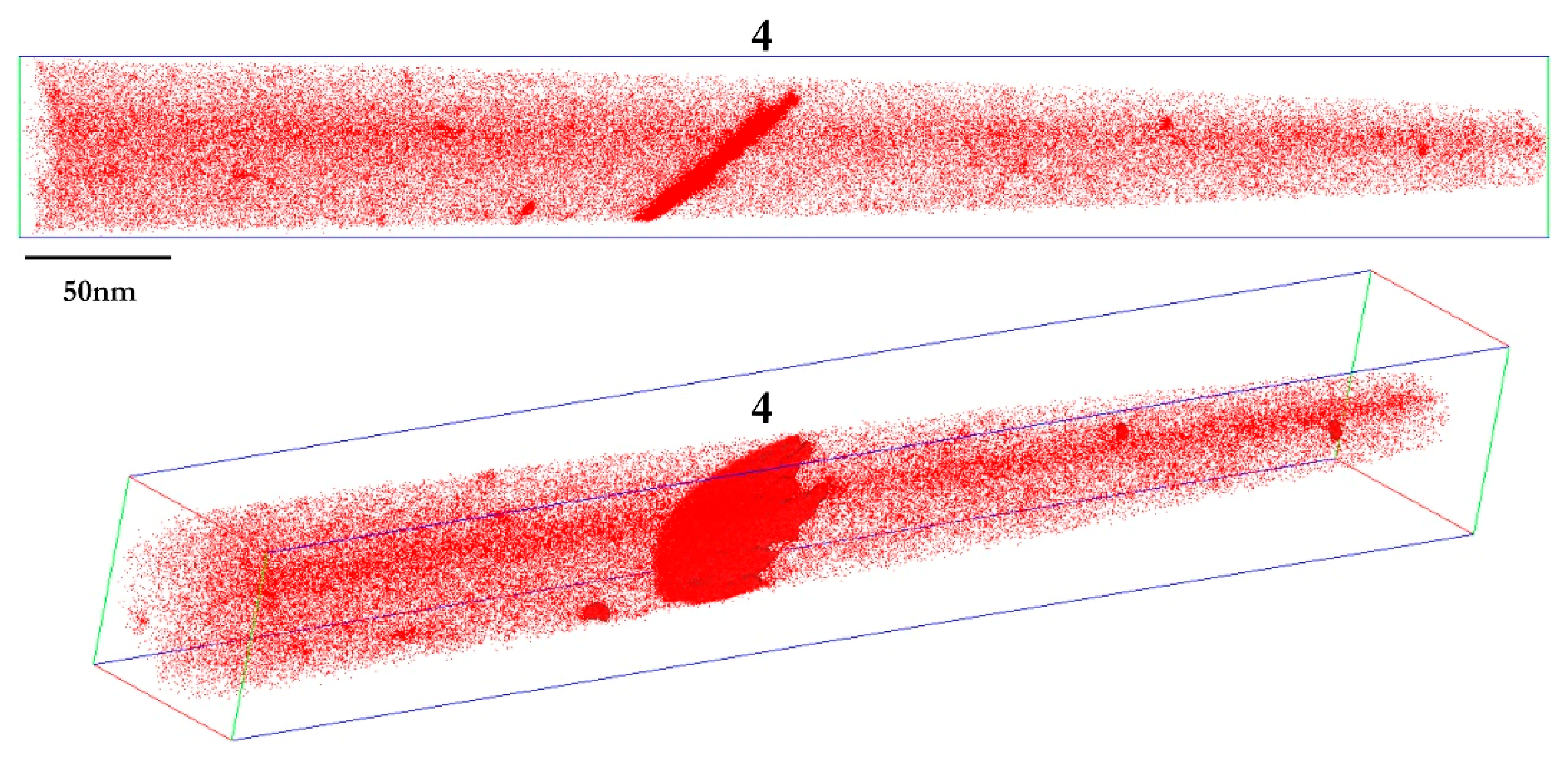
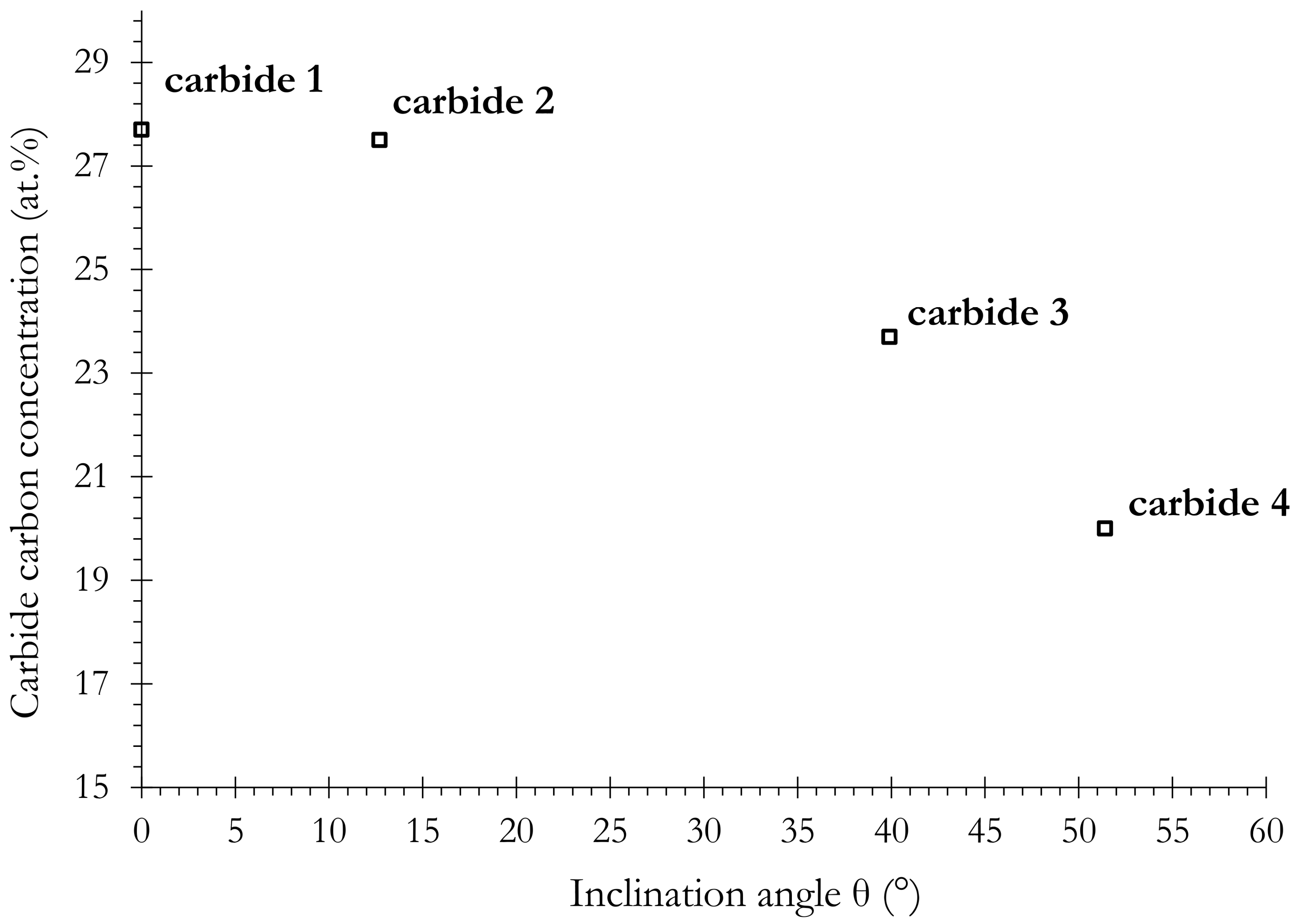
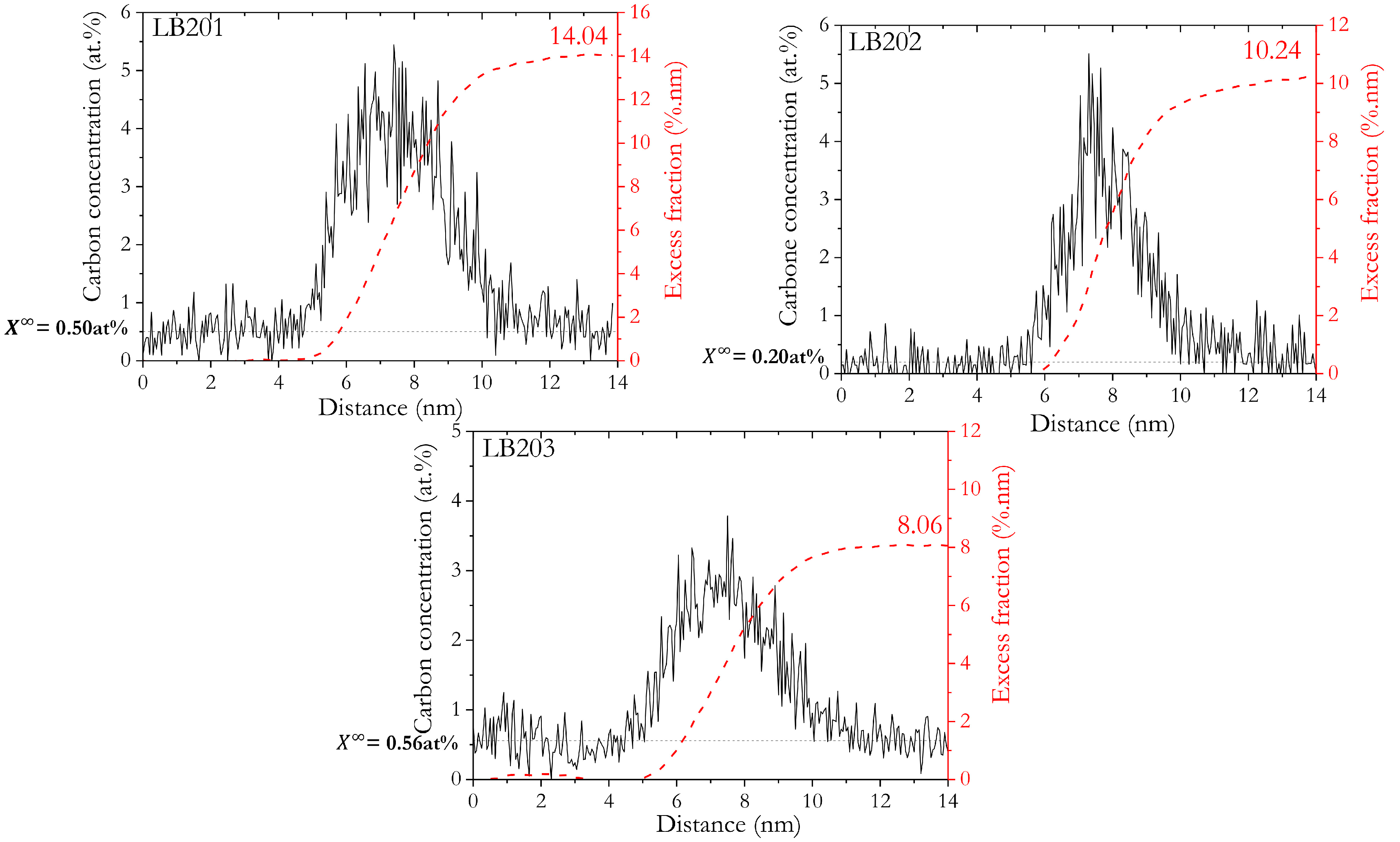
3.3.2. Carbon Segregation Evolution at Martensite Boundaries during Partitioning
4. Conclusions
- (1)
- Carbide-free BCT bainite is formed within a very short range during the reheating and partitioning step. Its transformation rate and kinetics depend on the QT. The highest transformation rate (45%) was measured at QT = 260 °C. As a consequence, a significant part of the carbon enrichment observed in austenite can be attributed to bainite transformation;
- (2)
- An image analysis methodology using SEM and EBSD was developed allowing the identification of bainite in Q&P steels;
- (3)
- A large part of carbon was shown to be trapped in martensite in the form of both Fe2.6C iron and segregation on lath boundaries. The energy for carbon segregation was determined to be 0.20 eV, and the carbon concentration on the lath boundaries was obtained to be around 25 at %. The carbon that is strongly trapped in martensite is a drag on carbon partitioning from martensite to austenite;
- (4)
- Thus, the carbon enrichment into austenite results from competitive reactions such as carbon partitioning from martensite, bainite transformation, and carbon trapping in martensite.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Iung, T.; Azuma, M.; Bouaziz, O.; Gouné, M.; Perlade, A.; Quidort, D. A Model for the Prediction of Microstructure and Mechanical Properties in Cold Rolled and Annealed TRIP Steels. Mater. Sci. Forum 2003, 426, 3849–3854. [Google Scholar] [CrossRef]
