Nematobacterial Complexes and Insect Hosts: Different Weapons for the Same War
Abstract
:1. Introduction
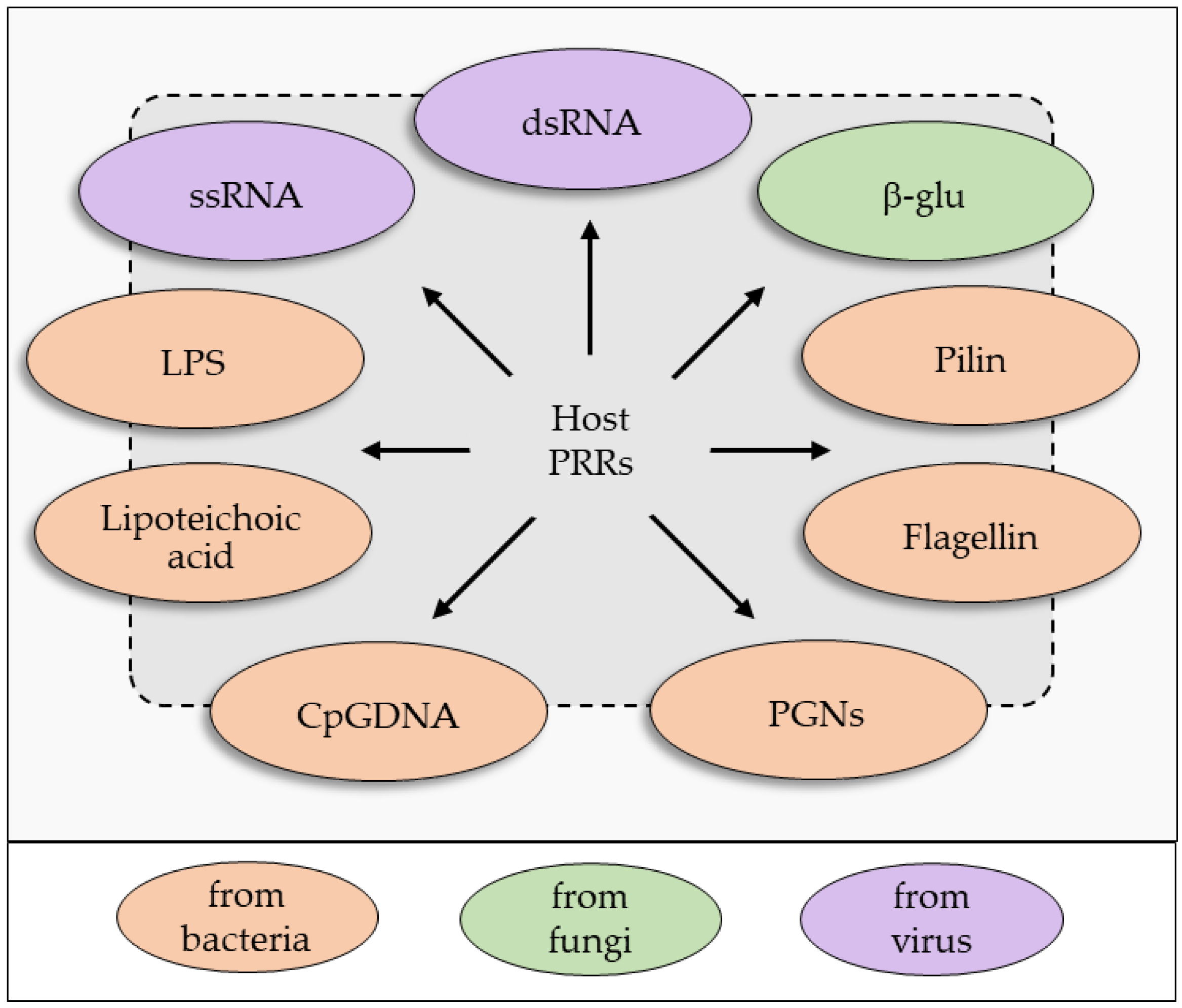
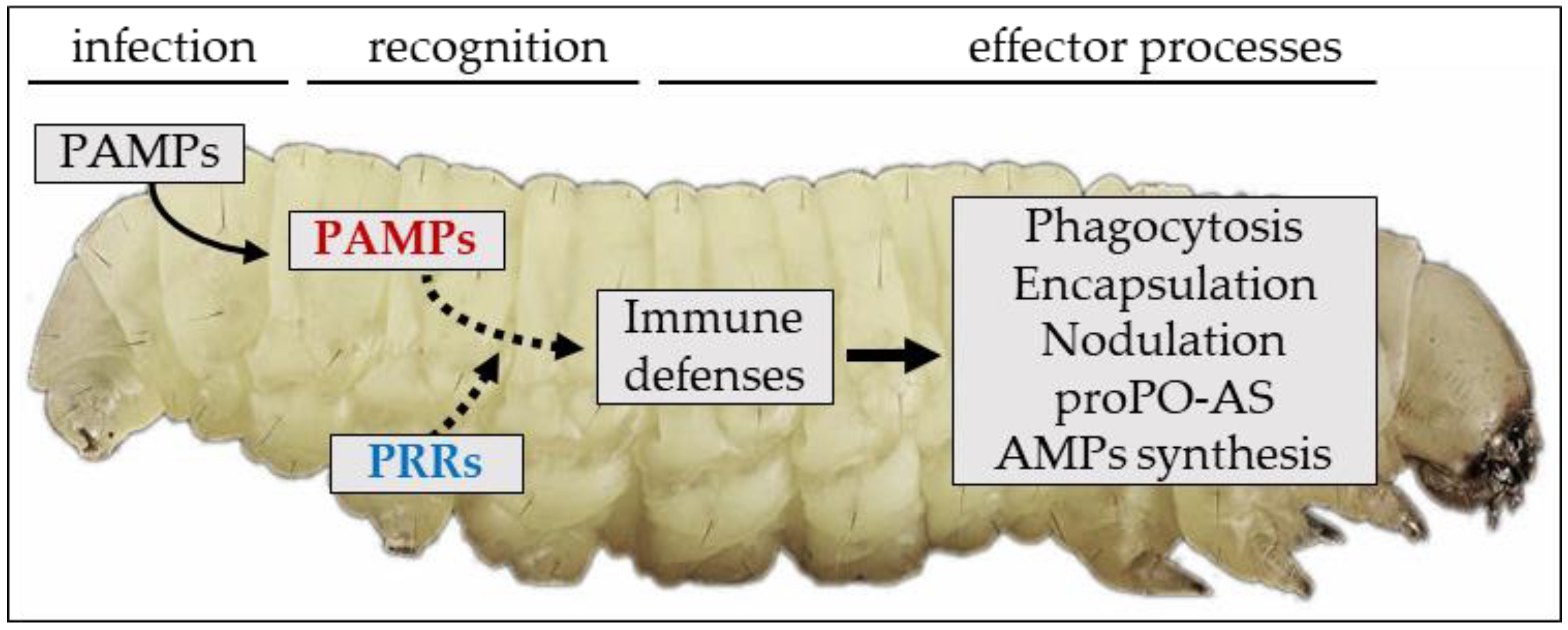
2. An Overview of the Insect Immune System: Sensing and Recognition of Non-Self
2.1. Humoral Immune Recognition
2.2. Cellular Immune Recognition
2.3. Effector Processes
2.4. Humoral Defence
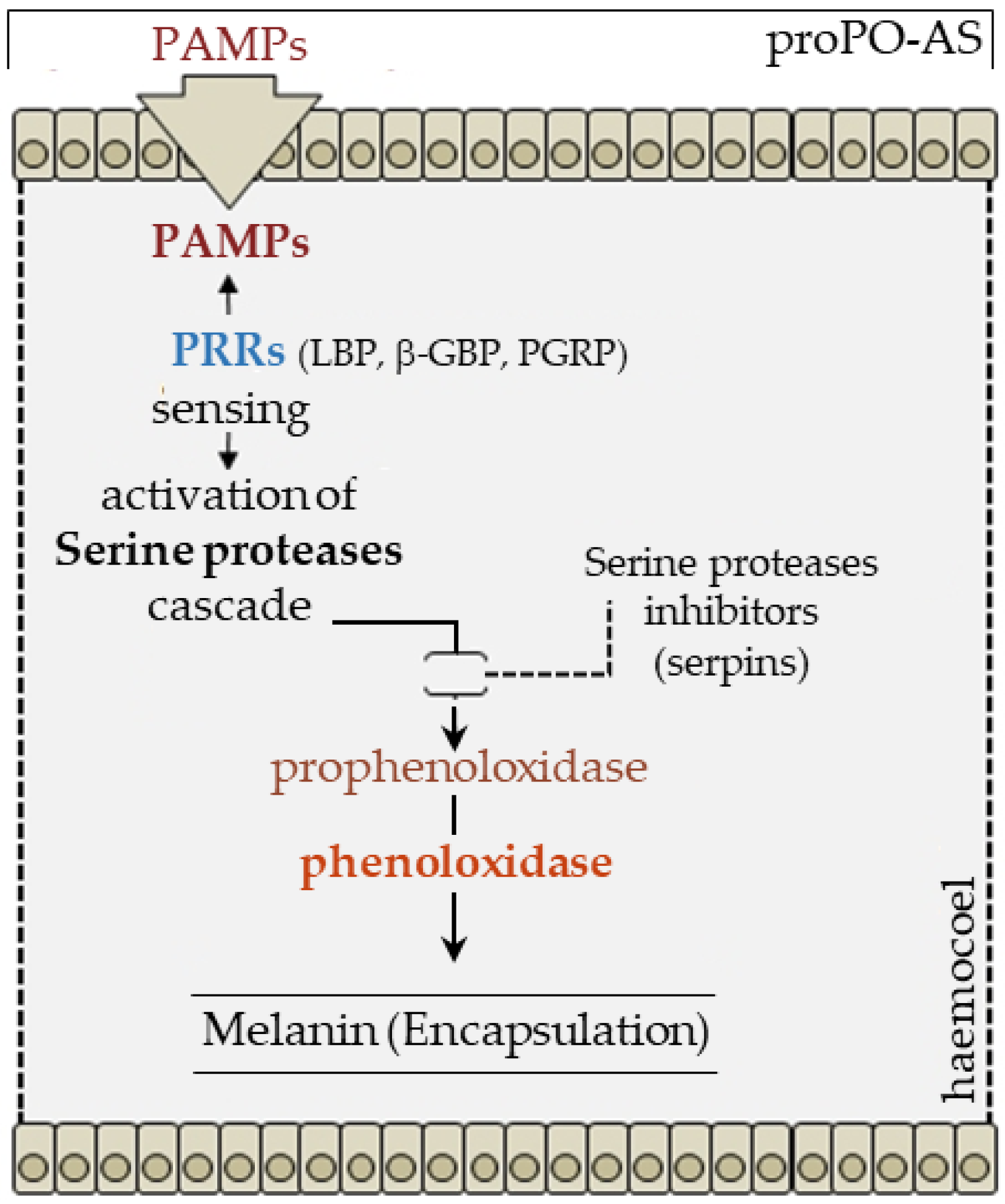
2.5. Melanization
2.6. Antimicrobial Peptides and Bacterial Clearance
2.7. Cellular Defenses
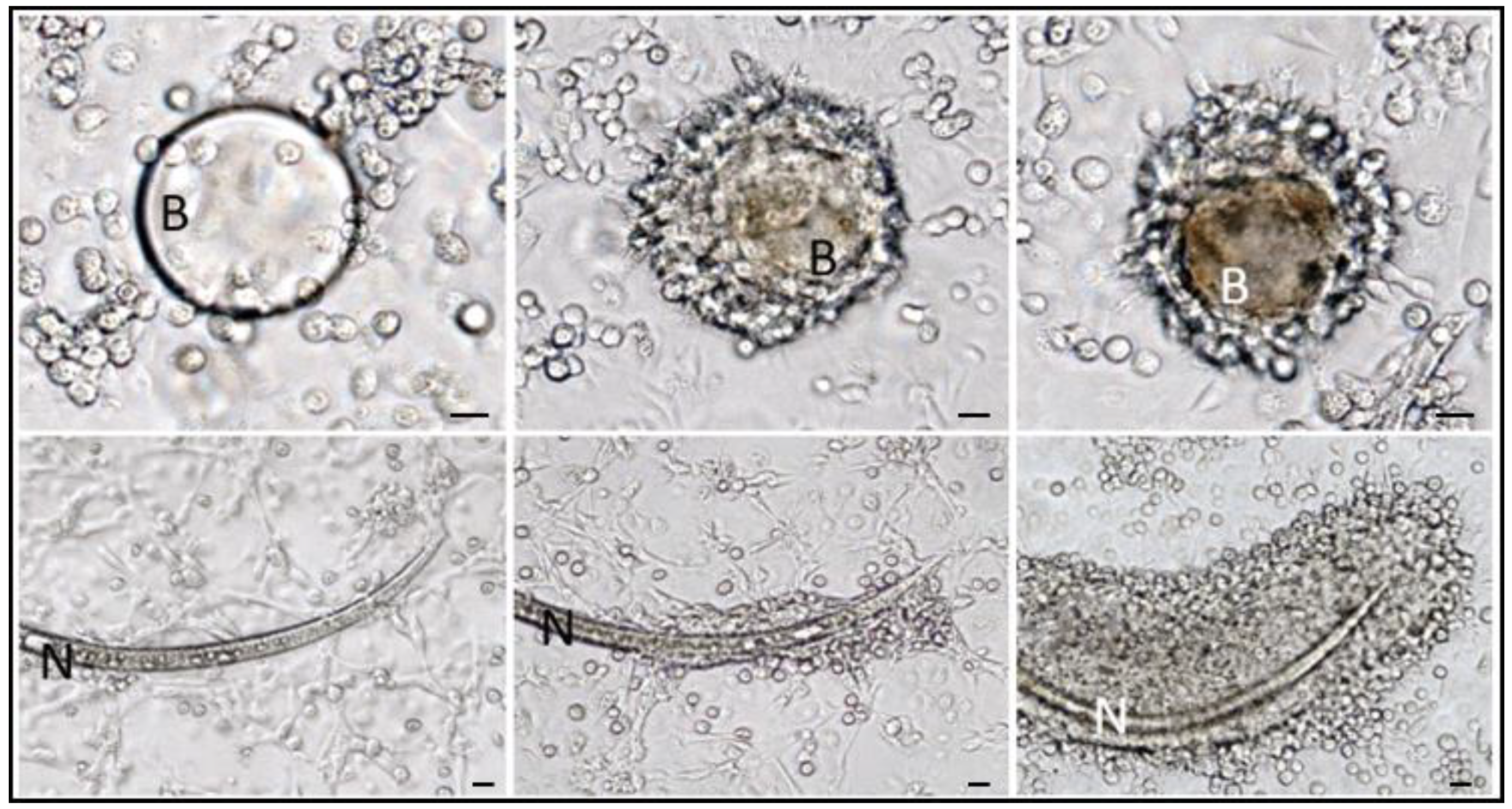
2.8. Phagocytosis
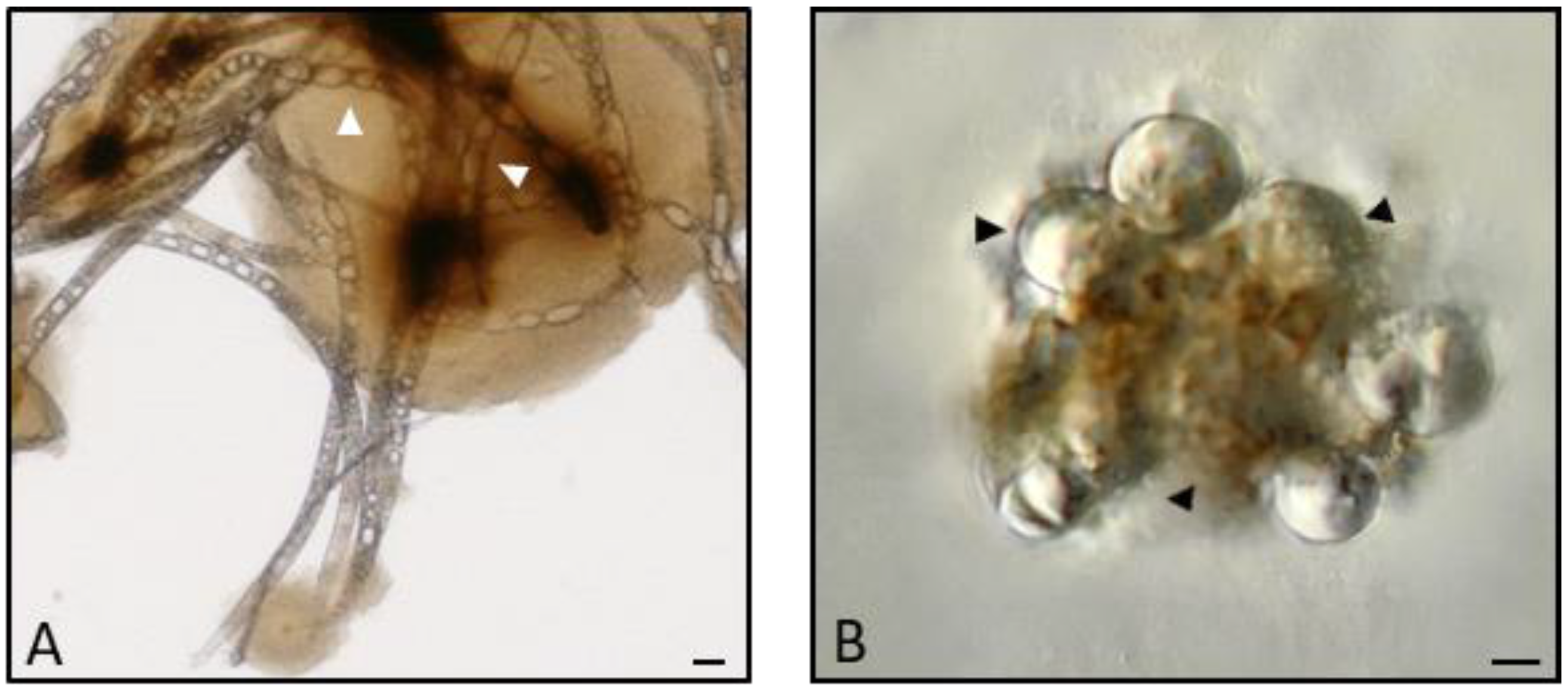
2.9. Encapsulation
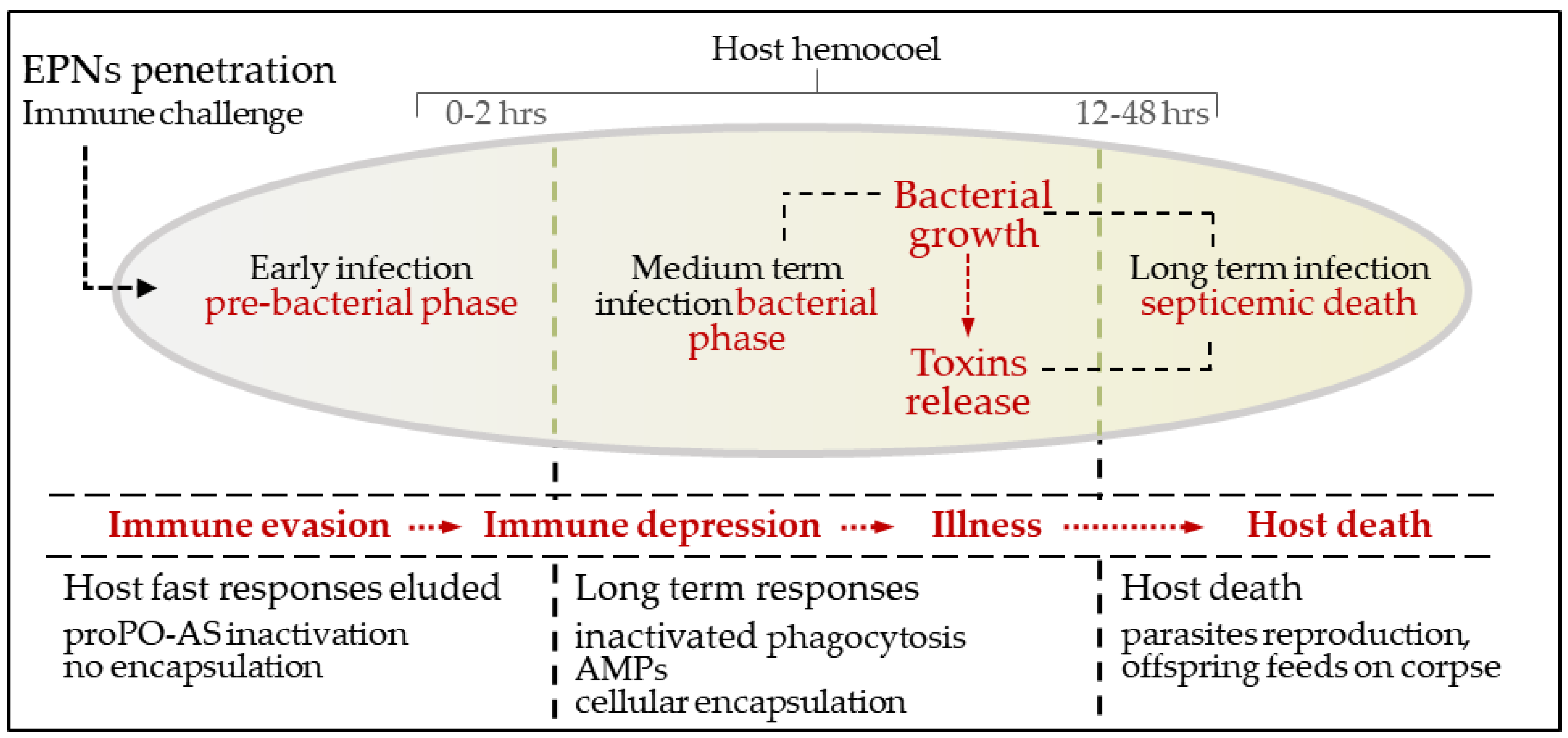
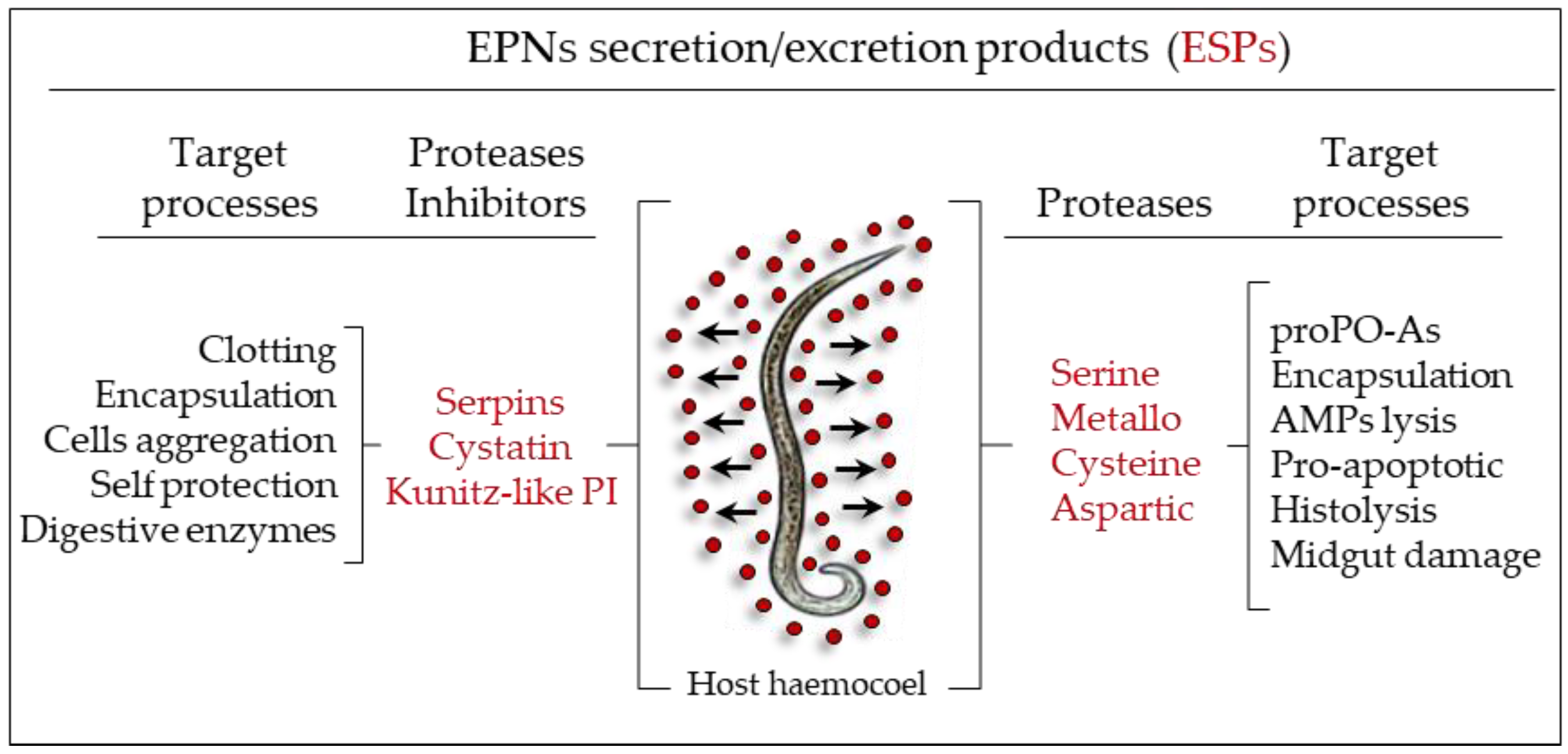
3. An Overview of Parasites’ Strategies
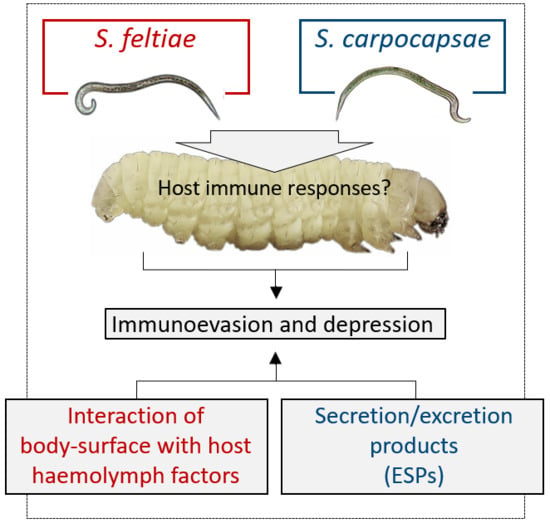
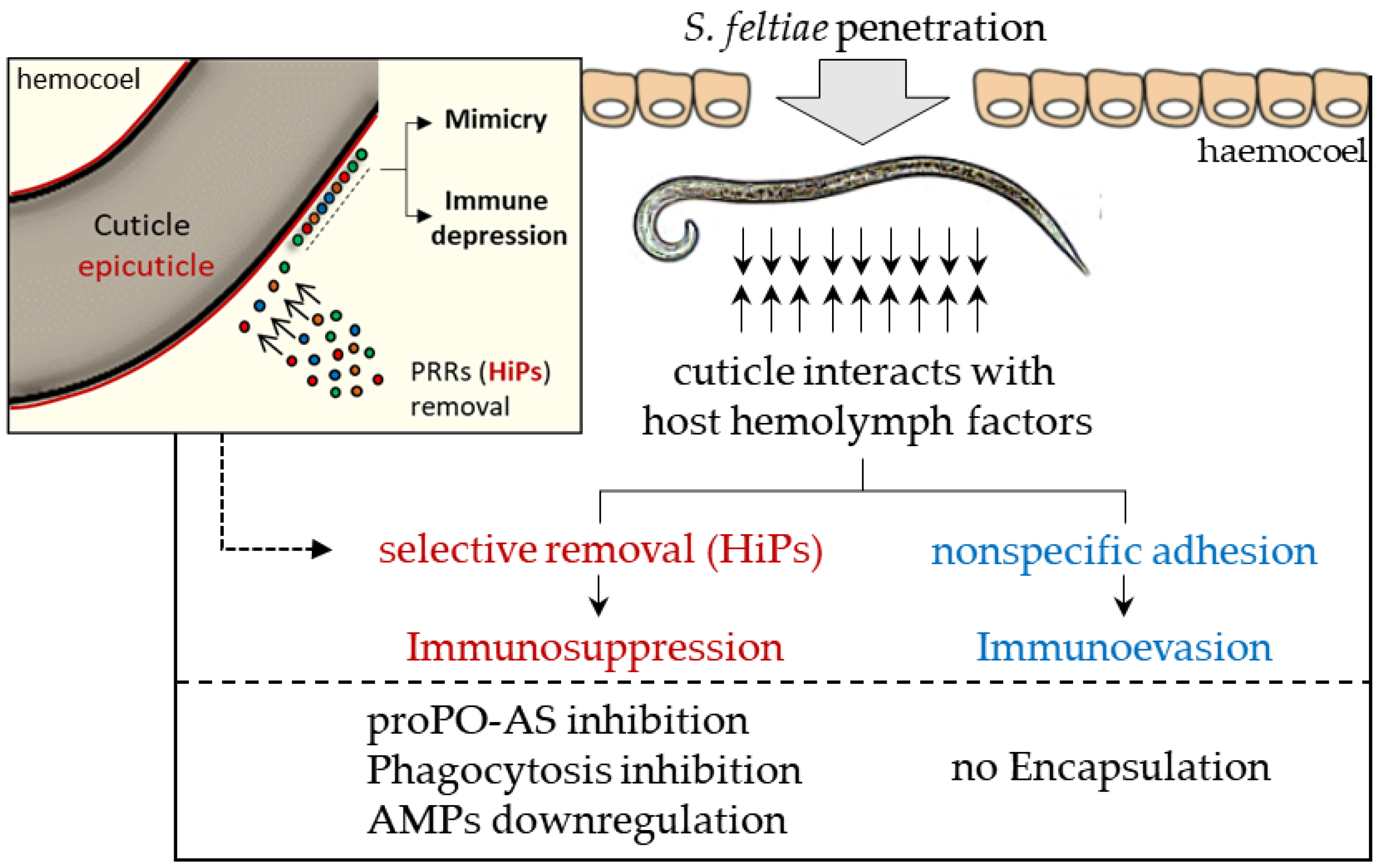
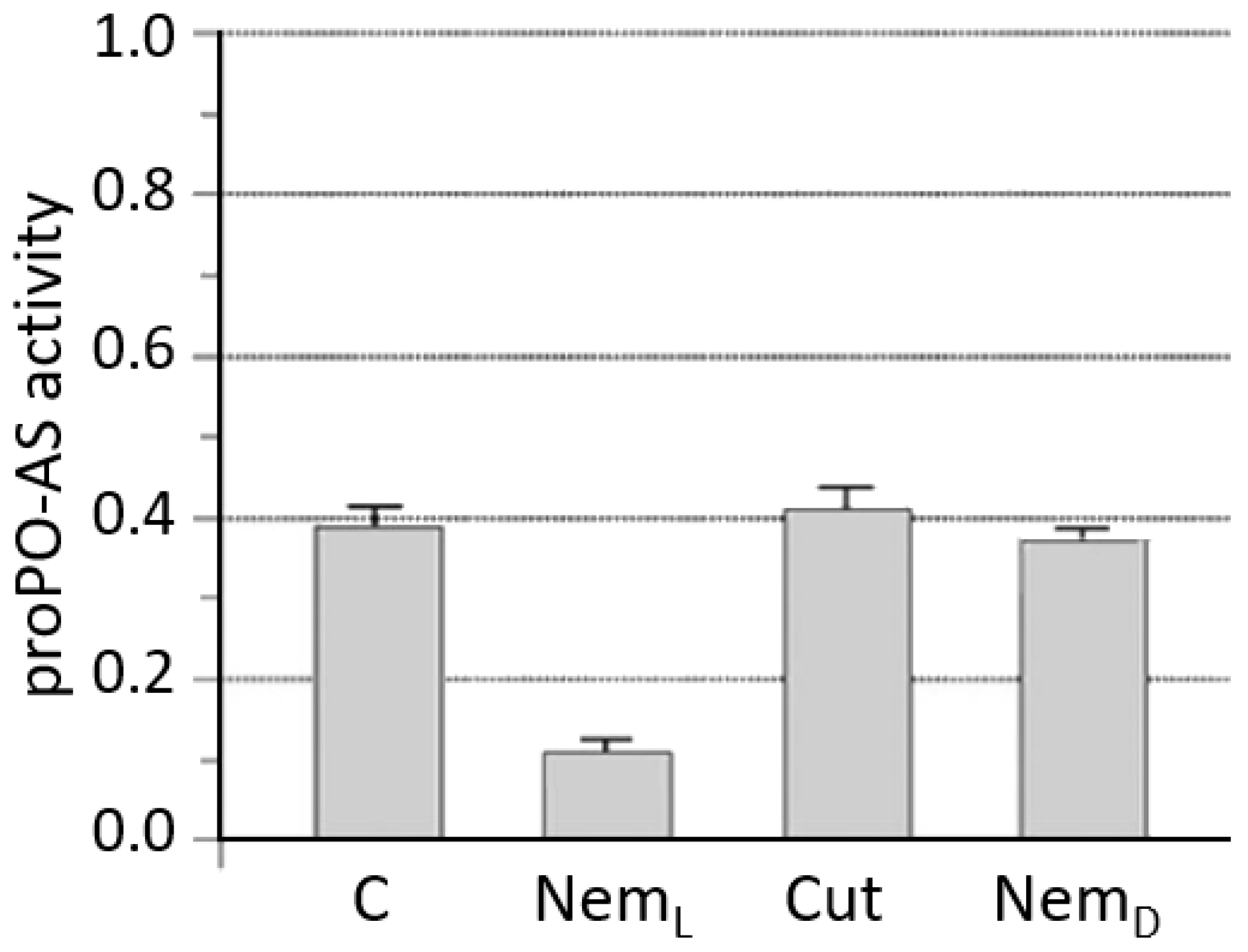
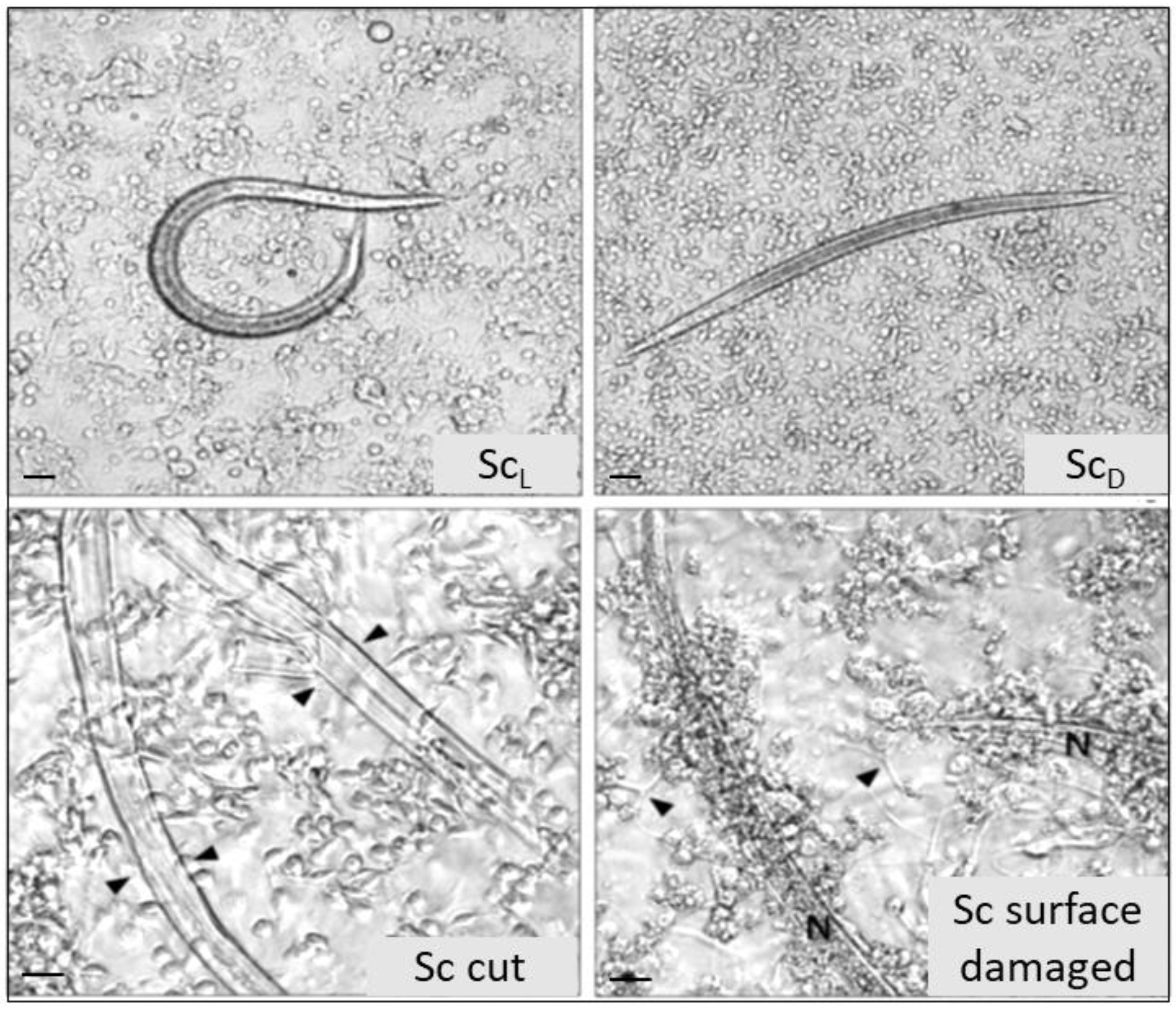
3.1. S. feltiae: Host Immunomodulation
3.2. S. carpocapsae: Host Immunomodulation
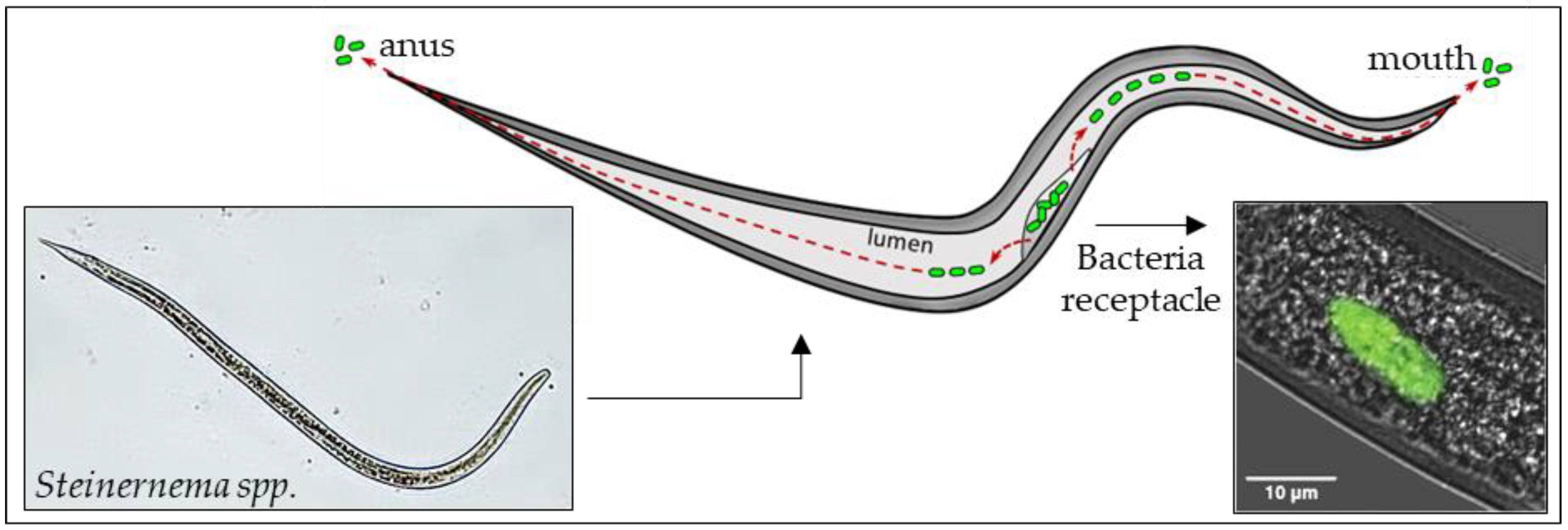
4. The Role of Bacterial Symbionts
5. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Blaxter, M.L. Nematoda: Genes, genomes and the evolution of parasitism. Adv. Parasitol. 2003, 54, 101–195. [Google Scholar] [PubMed]
- Blaxter, M.; De Ley, P.; Garey, J.; Liu, L.; Scheldeman, P.; Vierstraete, A.; Vanfleteren, J.; Mackey, L.; Dorris, M.; Frisse, L. A molecular evolutionary framework for the phylum Nematoda. Nature 1998, 392, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Schmid-Hempel, P. Parasite immune evasion: A momentous molecular war. Trends Ecol. Evol. 2008, 23, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Gaugler, R.; Kaya, H.K. (Eds.) Entomopathogenic Nematodes in Biological Control; CRC Press: Boca Raton, FL, USA, 1990. [Google Scholar]
- Georgis, R.; Manweiler, S.A. Entomopathogenic nematodes: A developing biological control technology. Agric. Zool. Rev. 1994, 6, 63–94. [Google Scholar]
- Poinar, G. Nematode biopesticides Fundam. Appl. Nematol. 1998, 21, 733–737. [Google Scholar]
- Poinar, G.O., Jr. Origins and phylogenetic relationships of the entomophilic rhabditids Heterorhabditis and Steinernema. Fundam. Appl. Nematol. 1993, 16, 333–338. [Google Scholar]
- Peters, A. The Natural Host Range of Steinernema and Heterorhabditis spp. and Their Impact on Insect Populations. Biocontrol. Sci. Technol. 1996, 6, 389–402. [Google Scholar] [CrossRef]
- Campbell, J.F.; Lewis, E.E.; Stock, S.P.; Nadler, S.; Kaya, H.K. Evolution of host search strategies in entomopathogenic nematodes. J. Nematol. 2003, 35, 142–145. [Google Scholar] [PubMed]
- Baiocchi, T.; Lee, G.; Choe, D.H.; Dillman, A.R. Host seeking parasitic nematodes use specific odors to assess host resources. Sci. Rep. 2017, 7, 6270. [Google Scholar] [CrossRef] [PubMed]
- Stock, S.P.; Chaerani, G.; Rani, T. Morphological and molecular characterisation of Steinernema hermaphroditumn. sp. (Nematoda: Steinernematidae), an entomopathogenic nematode from Indonesia, and its phylogenetic relationships with other members of the genus. Nematology 2004, 6, 401–412. [Google Scholar] [CrossRef]
- Chaudhuri, J.; Kache, V.; Pires-daSilva, A. Regulation of sexual plasticity in a nematode that produces males, females, and hermaphrodites. Curr. Biol. 2011, 21, 1548–1551. [Google Scholar] [CrossRef] [PubMed]
- Poinar, G.O., Jr. Nematodes for Biological Control of Insects; CRC Press: Boca Raton, FL, USA, 1979. [Google Scholar]
