Sex-Specific Sub-Lethal Effects and Immune Response in Ceratitis capitata Wied. (Diptera: Tephritidae) Challenged with Spinosad
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Bioassays
2.2. RT-qPCR Analysis
2.3. Data Analysis
3. Results
3.1. Lethal and Sub-Lethal Effects
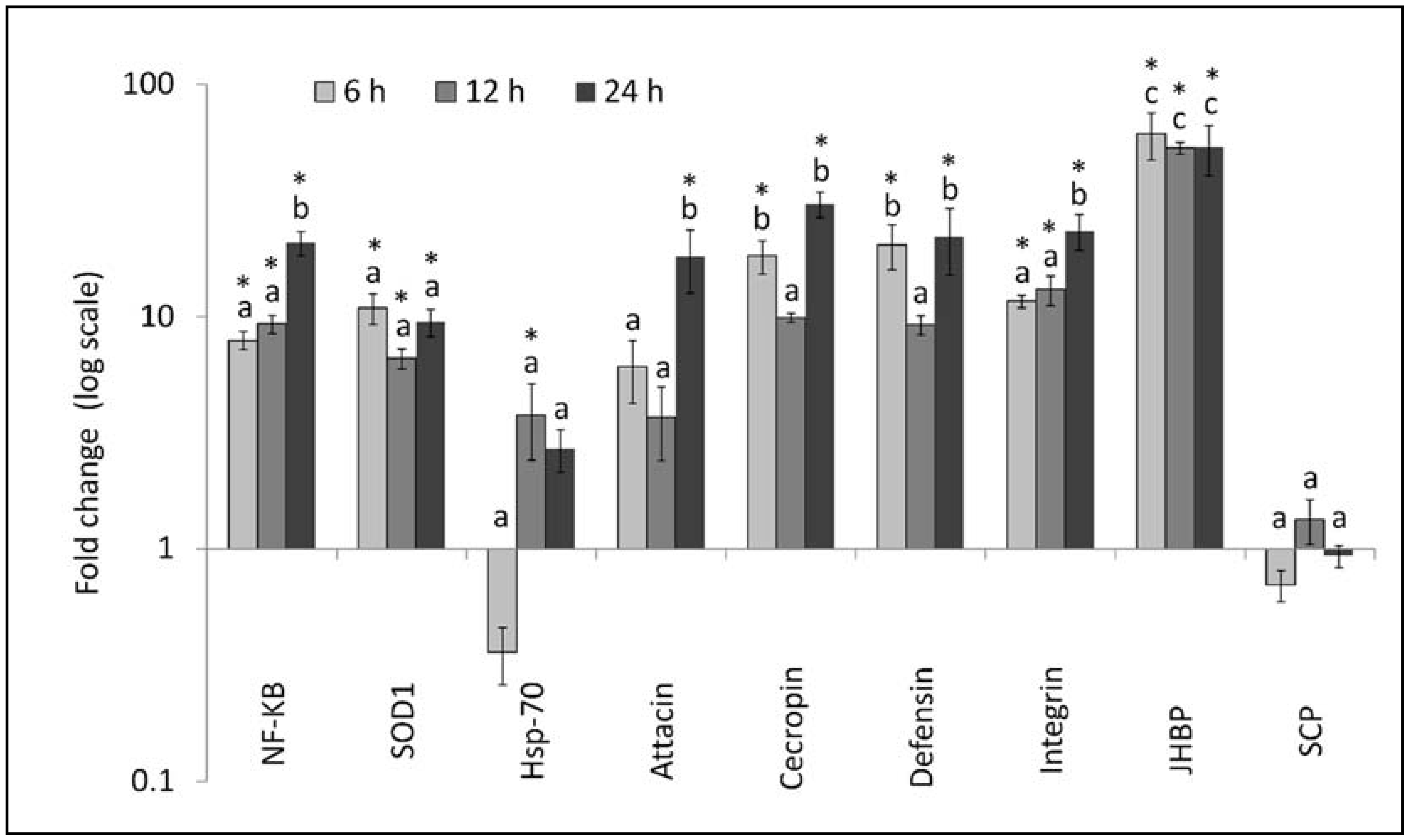
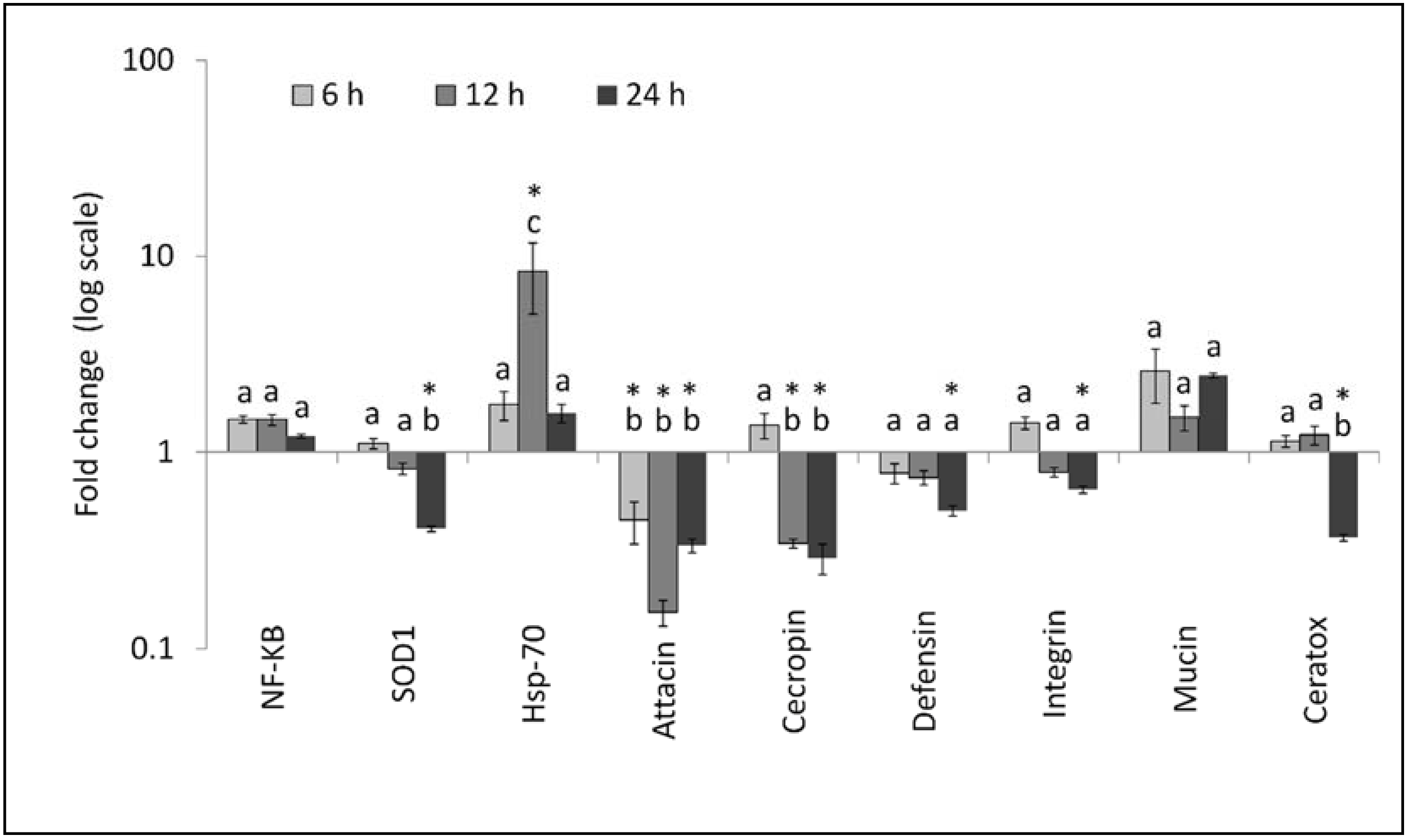
3.2. Gene Expression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mertz, F.P.; Yao, R.C. Saccharopolyspora spinosa sp. nov. isolated from soil collected in a sugar mill rum still. Int. J. Syst. Bacteriol. 1990, 40, 34–39. [Google Scholar] [CrossRef]
- Kirst, H.A.; Michel, K.H.; Martin, J.W.; Creemer, L.C.; Chio, E.H.; Yao, R.C.; Nakatsukasa, W.M.; Boeck, L.D.; Occolowitz, J.L.; Paschal, J.W.; et al. A83543A-D, unique fermentation-derived tetracyclic macrolides. Tetrahedron Lett. 1991, 32, 4839–4842. [Google Scholar] [CrossRef]
- Sparks, T.C.; Crouse, G.D.; Durst, G. Natural products as insecticides: The biology, biochemistry and quantitative structure-activity relationships of spinosyns and spinosoids. Pest Manag. Sci. 2001, 57, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Salgado, V.L.; Sparks, T.C. The spinosyns: Chemistry, biochemistry, mode of action, and resistance. In Comprehensive Molecular Insect Science; Gilbert, L.J., Iatrou, K., Gill, S.S., Eds.; Elsevier: Oxford, UK, 2005; Volume 6, pp. 137–173. [Google Scholar]
- López, J.D.; Latheef, M.A.; Hoffmann, W.C. Mortality and reproductive effects of ingested spinosad on adult bollworms. Pest Manag. Sci. 2011, 67, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, Y.-M.; Liu, H.-Y.; Xin, Z.; Xue, M. Lethal and sublethal effects of spinosad on Spodoptera exigua (Lepidoptera: Noctuidae). J. Econ. Entomol. 2013, 106, 1825–1831. [Google Scholar] [CrossRef] [PubMed]
- Rolff, J.; Reynolds, S.E. Insect Infection and Immunity, Evolution, Ecology, and Mechanisms; Oxford University Press: Oxford, UK, 2011. [Google Scholar]
- Lemaitre, B.; Hoffman, J. The host defense of Drosophila melanogaster. Annu. Rev. Immunol. 2007, 25, 697–743. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Wu, C.; Li, J.; Ren, G.; Huang, D.; Liu, F. Stress-induced HSP70 from Musca domestica plays a functionally significant role in the immune system. J. Insect Physiol. 2012, 58, 1226–1234. [Google Scholar] [CrossRef] [PubMed]