- Speer, J.G.; Matlock, D.K.; De Cooman, B.C.; Schroth, J.G. Carbon partitioning into austenite after martensite transformation. Acta Mater. 2003, 51, 2611–2622. [Google Scholar] [CrossRef]
- Speer, J.G.; Edmonds, D.V.; Rizzo, F.C.; Matlock, D.K. Partitioning of carbon from supersaturated plates of ferrite, with application to steel processing and fundamentals of the bainite transformation. Curr. Opin. Solid State Mater. Sci. 2004, 51, 2611–2622. [Google Scholar] [CrossRef]
- Hu, F.; Wu, K.M. Nanostructured high-carbon dual-phase steels. Scr. Mater. 2011, 65, 351–354. [Google Scholar] [CrossRef]
- De Knijf, D.; Petrov, R.; Föjer, C.; Kestens, L.A.I. Effect of fresh martensite on the stability of retained austenite in quenching and partitioning steel. Mater. Sci. Eng. A 2014, 615, 107–115. [Google Scholar] [CrossRef]
- Angeli, J.; Füreder, E.; Panholzer, M.; Kneissl, A.C. Etching Techniques for Characterizing the Phases of Low-Alloy Dual-Phase and TRIP Steels. Pract. Metallogr. 2006, 43, 489–504. [Google Scholar] [CrossRef]
- Clarke, A.J.; Speer, J.G.; Miller, M.K.; Hackenberg, R.E.; Edmonds, D.V.; Matlock, D.K.; Rizzo, F.C.; Clarke, K.D.; De Moor, E. Carbon partitioning to austenite from martensite or bainite during the quench and partition (Q&P) process: A critical assessment. Acta Mater. 2008, 56, 16–22. [Google Scholar]
- Gouné, M.; Danoix, F.; Allain, S.; Bouaziz, O. Unambiguous carbon partitioning from martensite to austenite in Fe-C-Ni alloys during quenching and partitioning. Scr. Mater. 2013, 68, 1004–1007. [Google Scholar] [CrossRef]