- Kaya, H.K.; Gaugler, R. Entomopathogenic nematodes. Annu. Rev. Entomol. 1993, 38, 181–206. [Google Scholar] [CrossRef]
- Forst, S.; Nealson, K. Molecular biology of the symbiotic-pathogenic bacteria Xenorhabdus spp. & Photorhabdus spp. Microbiol. Rev. 1996, 60, 21–43. [Google Scholar] [PubMed]
- Forst, S.; Dowds, B.; Boemare, N.; Stackebrandt, E. Xenorhabdus and Photorhabdus spp.: Bugs that kill bugs. Annu. Rev. Microbiol. 1997, 51, 47–72. [Google Scholar] [CrossRef] [PubMed]
- Forst, S.; Clarke, D. Bacteria-nematode symbiosis. In Entomopathogenic Nematology; Gaugler, R., Ed.; CAB International: London, UK, 2001; pp. 57–77. [Google Scholar]
- Silva, C.P.; Waterfield, N.R.; Daborn, P.J.; Dean, P.; Chilver, T.; Au, C.P.; Sharma, S.; Potter, U.; Reynolds, S.E.; Ffrench-Constant, R.H. Bacterial infection of a model insect: Photorhabdus luminescens and Manduca sexta. Cell. Microbiol. 2002, 4, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Poinar, G.O.; Grewal, P.S. History of entomopathogenic nematology. J. Nematol. 2012, 44, 153–161. [Google Scholar] [PubMed]
- Babic, I.; Fischer-Saux, M.; Giraud, E.; Boemare, N. Occurrence of natural dixenic associations between the symbiont Photorhabdus luminescens and bacteria related to Ochrobactrum spp. in tropical entomopathogenic Heterorhabditis spp. (Nematoda, Rhabditida). Microbiology 2000, 146, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Lysenko, O.B.; Weiser, J. Bacteria associated with the nematode Neoplectana carpocapsae and the pathogenicity of this complex for Galleria mellonella larvae. J. Invertebr. Pathol. 1974, 24, 332–336. [Google Scholar] [CrossRef]
- Enright, M.R.; Griffin, C.T. Specificity of association between Paenibacillus spp. and the entomopathogenic nematodes, Heterorhabditis spp. Microb. Ecol. 2004, 48, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, K.; Seo, J.; Shrestha, S.; Kim, H.H.; Nalini, M.; Yi, Y. Identification of an entomopathogenic bacterium, Serratia sp. ANU101, and its hemolytic activity. J. Microbiol. Biotechnol. 2009, 19, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Dillman, A.R.; Chaston, J.M.; Adams, B.J.; Ciche, T.A.; Goodrich-Blair, H.; Stock, S.P.; Sternberg, P.W. An entomopathogenic nematode by any other name. PLoS Pathog. 2012, 8, e1002527. [Google Scholar] [CrossRef] [PubMed]
- Mandel, M.J. Models and approaches to dissect host–symbiont specificity. Trends Microbiol. 2010, 18, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Dunphy, G.; Thurston, G. Insect immunity. In Entomopathogenic Nematodes in Biological Control; Gaugler, R., Kaya, H.K., Eds.; CRC Press: Boca Raton, FL, USA, 1990; pp. 301–326. [Google Scholar]
- Eleftherianos, I.; Yadav, S.; Kenney, E.; Cooper, D.; Ozakman, Y.; Patrnogic, J. Role of Endosymbionts in Insect-Parasitic Nematode Interactions. Trends Parasitol. 2018, 34, 430–444. [Google Scholar] [CrossRef] [PubMed]
- Schmid-Hempel, P. Immune defence, parasite evasion strategies and their relevance for macroscopic phenomena such as virulence. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Ligoxygakis, P. Advances in Insect Physiology, Insect Immunity; Academic Press: Cambridge, MA, USA, 2017; Volume 52, ISBN 9780128117750. [Google Scholar]
- Hoffmann, J.A.; Reichhart, J.M.; Hetru, C. Innate immunity in higher insects. Curr. Opin. Immunol. 1996, 8, 8–13. [Google Scholar] [CrossRef]