- Binggeli, O.; Neyen, C.; Poidevin, M.; Lemaitre, B. Prophenoloxidase activation is required for survival to microbial infections in Drosophila. PLoS Pathog. 2014, 10, e1004067. [Google Scholar] [CrossRef] [PubMed]
- James, R.R.; Xu, J. Mechanisms by which pesticides affect insect immunity. J. Invertebr. Pathol. 2012, 109, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Stark, J.D.; Vargas, R.; Miller, N. Toxicity of spinosad in protein bait to three economically important tephritid fruit fly species (Diptera: Tephritidae) and their parasitoids (Hymenoptera: Braconidae). J. Econ. Entomol. 2004, 97, 911–915. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Llopis, V.; Primo, J.; Vacas, S. Efficacy of attract-and-kill devices for the control of Ceratitis capitata. Pest Manag. Sci. 2013, 69, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Liquido, N.J.; Shinoda, L.A.; Cunningham, R.T. Host Plants of the Mediterranean Fruit Fly (Diptera Tephritidae): An Annotated World Review; Miscellaneous Publications; Entomological Society of America: Annapolis, MD, USA, 1991. [Google Scholar]
- Ruiu, L.; Falchi, G.; Floris, I.; Marche, M.G.; Mura, M.E.; Satta, A. Pathogenicity and characterization of a novel Bacillus cereus sensu lato isolate toxic to the Mediterranean fruit fly Ceratitis capitata Wied. J. Invertebr. Pathol. 2015, 126, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Papanicolaou, A.; Schetelig, M.F.; Arensburger, P.; Atkinson, P.W.; Benoit, J.B.; Bourtzis, K.; Castañera, P.; Cavanaugh, J.P.; Chao, H.; Childers, C.; et al. The whole genome sequence of the Mediterranean fruit fly, Ceratitis capitata (Wiedemann), reveals insights into the biology and adaptive evolution of a highly invasive pest species. Genome Biol. 2016, 17, 192. [Google Scholar] [CrossRef] [PubMed]
- Falchi, G.; Marche, M.G.; Mura, M.E.; Ruiu, L. Hydrophobins from aerial conidia of Beauveria bassiana interfere with Ceratitis capitata oviposition behavior. Biol. Control 2015, 81, 37–43. [Google Scholar] [CrossRef]
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- SAS Institute Inc. SAS/STAT 9.1, User’s Guide; SAS Institute Inc.: Cary, NC, USA, 2004. [Google Scholar]