- Caballero, F.G.; Miller, M.K.; Clarke, A.J.; Garcia-Mateo, C. Examination of carbon partitioning into austenite during tempering of bainite. Scr. Mater. 2010, 63, 442–445. [Google Scholar] [CrossRef] [Green Version]
- Allain, S.Y.P.; Geandier, G.; Hell, J.C.; Soler, M.; Danoix, F.; Gouné, M. In-situ investigation of quenching and partitioning by High Energy X-Ray Diffraction experiments. Scr. Mater. 2017, 131, 15–18. [Google Scholar] [CrossRef]
- Santofimia, M.J.; Zhao, L.; Sietsma, J. Overview of Mechanisms Involved During the Quenching and Partitioning Process in Steels. Met. Mater. Trans. A 2011, 42, 3620–3626. [Google Scholar] [CrossRef] [Green Version]
- Huyghe, P.; Caruso, M.; Collet, J.; Dépinoy, S.; Godet, S. Into the quenching & partitioning of a 0.2C steel: An in-situ synchrotron study. Mater. Sci. Eng. A 2019, 743, 175–184. [Google Scholar]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Young, R.A. Introduction to the Rietveld method. In The Rietveld Method; Young, R.A., Ed.; Oxford University Press: Oxônia, UK, 1993; pp. 1–38. [Google Scholar]
- Epp, J.; Hirsch, T.; Curfs, C. In situ X-Ray Diffraction Analysis of Carbon Partitioning during Quenching of Low Carbon Steel. Metall. Mater. Trans. A 2012, 43, 2210–2217. [Google Scholar] [CrossRef]
- Aoued, S. Study of the Mechanisms of Carbon Enrichment in Austenite in Q&P Steels. Ph.D. Thesis, Université de Bordeaux, Bordeaux, France, 2019. [Google Scholar]
- van Bohemen, S.M.C. Bainite and martensite start temperature calculated with exponential carbon dependence. Mater. Sci. Technol. 2012, 28, 487–495. [Google Scholar] [CrossRef]