- Chambers, M.C.; Schneider, D.S. Pioneering immunology: Insect style. Curr. Opin. Immunol. 2012, 24, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Loker, E.S. On being a parasite in an invertebrate host: A short survival course. J. Parasitol. 1994, 80, 728–747. [Google Scholar] [CrossRef] [PubMed]
- Schmid-Hempel, P. Natural insect host-parasite systems show immune priming and specificity: Puzzles to be solved. Bioessays 2005, 27, 1026–1034. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 2001, 1, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R.; Janeway, C.A., Jr. Decoding the patterns of self and nonself by the innate immune system. Science 2002, 296, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Kanost, M.R.; Jiang, H.; Qiang, Y.X. Innate immune responses of a lepidopteran insect, Manduca sexta. Immunol. Rev. 2004, 198, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Vilcinskas, A.; Kanost, M.R. Immunity in lepidopteran insects. Adv. Exp. Med. Biol. 2010, 708, 181–204. [Google Scholar] [PubMed]
- Janeway, C.A.; Medzhitov, R. Innate Immune Recognition. Annu. Rev. Immunol. 2002, 20, 197–216. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef] [PubMed]
- Raetz, C.R.; Whitfield, C. Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 2002, 71, 635–700. [Google Scholar] [CrossRef] [PubMed]
- Trent, M.S.; Stead, C.M.; Tran, A.X.; Hankins, J.V. Diversity of endotoxin and its impact on pathogenesis. J. Endotoxin Res. 2006, 12, 205–223. [Google Scholar] [CrossRef] [PubMed]
- Johannsen, L. Biological properties of bacterial peptidoglycan. APMIS 1993, 101, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Goodridge, H.S.; Wolf, A.J.; Underhill, D.M. Beta-glucan recognition by the innate immune system. Immunol. Rev. 2009, 230, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Morath, S.; von Aulock, S.; Hartung, T. Structure/function relationships of lipoteichoic acids. J. Endotoxin Res. 2005, 11, 348–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samatey, F.A.; Imada, K.; Nagashima, S.; Vonderviszt, F.; Kumasaka, T.; Yamamoto, M.; Namba, K. Structure of the bacterial flagellar protofilament and implications for a switch for supercoiling. Nature 2001, 410, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Akira, S. Innate immunity to virus infection. Immunol. Rev. 2009, 227, 75–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krieg, A.M. CpG motifs in bacterial DNA and their immune effects. Annu. Rev. Immunol. 2002, 20, 709–760. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, J.A.; Kafatos, F.C.; Janeway, C.A.; Ezekowitz, R.A. Phylogenetic perspectives in innate immunity. Science 1999, 284, 1313–1318. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, G. Insect immunity and its implication in mosquito-malaria interactions. Cell. Microbiol. 2003, 5, 3–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stokes, B.A.; Yadav, S.; Shokal, U.; Smith, L.C.; Eleftherianos, I. Bacterial and fungal pattern recognition receptors in homologous innate signaling pathways of insects and mammals. Front. Microbiol. 2015, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.K.; Kinoshita, K.; Ashida, M. Purification of a peptidoglycan recognition protein from hemolymph of the silkworm, Bombyx mori. J. Biol. Chem. 1996, 271, 13854–13860. [Google Scholar] [CrossRef] [PubMed]
- Royet, J.; Dziarski, R. Peptidoglycan recognition proteins: Pleiotropic sensors and effectors of antimicrobial defences. Nat. Rev. Microbiol. 2007, 5, 264–277. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.Q.; Zhu, Y.; Ma, C.; Fabrick, J.A.; Kanost, M.R. Pattern recognition proteins in Manduca sexta plasma. Insect Biochem. Mol. Biol. 2002, 32, 1287–1293. [Google Scholar] [CrossRef]