- Finney, D.J. Probit Analysis, 3rd ed.; Cambridge University: London, UK, 1971. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔC(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Juan-Blasco, M.; Sabater-Muñoz, B.; Argilés, R.; Jacas, J.A.; Ortego, F.; Urbaneja, A. Effects of pesticides used on citrus grown in Spain on the mortality of Ceratitis capitata (Diptera: Tephritidae) Vienna-8 strain sterile males. J. Econ. Entomol. 2013, 106, 1226–1233. [Google Scholar] [CrossRef] [PubMed]
- Gazit, Y.; Gavriel, S.; Akiva, R.; Timar, D. Toxicity of baited spinosad formulations to Ceratitis capitata: From the laboratory to the application. Entomol. Exp. Appl. 2013, 147, 120–125. [Google Scholar] [CrossRef]
- Stark, J.D.; Vargas, R.I.; Souder, S.L.; Fox, A.J.; Smith, T.R.; Mackey, B. A comparison of the bioinsecticide, spinosad, the semi-synthetic insecticide, spinetoram and synthetic insecticides as soil drenches for control of tephritid fruit flies. Biopestic. Int. 2013, 9, 120–126. [Google Scholar]
- Voudouris, C.C.; Mavridis, K.; Kalaitzaki, A.; Skouras, P.J.; Kati, A.N.; Eliopoulos, P.A.; Vontas, J.; Margaritopoulos, J.T. Susceptibility of Ceratitis capitata to deltamethrin and spinosad in Greece. J. Pest. Sci. 2018, 91, 861–871. [Google Scholar] [CrossRef]
- Yin, X.-H.; Wu, Q.-J.; Li, X.-F.; Zhang, Y.-J.; Xu, B.-Y. Sublethal effects of spinosad on Plutella xylostella (Lepidoptera: Yponomeutidae). Crop Prot. 2008, 27, 1385–1391. [Google Scholar] [CrossRef]
- Galvan, T.L.; Koch, R.L.; Hutchison, W.D. Effects of spinosad and indoxacarb on survival, development, and reproduction of the multicolored Asian lady beetle (Coleoptera: Coccinellidae). Biol. Control 2005, 34, 108–114. [Google Scholar] [CrossRef]
- Kirst, H.A. The spinosyn family of insecticides: Realizing the potential of natural products research. J. Antibiot. 2010, 63, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Mamali, I.; Lamprou, I.; Karagiannis, F.; Karakantza, M.; Lampropoulou, M.; Marmaras, V.J. A beta integrin subunit regulates bacterial phagocytosis in medfly haemocytes. Dev. Comp. Immunol. 2009, 33, 858–866. [Google Scholar] [CrossRef] [PubMed]