- Allain, S.Y.P.; Aoued, S.; Quintin-Poulon, A.; Gouné, M.; Danoix, F.; Hell, J.-C.; Bouzat, M.; Soler, M.; Geandier, G. In Situ Investigation of the Iron Carbide Precipitation Process in a Fe-C-Mn-Si Q&P Steel. Materials 2018, 11, 1087. [Google Scholar] [CrossRef] [Green Version]
- Navarro-Lopez, A.; Hidalgo, J.; Sietsma, J.; Santofimia, M.J. Characterization of bainitic/martensitic structures formed in isothermal treatments below the MS temperature. Mater. Charact. 2017, 128, 248–256. [Google Scholar] [CrossRef]
- ASTM E562-02. Standard Test Method for Determining Volume Fraction by Systematic Manual Point Count; ASTM International: West Conshohocken, PA, USA, 2002; ISBN 5957529001. [Google Scholar]
- Hulme-Smith, C.N.; Lonardelli, I.; Dippel, A.C.; Bhadeshia, H.K.D.H. Experimental evidence for non-cubic bainitic ferrite. Scr. Mater. 2013, 69, 409–412. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Mateo, C.; Jimenez, J.A.; Yen, H.W.; Miller, M.K.; Morales-Rivas, L.; Kuntz, M.; Ringer, S.P.; Yang, J.R.; Caballero, F.G. Low temperature bainitic ferrite: Evidence of carbon super-saturation and tetragonality. Acta Mater. 2015, 91, 162–173. [Google Scholar] [CrossRef] [Green Version]
- Zener, C. Theory of strain interaction of solute atoms. Phys. Rev. 1948, 74, 639–647. [Google Scholar] [CrossRef]
- Caballero, F.G.; Garcia-Mateo, C.; Santofimia, M.J.; Miller, M.K.; García de Andrés, C. New experimental evidence on the incomplete transformation phenomenon in steel. Acta Mater. 2009, 57, 8–17. [Google Scholar] [CrossRef] [Green Version]