- Zhu, Y.; Johnson, T.J.; Myers, A.A.; Kanost, M.R. Identification by subtractive suppression hybridization of bacteria-induced genes expressed in Manduca sexta fat body. Insect Biochem. Mol. Biol. 2003, 33, 541–559. [Google Scholar] [CrossRef]
- Ma, C.; Kanost, M.R. A β1,3-glucan recognition protein from an insect, Manduca sexta, agglutinates microorganisms and activates the phenoloxidase cascade. J. Biol. Chem. 2000, 275, 7505–7514. [Google Scholar] [CrossRef] [PubMed]
- Weis, W.I.; Taylor, M.E.; Drickamer, K. The C-type lectin superfamily in the immune system. Immunol. Rev. 1998, 163, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Vasta, G.R.; Quesenberry, M.; Ahmed, H.; O’Leary, N. C-type lectins and galectins mediate innate and adaptive immune functions: Their roles in the complement activation pathway. Dev. Comp. Immunol. 1999, 23, 401–420. [Google Scholar] [CrossRef]
- Yu, X.Q.; Kanost, M.R. Manduca sexta lipopolysaccharide-specific immulectin-2 protects larvae from bacterial infection. Dev. Comp. Immunol. 2003, 27, 189–196. [Google Scholar] [CrossRef]
- Yu, X.Q.; Kanost, M.R. Immulectin-2, a pattern recognition receptor that stimulates hemocytes encapsulation and melanization in the tobacco hornworm, Manduca sexta. Dev. Comp. Immunol. 2004, 28, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Dunphy, G.; Halwani, A. Haemolymph proteins of larvae of Galleria mellonella detoxify endotoxins of the insect pathogenic bacteria Xenorhabdus nematophilus (Enterobacteriaceae). J. Insect Physiol. 1997, 43, 1023–1029. [Google Scholar] [CrossRef]
- Wiesner, A.; Losen, S.; Kopacek, P.; Weise, C.; Götz, P. Isolated apolipophorin III from Galleria mellonella stimulates the immune reactions of the insect. J. Insect Physiol. 1997, 43, 383–391. [Google Scholar] [CrossRef]
- Niere, M.; Meisslitzer, C.; Dettloff, M.; Weise, C.; Ziegler, M.; Wiesner, A. Insect immune activation by recombinant Galleria mellonella apolipophorin III. Biochem. Biophys. Acta 1999, 1433, 16–26. [Google Scholar] [CrossRef]
- Zdybicka-Barabas, A.; Januszanis, B.; Mak, P.; Cytryńska, M. An atomic force microscopy study of Galleria mellonella apolipophorin III effect on bacteria. Biochim. Biophys. Acta 2011, 1808, 1896–1906. [Google Scholar] [CrossRef] [PubMed]
- Zdybicka-Barabas, A.; Mak, P.; Klys, A.; Skrzypiec, K.; Mendyk, E.; Fiołka, M.; Cytryńska, M. Synergistic action of Galleria mellonella anionic peptide 2 and lysozyme against Gram-negative bacteria. Biochim. Biophys. Acta 2012, 1818, 2623–2635. [Google Scholar] [CrossRef] [PubMed]
- Marmaras, V.J.; Lampropoulou, M. Regulators and signalling in insect haemocyte immunity. Cell. Signal. 2009, 21, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Hultmark, D. Drosophila immunity: Paths and patterns. Curr. Opin. Immunol. 2003, 15, 12–19. [Google Scholar] [CrossRef]
- Schmidt, O.; Theopold, U.; Strand, M.R. Innate immunity and its evasion and suppression by hymenopteran endoparasitoids. Bioessays 2001, 23, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Dubovskiy, I.M.; Kryukova, N.A.; Glupov, V.V.; Ratcliffe, N.A. Encapsulation and nodulation in insects. Invertebr. Surv. J. 2016, 13, 229–246. [Google Scholar]