- Kalosaka, K.; Soumaka, E.; Politis, N.; Mintzas, A.C. Thermotolerance and Hsp70 expression in the Mediterranean fruit fly Ceratitis capitata. J. Insect Physiol. 2009, 55, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Orr, W.C.; Sohal, R.S. Extension of life-span by overexpression of superoxide dismutase and catalase in Drosophila melanogaster. Science 1994, 263, 1128–1130. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Liang, H.; Hou, Y. Functional analysis of a NF-κB transcription factor in the immune defense of Oriental fruit fly, Bactrocera dorsalis Hendel (Diptera: Tephritidae). Bull. Entomol. Res. 2017, 107, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Kouloussis, N.A.; Damos, P.T.; Ioannou, C.S.; Tsitsoulas, C.; Papadopoulos, N.T.; Nestel, D.; Koveos, D.S. Age related assessment of sugar and protein intake of Ceratitis capitata in ad libitum conditions and modeling its relation to reproduction. Front. Physiol. 2017, 8, 271. [Google Scholar] [CrossRef] [PubMed]
- Shaurub, E.-S.H. Immunomodulation in insects post-treatment with abiotic agents: A review. Eur. J. Entomol. 2012, 109, 303–316. [Google Scholar] [CrossRef]
- Figueiredo, M.B.; Castro, D.P.; Nogueira, N.F.S.; Garcia, E.S.; Azambuja, P. Cellular immune response in Rhodnius prolixus: Role of ecdysone in hemocyte phagocytosis. J. Insect Physiol. 2006, 52, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Adams, T.S.; Kelly, T.J.; Woods, C.W. Relationship between the corpus cardiacum-allatum complex and ovaries with the haemolymph ecdysteroid profile in the housefly, Musca domestica. J. Insect Physiol. 1988, 34, 1105–1109. [Google Scholar] [CrossRef]
- Ishaaya, I.; Barazani, A.; Kontsedalov, S.; Horowitz, A.R. Insecticides with novel modes of action: Mechanism, selectivity and cross-resistance. Entomol. Res. 2007, 37, 148–152. [Google Scholar] [CrossRef]
- Salokhe, S.; Sarkar, A.; Kulkarni, A.; Mukherjee, S.; Pal, J.K. Flufenoxuron, an acylurea insect growth regulator, alters development of Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae) by modulating levels of chitin, soluble protein content, and HSP70 and p34cdc2 in the larval tissues. Pestic. Biochem. Physiol. 2006, 85, 84–90. [Google Scholar] [CrossRef]
- Nasr, H.M.; Badway, M.E.I.; Rabea, E.I. Toxicity and biochemical study of two insect growth regulators, buprofezin and pyriproxyfen, on cotton leafworm Spodoptera littoralis. Pestic. Biochem. Physiol. 2010, 98, 198–205. [Google Scholar] [CrossRef]
- George, P.J.E.; Ambrose, D.P. Impact of insecticides on the haemogram of Rhynocoris kumarii Ambrose and Livingstone (Hem., Reduviidae). J. Appl. Entomol. 2004, 128, 600–604. [Google Scholar] [CrossRef]