- Caballero, F.G.; Miller, M.K.; Garcia-Mateo, C.; Cornide, J.; Santofimia, M.J. Temperature dependence of carbon supersaturation of ferrite in bainitic steels. Scr. Mater. 2012, 67, 846–849. [Google Scholar] [CrossRef] [Green Version]
- Mirzayev, D.A.; Mirzoev, A.A.; Buldashev, I.V.; Okishev, K.Y. Thermodynamic analysis of the formation of tetragonal bainite in steels. Phys. Met. Metallogr. 2017, 118, 517–523. [Google Scholar] [CrossRef]
- Toji, Y.; Miyamoto, G.; Raabe, D. Carbon partitioning during quenching and partitioning heat treatment accompanied by carbide precipitation. Acta Mater. 2015, 86, 137–147. [Google Scholar] [CrossRef]
- Larson, D.J.; Prosa, T.J.; Ulfig, R.M.; Geiser, B.P.; Kelly, T.F. Local Electrode Atom Probe Tomography; Springer: New York, NY, USA, 2013; ISBN 978-1-4614-8720-3. [Google Scholar]
- Pierce, D.T.; Coughlin, D.R.; Williamson, D.L.; Clarke, K.D.; Clarke, A.J.; Speer, J.G.; Moor, E. De Characterization of transition carbides in quench and partitioned steel microstructures by Mössbauer spectroscopy and complementary techniques. Acta Mater. 2015, 90, 417–430. [Google Scholar] [CrossRef] [Green Version]
- Pierce, D.T.; Coughlin, D.R.; Williamson, D.L.; Kähkönen, J.; Clarke, A.J.; Clarke, K.D.; Speer, J.G.; Moor, E. De Quantitative investigation into the influence of temperature on carbide and austenite evolution during partitioning of a quenched and partitioned steel. Scr. Mater. 2016, 121, 5–9. [Google Scholar] [CrossRef] [Green Version]
- Lu, W.; Herbig, M.; Liebscher, C.H.; Morsdorf, L.; Marceau, R.K.W.; Dehm, G.; Raabe, D. Formation of eta carbide in ferrous martensite by room temperature aging. Acta Mater. 2018, 158, 297–312. [Google Scholar] [CrossRef]