- Kocks, C.; Cho, J.H.; Nehme, N.; Ulvila, J.; Pearson, A.M.; Meister, M.; Strom, C.; Conto, S.L.; Hetru, C.; Stuart, L.M.; et al. Eater, a transmembrane protein mediating phagocytosis of bacterial pathogens in Drosophila. Cell 2005, 23, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, J.A. The immune response of Drosophila. Nature 2003, 426, 33–38. [Google Scholar] [CrossRef] [PubMed]
- De Gregorio, E.; Spellman, P.T.; Tzou, P.; Rubin, G.M.; Lemaitre, B. The Toll and Imd pathways are the major regulators of the immune response in Drosophila. EMBO J. 2002, 21, 2568–2579. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, J.A.; Reichhart, J.M. Drosophila innate immunity: An evolutionary perspective. Nat. Immunol. 2002, 3, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Leulier, F.; Parquet, C.; Pili-Floury, S.; Ryu, J.H.; Caroff, M.; Lee, W.J.; Mengin-Lecreulx, D.; Lemaitre, B. The Drosophila immune system detects bacteria through specific peptidoglycan recognition. Nat. Immunol. 2003, 4, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Kurata, S. Extracellular and intracellular pathogen recognition by Drosophila PGRP-LE and PGRP-LC. Int. Immunol. 2010, 22, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Georgel, P.; Naitza, S.; Kappler, C.; Ferrandon, D.; Zachary, D.; Swimmer, C.; Kopczynski, C.; Duyk, G.; Reichhart, J.M.; Hoffmann, J.A. Drosophila immune deficiency (IMD) is a death domain protein that activates antibacterial defense and can promote apoptosis. Dev. Cell 2001, 4, 503–514. [Google Scholar] [CrossRef]
- Dziarski, R. Peptidoglycan recognition proteins (PGRPs). Mol. Immunol. 2004, 40, 877–886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choe, K.M.; Lee, H.; Anderson, K.V. Drosophila peptidoglycan recognition protein LC (PGRP-LC) acts as a signal-transducing innate immune receptor. Proc. Natl. Acad. Sci. USA 2005, 102, 1122–1126. [Google Scholar] [CrossRef] [PubMed]
- Tzou, P.; De Gregorio, E.; Lemaitre, B. How Drosophila combats microbial infection: A model to study innate immunity and host-pathogen interactions. Curr. Opin. Microbiol. 2002, 5, 102–110. [Google Scholar] [CrossRef]
- Tanji, T.; Ip, Y.T. Regulators of the Toll and Imd pathways in the Drosophila innate immune response. Trends Immunol. 2005, 26, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Tanji, T.; Hu, X.; Weber, A.N.; Ip, Y.T. Toll and IMD pathways synergistically activate an innate immune response in Drosophila melanogaster. Mol. Cell. Biol. 2007, 27, 4578–4588. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, K.; Silverman, N. Positive and negative regulation of the Drosophila immune response. BMB Rep. 2008, 41, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.C.; Åsling, B.; Faye, I. Organization and expression of the immunoresponsive lysozyme gene in a giant silk moth, Hyalophora cecropia. J. Biol. Chem. 1991, 266, 6644–6649. [Google Scholar] [PubMed]
- Hetru, C.; Hoffmann, J.A. NF-kappaB in the immune response of Drosophila. Cold Spring Harb. Perspect. Biol. 2009, 1, a000232. [Google Scholar] [CrossRef] [PubMed]
- Cherry, S.; Silverman, N. Host-pathogen interactions in Drosophila: New tricks from an old friend. Nat. Immunol. 2006, 7, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Vey, A. Humoral Encapsulation. In Insect Immunity; Pathak, J.P.N., Ed.; Series Entomologica; Springer: Dordrecht, The Netherlands, 1993; p. 48. [Google Scholar] [CrossRef]
- Brivio’s Lab; Laboratory of Comparative Immunology and Parasitology, Department of Theoretical and Applied Sciences, University of Insubria, Varese, Italy. Personal communication, 2018.