- Büyükgüzel, E. Evidence of oxidative and antioxidative responses by Galleria mellonella larvae to Malathion. J. Econ. Entomol. 2009, 102, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Wang, B.; Gao, J.; Zhang, Y.; Xu, W.; Tao, L. Spinosad induces programmed cell death involves mitochondrial dysfunction and cytochrome C release in Spodoptera frugiperda Sf9 cells. Chemosphere 2017, 169, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Hao, Y.; Gao, J.; Zhang, Y.; Xu, W.; Tao, L. Spinosad induces autophagy of Spodoptera frugiperda Sf9 cells and the activation of AMPK/mTOR signaling pathway. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2017, 195, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Gomulski, L.M.; Dimopoulos, G.; Xi, Z.; Scolari, F.; Gabrieli, P.; Siciliano, P.; Clarke, A.R.; Malacrida, A.R.; Gasperi, G. Transcriptome profiling of sexual maturation and mating in the Mediterranean Fruit Fly, Ceratitis capitata. PLoS ONE 2012, 7, e30857. [Google Scholar] [CrossRef] [PubMed]
- Scolari, F.; Gomulski, L.M.; Ribeiro, J.M.C.; Siciliano, P.; Meraldi, A.; Falchetto, M.; Bonomi, A.; Manni, M.; Gabrieli, P.; Malovini, A.; et al. Transcriptional Profiles of Mating-Responsive Genes from Testes and Male Accessory Glands of the Mediterranean Fruit Fly, Ceratitis capitata. PLoS ONE 2012, 7, e46812. [Google Scholar] [CrossRef] [PubMed]
- Rosetto, M.; Marchini, D.; de Filippis, T.; Ciolfi, S.; Frati, F.; Quilici, S.; Dallai, R. The ceratotoxin gene family in the medfly Ceratitis capitata and the Natal fruit fly Ceratitis rosa (Diptera: Tephritidae). Heredity 2003, 90, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Mura, M.E.; Ruiu, L. Brevibacillus laterosporus pathogenesis and local immune response regulation in the house fly midgut. J. Invertebr. Pathol. 2017, 145, 55–61. [Google Scholar] [CrossRef] [PubMed]

| Protein Product | Gene ID | Accession Number a | Primer Pair Sequences | Annealing Temperature (°C) |
|---|---|---|---|---|
| Heat shock protein | Hsp-70 | Y08955.1 | F: 5′CAAAGTGGAGATTATCGCCAACGACCA 3′ R: 5′CACTGTGATGACGGCGTCTGTTACTGT 3′ | 60 |
| SODCu/Zn | SOD1 | M76975.1 | F: 5′CCCGTCCTAGTCACTGGTGAGGTTAAC 3′ R: 5′CTTCGTTGCTCCATCGCCACTGG 3′ | 62 |