- Maugis, P.; Hoummada, K. A methodology for the measurement of the interfacial excess of solute at a grain boundary. Scr. Mater. 2016, 120, 90–93. [Google Scholar] [CrossRef]
- Pierce, D.T.; Coughlin, D.R.; Clarke, K.D.; De Moor, E.; Poplawsky, J.; Williamson, D.L.; Mazumder, B.; Speer, J.G.; Hood, A.; Clarke, A.J. Microstructural evolution during quenching and partitioning of 0.2C–1.5Mn-1.3Si steels with Cr or Ni additions. Acta Mater. 2018, 151, 454–469. [Google Scholar] [CrossRef]
- Clarke, A.J.; Miller, M.K.; Field, R.D.; Coughlin, D.R.; Gibbs, P.J.; Clarke, K.D.; Alexander, D.J.; Powers, K.A.; Papin, P.A.; Krauss, G. Atomic and nanoscale chemical and structural changes in quenched and tempered 4340 steel. Acta Mater. 2014, 77, 17–27. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, W.; Zhao, H.S.; Lu, X.W.; Jin, X.J. Investigation of carbon segregation during low temperature tempering in a medium carbon steel. Mater. Charact. 2016, 117, 84–90. [Google Scholar] [CrossRef]
- Da Rosa, G. Mécanismes et Conséquences de La Ségrégation du Bore Aux Joints de Grains Austénitiques Dans Les Aciers à Très Haute Résistance. Ph.D. Thesis, Université d’Aix-Marseille, Marseille, France, 2018. [Google Scholar]
- Jang, J.H.; Bhadeshia, H.K.D.H.; Suh, D.W. Solubility of carbon in tetragonal ferrite in equilibrium with austenite. Scr. Mater. 2013, 68, 195–198. [Google Scholar] [CrossRef]
- Clouet, E.; Garruchet, S.; Nguyen, H.; Perez, M.; Becquart, C.S. Dislocation interaction with C in α-Fe: A comparison between atomic simulations and elasticity theory. Acta Mater. 2008, 56, 3450–3460. [Google Scholar] [CrossRef] [Green Version]