- Cerenius, L.; Lee, B.L.; Söderhäll, K. The proPO-system: Pros and cons for its role in invertebrate immunity. Trends Immunol. 2008, 29, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Nappi, A.J.; Kohler, L.; Mastore, M. Signaling pathways implicated in the cellular innate immune responses of Drosophila. Invertebr. Surv. J. 2004, 1, 5–33. [Google Scholar]
- Ashida, M. The prophenoloxidase cascade in insect immunity. Res. Immunol. 1990, 90, 908–910. [Google Scholar] [CrossRef]
- Brivio, M.F.; Mazzei, C.; Scarì, G. proPO System of Allogamus auricollis (Insecta): Effects of Various Compounds on Phenoloxidase Activity. Comp. Biochem. Physiol. B 1996, 113, 281–287. [Google Scholar] [CrossRef]
- Dimopoulous, G.; Müller, H.M.; Levashina, E.A.; Kafatos, F.C. Innate immune defense against malaria infection in the mosquito. Curr. Opin. Immunol. 2001, 13, 79–88. [Google Scholar] [CrossRef]
- Jomori, T.; Natori, S. Function of the lipopolysaccharide-binding protein of Periplaneta americana as an opsonin. FEBS Lett. 1992, 296, 283–286. [Google Scholar] [CrossRef]
- Söderhäll, K. Invertebrate immunity. Dev. Comp. Immunol. 1999, 23, 263–266. [Google Scholar] [PubMed]
- Bulet, P.; Stöcklin, R. Insect antimicrobial peptides: Structures, properties and gene regulation. Protein Pept. Lett. 2005, 12, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Hull, R.; Katete, R.; Ntwasa, M. Therapeutic potential of antimicrobial peptides from insects. Biotechnol. Mol. Biol. Rev. 2012, 7, 31–47. [Google Scholar]
- Yeung, A.T.; Gellatly, S.L.; HancocK, R.E. Multifunctional cationic host defence peptides and their clinical applications. Cell. Mol. Life Sci. 2011, 68, 2161–2176. [Google Scholar] [CrossRef] [PubMed]
- Bulet, P.; Stöcklin, R.; Menin, L. Anti-microbial peptides: From invertebrates to vertebrates. Immunol. Rev. 2004, 198, 169–184. [Google Scholar] [CrossRef] [PubMed]
- Hultmark, D. Insect lysozymes. EXS 1996, 75, 87–102. [Google Scholar] [PubMed]
- Brivio, M.F.; Moro, M.; Mastore, M. Down-regulation of antibacterial peptide synthesis in an insect model induced by the body-surface of an entomoparasite (Steinernema feltiae). Dev. Comp. Immunol. 2006, 30, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Strand, M.R. The insect cellular immune response. Insect Sci. 2008, 15, 1–14. [Google Scholar] [CrossRef]
- Bulet, P.; Hetru, C.; Dimarcq, J.L.; Hoffmann, D. Antimicrobial peptides in insects; structure and function. Dev. Comp. Immunol. 1999, 23, 329–344. [Google Scholar] [CrossRef]
- Dubovskiy, I.M.; Grizanova, E.V.; Whitten, M.M.; Mukherjee, K.; Greig, C.; Alikina, T.; Kabilov, M.; Vilcinskas, A.; Glupov, V.V.; Butt, T.M. Immuno-physiological adaptations confer wax moth Galleria mellonella resistance to Bacillus thuringiensis. Virulence 2016, 7, 860–870. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.P.; Kerkut, G.A.; Gilbert, L.I. (Eds.) Comprehensive Insect Physiology, Biochemistry, and Pharmacology; Pergamon Press: Oxford, UK, 1985; Volume 3. [Google Scholar]
- Brehélin, M.; Zachary, D. Insect haemocytes: A new classification to rule out the controversy. In Immunity in Invertebrates; Brehélin, M., Ed.; Springer: Berlin, Germany, 1986; pp. 36–48. [Google Scholar]
- Vilmos, P.; Nagy, I.; Kurucz, E.; Hultmark, D.; Gateff, E.; Andó, I. A rapid rosetting method for separation of hemocyte sub-populations of Drosophila melanogaster. Dev. Comp. Immunol. 2004, 28, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Willott, E.; Trenczek, T.; Thrower, L.W.; Kanost, M.R. Immunochemical identification of insect hemocyte populations: Monoclonal antibodies distinguish four major hemocyte types in Manduca sexta. Eur. J. Cell Biol. 1994, 65, 417–423. [Google Scholar] [PubMed]
- Strand, M.R.; Johnson, J.A. Characterization of monoclonal antibodies to hemocytes of Pseudoplusia includens. J. Insect Physiol. 1996, 42, 21–31. [Google Scholar] [CrossRef]
- Gardiner, E.M.M.; Strand, M.R. Monoclonal antibodies bind distinct classes of hemocytes in the moth Pseudoplusia includens. J. Insect Physiol. 1999, 45, 113–126. [Google Scholar] [CrossRef]
- Lebestky, T.; Chang, T.; Hartenstein, V.; Banerjee, U. Specification of Drosophila hematopoietic lineage by conserved transcription factors. Science 2000, 288, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Wood, W.; Jacinto, A. Drosophila melanogaster embryonic haemocytes: Masters of multitasking. Nat. Rev. Mol. Cell Biol. 2007, 8, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Ratcliffe, N.A.; Rowley, A.F.; Fitzgerald, S.W. Invertebrate immunity: Basic concepts and recent advances. Int. Rev. Cytol. 1985, 97, 183–349. [Google Scholar]
- Tepass, U.; Fessler, L.I.; Aziz, A.; Hartenstein, V. Embryonic origin of hemocytes and their relationship to cell death in Drosophila. Development 1994, 120, 1829–1837. [Google Scholar] [PubMed]
- Lavine, M.D.; Strand, M.R. Insect hemocytes and their role in immunity. Insect Biochem. Mol. Biol. 2002, 32, 1295–1309. [Google Scholar] [CrossRef]
- Sass, M.; Kiss, A.; Locke, M. Integument and hemocyte peptides. J. Insect Physiol. 1994, 40, 407–421. [Google Scholar] [CrossRef]
- Stuart, L.M.; Ezekowitz, R.A. Phagocytosis and comparative innate immunity: Learning on the fly. Nat. Rev. Immunol. 2008, 8, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Tojo, S.; Naganuma, F.; Arakawa, K.; Yokoo, S. Involvement of both granular cells and plasmatocytes in phagocytic reactions in the greater wax moth, Galleria mellonella. J. Insect Physiol. 2000, 46, 1129–1135. [Google Scholar] [CrossRef]
- Rittig, M.G.; Kuhn, K.H.; Dechant, C.A.; Gauckler, A.; Modolell, M.; Ricciardi-Castagnoli, P.; Krause, A.; Burmester, G.R. Phagocytes from both vertebrate and invertebrate species use pooling-phagocytosis. Dev. Comp. Immunol. 1996, 20, 393–406. [Google Scholar] [CrossRef]