| Attacin-B | attacin | XM_004520300.2 | F: 5′TGGAGCTCGCTAAAGGTGTGGGCACA3′ R: ′CTTTGTGAGCGAATCACTTATGCCGGGTGT 3′ | 63 |
| Cecropin-1 | cecropin | XM_004534275.2 | F: 5′CTCCTCGCTGTCATCTTTGCCGTTTTC3′ R: 5′AGCAACATTGGCCGCCTGTTGGGC3 | 52 |
| Defensin-A | defensin | XM_004537571.2 | F: 5′TTATCGCCGGACTCATCCTCTGCTCCT3′ R: 5′ATGTACCGTTCCTGCAGTAACCGCCAC3′ | 63 |
| Integrin alpha-PS | integrin | XM_004521659.1 | F: 5′TGCAACCTGATCTGGGCGATTCGC3′ R: 5′ATGAGCTACTGCTATACGATGAGCCAGCCT3′ | 62 |
| Mucin C | mucin | XM_004533242.1 | F: 5′GGGGAGTACATAACCAATCCGGAAGCAACT3′ R: 5′GACTTGAGCGGTCGTTGTTGATGATGCAGT3′ | 60 |
| Ceratotoxin A | ceratotox | AJ272446.1 | F: 5′TAGTAGCCGAACCTGCTGCCGAAGATT3′ R: 5′AACGGGTAGAGCAGCCTTTGCAATGGGTAA3′ | 62 |
| NF-kB | NF-kB | XM_004517947.2 | F: 5′ACAAAGTTCTCAATGCCCACAATG3′ R: 5′GTTCCTTAACAGCGATATGTAGTGC3′ | 55 |
| SCP_17a | SCP_17a | DQ406807.1 | F: 5′AATATTCTTGTCGGCAACCATAA3′ R: 5′CAGAATTTGGCATGGATCAGT3′ | 50 |
| JHBP_28C | JHBP_28C | DQ406809.1 | F: 5′TGAACGGACCAGATATCCAA3′ R: 5′ACCATGCAGATAGGCAGGAC3′ | 52 |
| Spinosad Concentration (ppm) a | Fecundity (No. Eggs/Female) | Egg Hatch b % | Longevity (Days) c | |
|---|---|---|---|---|
| Male | Female | |||
| Control | 293.8 ± 17.1 a,d | 93.7 ± 1.1 a | 24.0 ± 1.0 a | 28.6 ± 0.8 a |
| 0.1 | 209.8 ± 11.8 b | 88.3 ± 0.9 b | 20.8 ± 0.9 b | 23.0 ± 0.8 b |
| 0.4 | 139.8 ± 9.3 c | 82.5 ± 1.4 c | 16.6 ± 0.5 c | 19.0 ± 0.7 c |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mura, M.E.; Ruiu, L. Sex-Specific Sub-Lethal Effects and Immune Response in Ceratitis capitata Wied. (Diptera: Tephritidae) Challenged with Spinosad. Insects 2018, 9, 73. https://doi.org/10.3390/insects9030073
Mura ME, Ruiu L. Sex-Specific Sub-Lethal Effects and Immune Response in Ceratitis capitata Wied. (Diptera: Tephritidae) Challenged with Spinosad. Insects. 2018; 9(3):73. https://doi.org/10.3390/insects9030073
Chicago/Turabian StyleMura, Maria Elena, and Luca Ruiu. 2018. "Sex-Specific Sub-Lethal Effects and Immune Response in Ceratitis capitata Wied. (Diptera: Tephritidae) Challenged with Spinosad" Insects 9, no. 3: 73. https://doi.org/10.3390/insects9030073
APA StyleMura, M. E., & Ruiu, L. (2018). Sex-Specific Sub-Lethal Effects and Immune Response in Ceratitis capitata Wied. (Diptera: Tephritidae) Challenged with Spinosad. Insects, 9(3), 73. https://doi.org/10.3390/insects9030073