- Cochardt, A.W.; Schoek, G.; Wiedersich, H. Interaction between dislocations and interstitial atoms in body-centered cubic metals. Acta Metall. 1955, 3, 533–537. [Google Scholar] [CrossRef]
- Kalish, D.; Cohen, M. Structural changes and strengthening in the strain tempering of martensite. Mater. Sci. Eng. 1970, 6, 156–166. [Google Scholar] [CrossRef]
- Speich, G.R. Tempering of low-carbon martensite. Trans. TMS-AIME 1969, 245, 2553–2564. [Google Scholar]



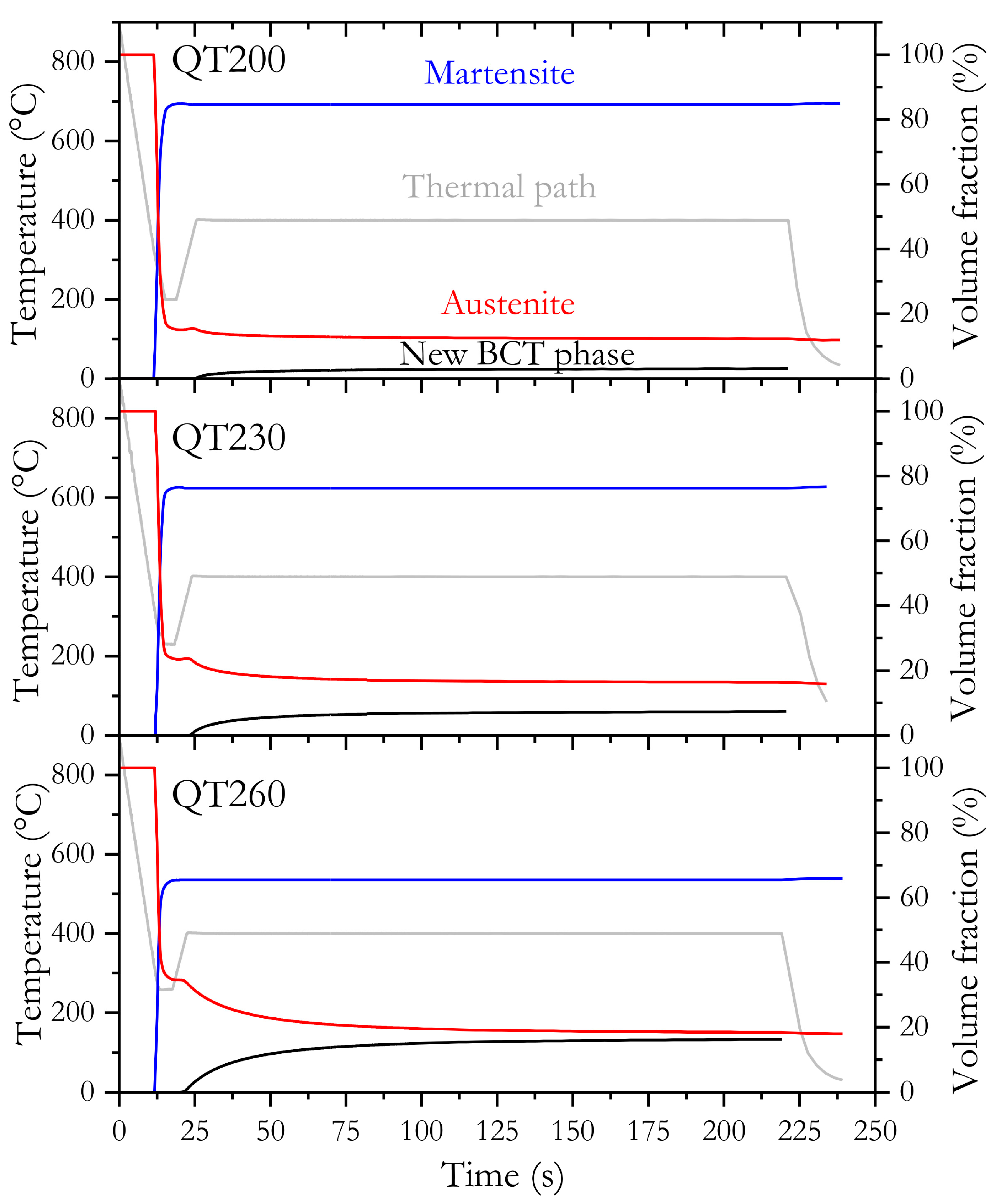









| Quenching Condition | New BCT Start Temperature |
|---|---|
| QT200 | 381 °C |
| QT230 | 369 °C |
| QT260 | 335 °C |
| Feature | Characteristics |
|---|---|
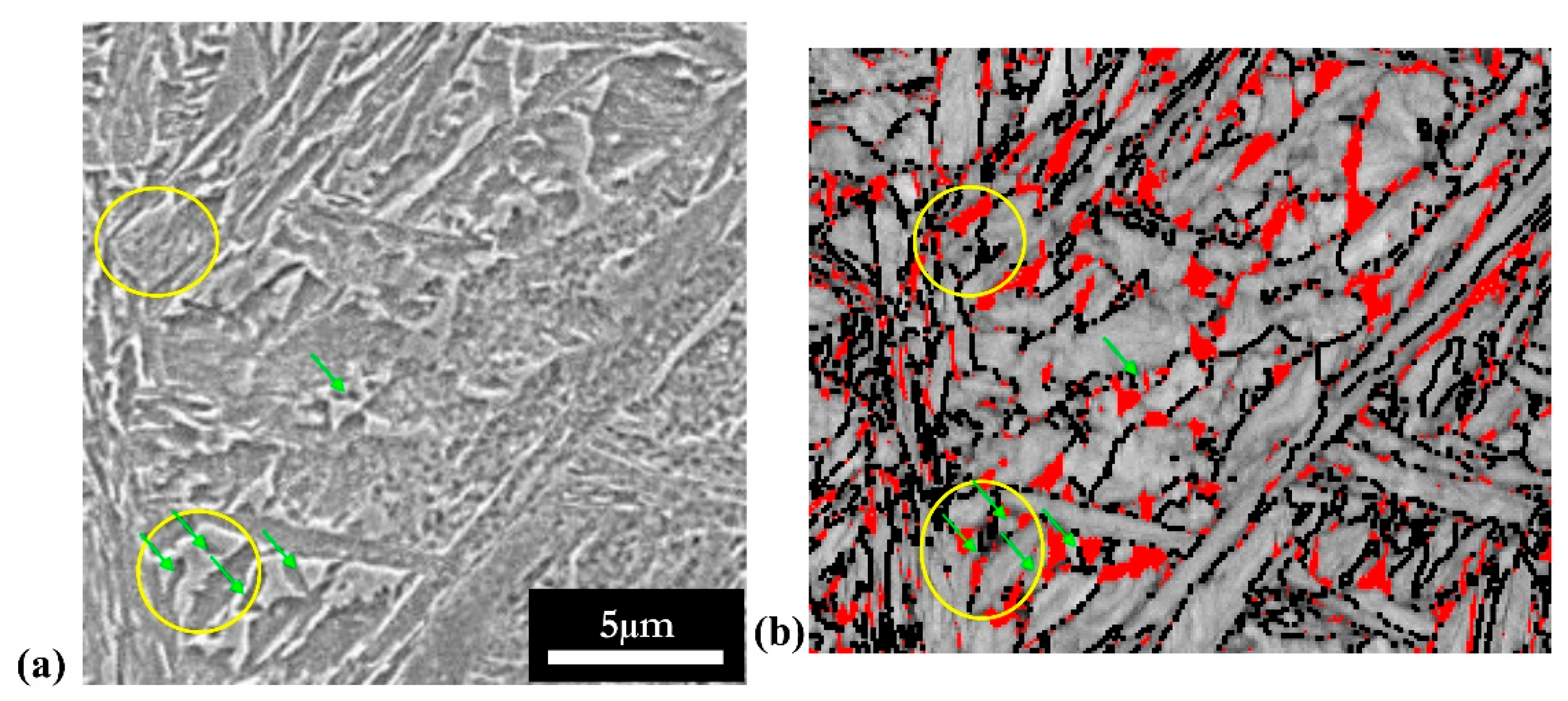
Large islands | Large block of martensite/austenite Smooth surface Length: 400 nm to 6 µm May present internal structure Phase: fresh martensite + retained austenite |
Packets of small laths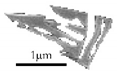 | Groups of thin laths Length of individual laths: 200 to 900 nm Phase: retained austenite |
Long laths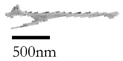 | Long thin laths surrounded by other BCT phases Appears quite bright Length: 1–2.5 µm Very high aspect ratio Phase: retained austenite |
| Feature | Characteristics |
|---|---|
Large laths with carbides  | Long laths Contain carbides Length: 1–5 µm Phase: tempered martensite |
Small carbide-free laths  | Thin acicular shaped laths Carbide free Length: 200–800 nm Phase: bainite |