- Ni, Y.; Tizard, I. Lectin-carbohydrate interactions in the immune system. Vet. Immunol. Immunopathol. 1996, 55, 205–223. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, H. Prophenoloxidase (PPO) activation in Manduca sexta: An initial analysis of molecular interactions among PPO, PPO-activating proteinase-3 (PAP-3), and a cofactor. Insect Biochem. Mol. Biol. 2004, 34, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Marmaras, V.J.; Charalambidis, N.D. Certain hemocyte proteins of the Medfly, Ceratitis capitata, are responsible for nonself recognition and immobilization of Escherichia coii in vitro. Arch. Insect Biochem. Physiol. 1992, 21, 281–288. [Google Scholar] [CrossRef]
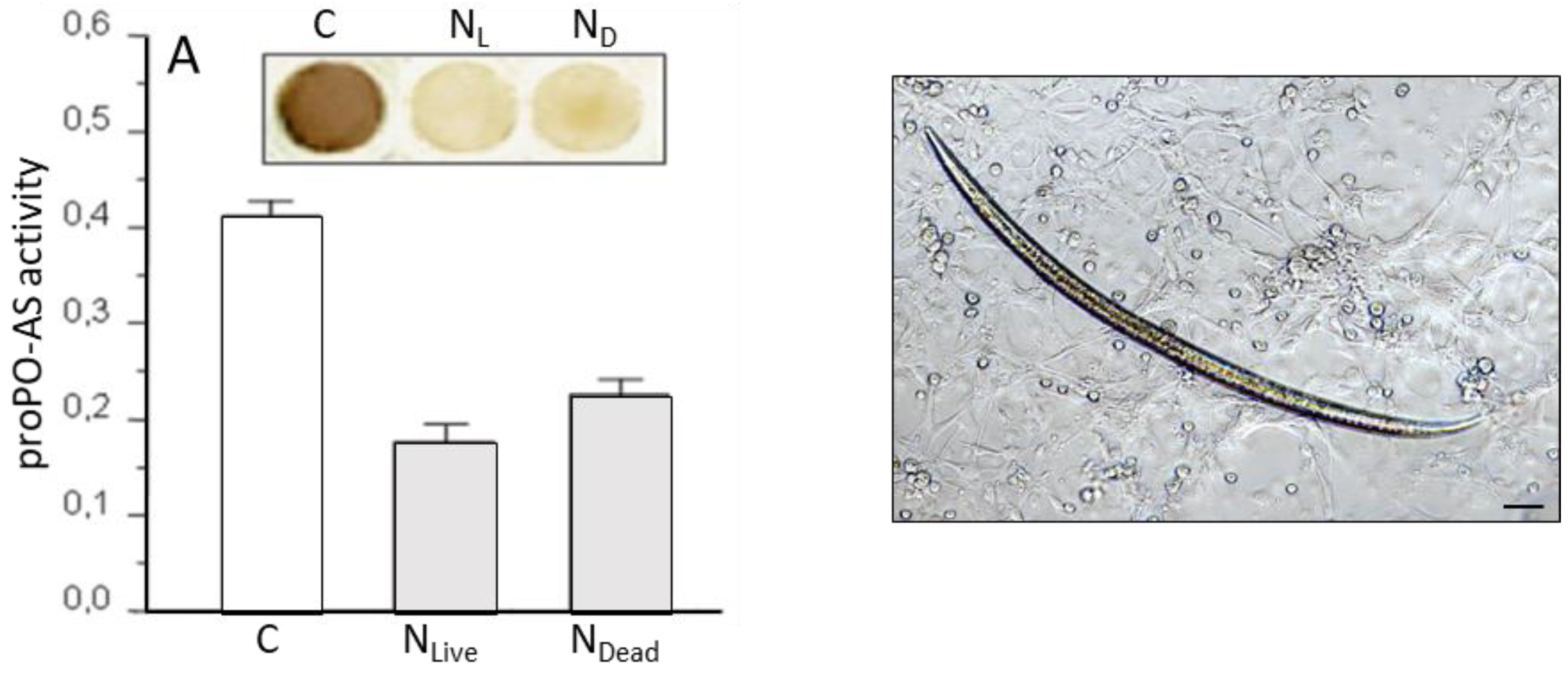
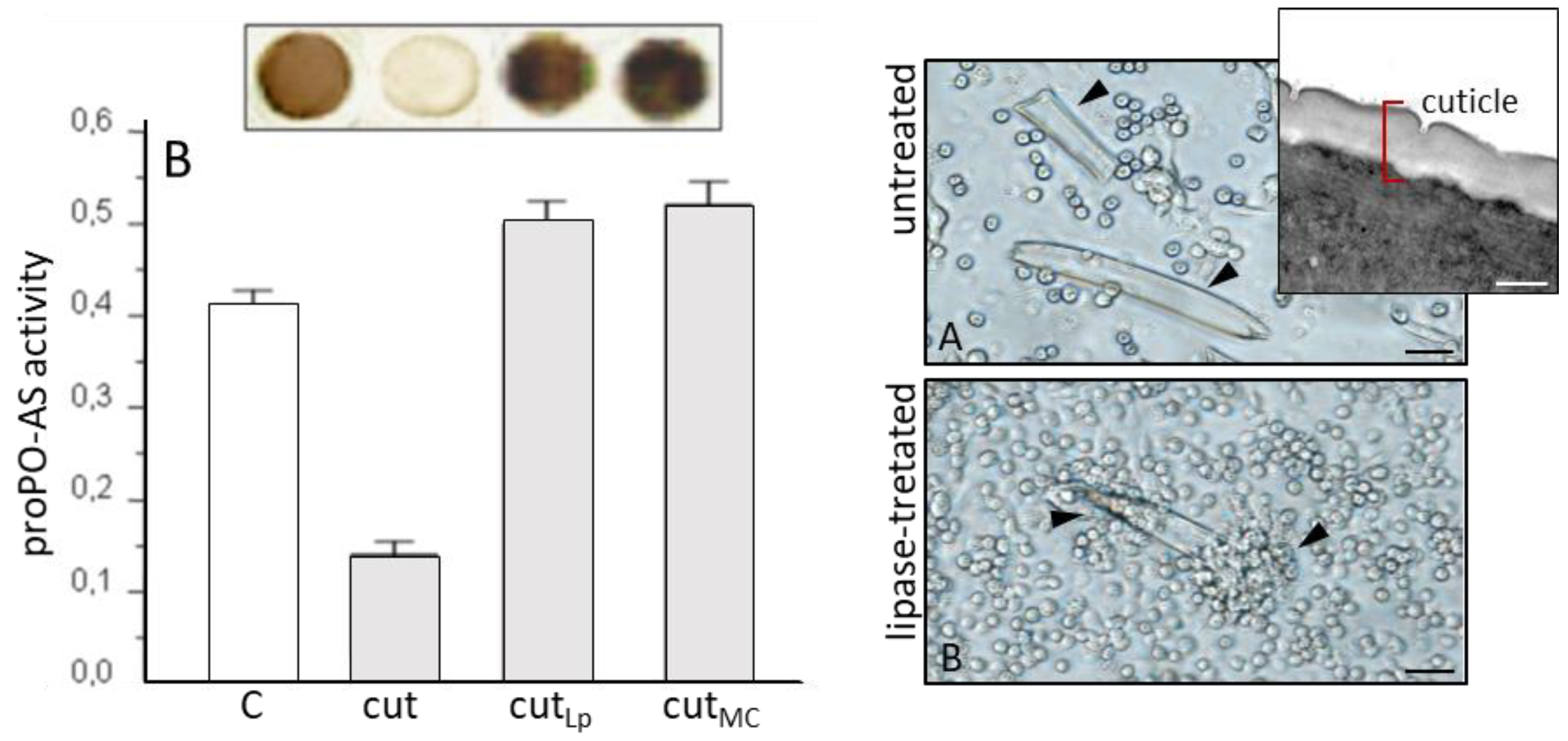
- Mastore, M.; Brivio, M.F. Cuticular surface lipids are responsible for disguise properties of an entomoparasite against host cellular responses. Dev. Comp. Immunol. 2008, 32, 1050–1062. [Google Scholar] [CrossRef] [PubMed]
- Clark, K.D.; Volkman, B.F.; Thoetkiattiku, H.; Hayakawa, Y.; Strand, M.R. N-terminal residues of plasmatocyte spreading peptide possess specific determinants required for biological activity. J. Biol. Chem. 2001, 276, 37431–37435. [Google Scholar] [CrossRef] [PubMed]
- Clark, K.D.; Volkman, B.F.; Thoetkiattikul, H.; King, D.; Hayakawa, Y.; Strand, M.R. Alanine-scanning Mutagenesis of Plasmatocyte Spreading Peptide Identifies Critical Residues for Biological Activity. J. Biol. Chem. 2001, 276, 18491–18496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srikanth, K.; Park, J.; Stanley, D.W.; Kim, Y. Plasmatocyte-spreading peptide influences hemocyte behavior via eicosanoids. Arch. Insect Biochem. Physiol. 2011, 78, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Salt, G. Teratocytes as a means of resistance to cellular defense reactions. Nature 1971, 232, 639. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, J.P.; Kanost, M.R.; Trenczek, T. Biological mediators of insect immunity. Annu. Rev. Entomol. 1997, 42, 611–614. [Google Scholar] [CrossRef] [PubMed]
- Vass, E.; Nappi, A.J. Developmental and immunological aspects of Drosophila-parasitoid relationships. J. Parasitol. 2000, 86, 1259–1270. [Google Scholar] [CrossRef] [PubMed]
- Nappi, A.J.; Vass, E. Cytotoxic reactions associated with insect immunity. Adv. Exp. Med. Biol. 2001, 484, 329–348. [Google Scholar] [PubMed]
- Griffin, C.T. Perspectives on the Behavior of Entomopathogenic Nematodes from Dispersal to Reproduction: Traits Contributing to Nematode Fitness and Biocontrol Efficacy. J. Nematol. 2012, 44, 177–184. [Google Scholar] [PubMed]
- Castillo, J.C.; Shokal, U.; Eleftherianos, I. A novel method for infecting Drosophila adult flies with insect pathogenic nematodes. Virulence 2012, 3, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Peña, J.M.; Carrillo, M.A.; Hallem, E.A. Variation in the susceptibility of Drosophila to different entomopathogenic nematodes. Infect. Immun. 2015, 83, 1130–1138. [Google Scholar] [CrossRef] [PubMed]
- Bayne, C.J.; Boswell, C.A.; Yui, M.A. Widespread antigenic cross-reactivity between plasma proteins of a gastropod, and its trematode parasite. Dev. Comp. Immunol. 1987, 11, 321–329. [Google Scholar] [CrossRef]
- Damian, R.T. Tropomyosin and molecular mimicry. Parasitol. Today 1991, 7, 96. [Google Scholar] [CrossRef]
- Weston, D.; Allen, B.; Thakur, A.; LoVerde, P.T.; Kemp, W.M. Invertebrate host-parasite relationships: Convergent evolution of a tropomyosin epitope between Schistosoma sp., Fasciola hepatica, and certain pulmonate snails. Exp. Parasitol. 1994, 78, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Strand, M.R.; Pech, L.L. Immunological basis for compatibility in parasitoid-host relationships. Annu. Rev. Entomol. 1995, 40, 31–56. [Google Scholar] [CrossRef] [PubMed]
- Kathirithamby, J.; Ross, L.D.; Johnston, J.S. Masquerading as self? Endoparasitic Strepsiptera (Insecta) enclose themselves in host-derived epidermal bag. Proc. Natl. Acad. Sci. USA 2003, 100, 7655–7659. [Google Scholar] [CrossRef] [PubMed]
- Simões, N.; Rosa, J.S. Pathogenicity and host specificity of entomopathogenic nematodes. Biocontrol Sci. Technol. 1996, 6, 403–411. [Google Scholar] [CrossRef]
- Balasubramanian, N.; Hao, Y.J.; Toubarro, D.; Nascimento, G.; Simões, N. Purification, biochemical and molecular analysis of a chymotrypsin protease with prophenoloxidase suppression activity from the entomopathogenic nematode Steinernema carpocapsae. Int. J. Parasitol. 2009, 39, 975–984. [Google Scholar] [CrossRef] [PubMed]
- Politz, S.M.; Philipp, M. Caenorhabditis elegans as a model for parasitic nematodes: A focus on the cuticle. Parasitol. Today 1992, 8, 6–12. [Google Scholar] [CrossRef]
- Akhurst, R.J.; Dunphy, G.B. Tripartite interactions between symbiotically associated entomopathogenic bacteria, nematodes, and their insect hosts. In Parasites and Pathogens of Insects; Academic Press: Cambridge, MA, USA, 1993; Volume 2, pp. 1–23. [Google Scholar]
- Blaxter, M.L.; Page, A.P.; Rudin, W.; Maizels, R.M. Nematode surface coats: actively evading immunity. Parasitol. Today 1992, 8, 243–247. [Google Scholar] [CrossRef]
- Cox, G.N.; Kusch, M.; DeNevi, K.; Edgar, R.S. Temporal regulation of cuticle synthesis during development of Caenorhabditis elegans. Dev. Biol. 1981, 84, 277–285. [Google Scholar] [CrossRef]
- Cox, G.N.; Kusch, M.; Edgar, R.S. Cuticle of Caenorhabditis elegans: Its isolation and partial characterisation. J. Cell Biol. 1981, 90, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Dunphy, G.; Webster, J. Partially characterized components of the epicuticle of dauer juvenile Steinernema feltiae and their influence on the hemocyte activity in Galleria mellonella. J. Parasitol. 1987, 73, 584–588. [Google Scholar] [CrossRef]
- Brivio, M.F.; Pagani, M.; Restelli, S. Immune suppression of Galleria mellonella (Insecta, Lepidoptera) humoral defenses induced by Steinernema feltiae (Nematoda, Rhabditida): Involvement of the parasite cuticle. Exp. Parasitol. 2002, 101, 149–156. [Google Scholar] [CrossRef]
- Brivio, M.F.; Mastore, M.; Moro, M. The role of Steinernema feltiae body-surface lipids in host–parasite immunological interactions. Mol. Biochem. Parasitol. 2004, 135, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Halwani, A.E.; Dunphy, G.B. Apolipophorin-III in Galleria mellonella potentiates hemolymph lytic activity. Dev. Comp. Immunol. 1999, 23, 563–570. [Google Scholar] [CrossRef]