Small laths in large MA Islands  | MA Islands (in grey) with embedded bainite (yellow contouring) Length: 200–800 na Phase: bainite |
| Heading | Heading | (%.nm) | (at.nm−2) | (at%) | (%) | Es (eV) | %C (%at) |
|---|---|---|---|---|---|---|---|
| QT200_400/0 s | LB04 (tip n°1) | 10.25 | 8.24 | 0.52 | 11.45 | 0.179 | 25.41 |
| LB05 (tip n°2) | 10.25 | 8.24 | 0.90 | 11.45 | 0.149 | 25.41 | |
| Average | 10.25 | 8.24 | 0.71 | 11.45 | 0.164 | 25.41 | |
| QT200_400/200 s | LB201 (tip n°3) | 14.04 | 11.30 | 0.50 | 15.68 | 0.202 | 31.82 |
| LB202 (tip n°4) | 10.24 | 8.24 | 0.20 | 11.44 | 0.233 | 25.40 | |
| LB203 (tip n°5) | 8.06 | 6.48 | 0.56 | 9.00 | 0.160 | 21.13 | |
| Average | 9.15 | 7.36 | 0.38 | 10.22 | 0.197 | 23.26 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aoued, S.; Danoix, F.; Allain, S.Y.P.; Gaudez, S.; Geandier, G.; Hell, J.-C.; Soler, M.; Gouné, M. Microstructure Evolution and Competitive Reactions during Quenching and Partitioning of a Model Fe–C–Mn–Si Alloy. Metals 2020, 10, 137. https://doi.org/10.3390/met10010137
Aoued S, Danoix F, Allain SYP, Gaudez S, Geandier G, Hell J-C, Soler M, Gouné M. Microstructure Evolution and Competitive Reactions during Quenching and Partitioning of a Model Fe–C–Mn–Si Alloy. Metals. 2020; 10(1):137. https://doi.org/10.3390/met10010137
Chicago/Turabian StyleAoued, Samy, Frédéric Danoix, Sébastien Y.P. Allain, Steve Gaudez, Guillaume Geandier, Jean-Christophe Hell, Michel Soler, and Mohamed Gouné. 2020. "Microstructure Evolution and Competitive Reactions during Quenching and Partitioning of a Model Fe–C–Mn–Si Alloy" Metals 10, no. 1: 137. https://doi.org/10.3390/met10010137