- Finnerty, C.M.; Karplus, P.A.; Granados, R.R. The insect immune protein scolexin is a novel serine proteinase homolog. Protein Sci. 1999, 8, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Ryan, R.O.; Van der Horst, D.J. Lipid transport bio-chemistry and its role in energy production. Annu. Rev. Entomol. 2000, 45, 233–260. [Google Scholar] [CrossRef] [PubMed]
- Zakarian, R.J.; Dunphy, G.B.; Albert, P.J.; Rau, M.E. Apolipophorin-III affects the activity of the haemocytes of Galleria mellonella larvae. J. Insect Physiol. 2002, 48, 715–723. [Google Scholar] [CrossRef]
- Rahatkhah, Z.; Karimi, J.; Ghadamyari, M.; Brivio, M. Immune defences of Agriotes lineatus larvae against entomopathogenic nematodes. BioControl 2015, 60, 641–653. [Google Scholar] [CrossRef]
- Abdolmaleki, A.; Dastjerdi, H.R.; Tanha Maafi, Z.; Naseri, B. Cellular and humoral responses of Pieris brassicae to infection by Steinernema feltiae, its symbiont bacteria, and their metabolites. Nematology 2017, 19, 477–487. [Google Scholar] [CrossRef]
- Ebrahimi, L.; Shiri, M.; Dunphy, G.B. Effect of entomopathogenic nematode, Steinernema feltiae, on survival and plasma phenoloxidase activity of Helicoverpa armigera (Hb) (Lepidoptera: Noctuidae) in laboratory conditions. Egypt. J. Biol. Pest Control 2018, 28, 12. [Google Scholar] [CrossRef]
- Li, X.Y.; Cowles, R.S.; Cowles, E.A.; Gaugler, R.; Cox-Foster, D.L. Relationship between the successful infection by entomopathogenic nematodes and the host immune response. Int. J. Parasitol. 2007, 37, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, L.; Niknam, G.; Dunphy, G.B. Hemocyte responses of the Colorado potato beetle, Leptinotarsa decemlineata, and the greater wax moth, Galleria mellonella, to the entomopathogenic nematodes, Steinernema feltiae and Heterorhabditis bacteriophora. J. Insect Sci. 2011, 11, 75. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.; Ehlers, R.U. Encapsulation of the entomopathogenic nematode Steinernema feltiae in Tipula oleracea. J. Invertebr. Pathol. 1997, 69, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Cruz, N.; Rosa, J.S.; Simões, N. Encapsulation response of 6th instar of Pseudaletia unipuncta (Lepidoptera: Noctuidae) to Steinernema carpocapsae (Nematoda: Steinernematidae). J. Invertebr. Pathol. 2001, 78, 272–274. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gaugler, R. Steinernema glaseri surface coat protein suppresses the immune response of Popillia japonica (Coleoptera: Scarabaeidae) larvae. Biol. Control 1999, 14, 45–50. [Google Scholar] [CrossRef]
- Gotz, P.; Boman, A.; Boman, H.G. Interactions between insect immunity and an insect-pathogenic nematode with symbiotic bacteria. Proc. R. Soc. Lond. B Biol. Sci. 1981, 212, 333–350. [Google Scholar] [CrossRef]
- Laumond, C.; Simões, N.; Boemare, N. Toxins of entomoparasitic nematodes. Pathogenicity of Steinernema carpocapsae–prospectives of genetic engineering. C. R. Acad. Agric. Fr. 1989, 75, 135–138. [Google Scholar]
- Snyder, H.; Stock, S.P.; Kim, S.K.; Flores-Lara, Y.; Forst, S. New insights into the colonization and release processes of Xenorhabdus nematophila and the morphology and ultrastructure of the bacterial receptacle of its nematode host, Steinernema carpocapsae. Appl. Environ. Microbiol. 2007, 73, 5338–5346. [Google Scholar] [CrossRef] [PubMed]
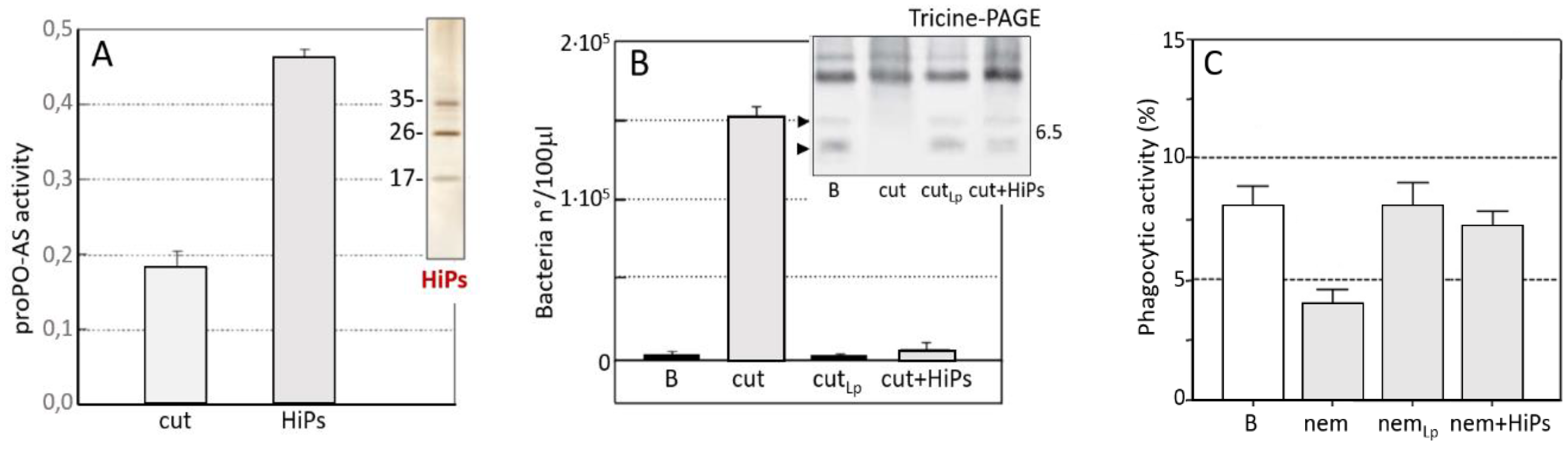
- Mastore, M.; Arizza, V.; Manachini, B.; Brivio, M.F. Modulation of immune responses of Rhynchophorus ferrugineus (Insecta: Coleoptera) induced by the entomopathogenic nematode Steinernema carpocapsae (Nematoda: Rhabditida). Insect Sci. 2015, 22, 748–760. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Macchietto, M.; Chang, D.; Barros, M.M.; Baldwin, J.; Mortazavi, A.; Dillman, A.R. Activated entomopathogenic nematode infective juveniles release lethal venom proteins. PLoS Pathog. 2017, 13, e1006302. [Google Scholar] [CrossRef] [PubMed]
- Toubarro, D.; Lucena-Robles, M.; Nascimento, G.; Costa, G.; Montiel, R.; Coelho, A.V.; Simões, N. An apoptosis-inducing serine protease secreted by the entomopathogenic nematode Steinernema carpocapsae. Int. J. Parasitol. 2009, 39, 1319–1330. [Google Scholar] [CrossRef] [PubMed]
- Toubarro, D.; Avila, M.M.; Montiel, R.; Simões, N.A. pathogenic nematode targets recognition protein to avoid insect defenses. PLoS ONE 2013, 8, e75691. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.J.; Montiel, R.; Nascimento, G.; Toubarro, D.; Simoes, N. Identification and expression analysis of the Steinernema carpocapsae elastase-like serine protease gene during the parasitic stage. Exp. Parasitol. 2009, 122, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.J.; Montiel, R.; Abubucker, S.; Mitreva, M.; Simões, N. Transcripts analysis of the entomopathogenic nematode Steinernema carpocapsae induced in vitro with insect haemolymph. Mol. Biochem. Parasitol. 2010, 169, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Toubarro, D.; Hao, Y.; Simões, N. Cloning, characterisation and heterologous expression of an astacin metalloprotease, Sc-AST, from the entomoparasitic nematode Steinernema carpocapsae. Mol. Biochem. Parasitol. 2010, 174, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.J.; Montiel, R.; Nascimento, G.; Toubarro, D.; Simoes, N. Identification, characterization of functional candidate genes for host-parasite interactions in entomopathogenetic nematode Steinernema carpocapsae by suppressive subtractive hybridization. Parasitol. Res. 2008, 103, 671–683. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, N.; Toubarro, D.; Nascimento, G.; Ferreira, R.; Simões, N. Purification, molecular characterization and gene expression analysis of an aspartic protease (Sc-ASP113) from the nematode Steinernema carpocapsae during the parasitic stage. Mol. Biochem. Parasitol. 2012, 182, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, N.; Nascimento, G.; Ferreira, R.; Martinez, M.; Simões, N. Pepsin-like aspartic protease (Sc-ASP155) cloning, molecular characterization and gene expression analysis in developmental stages of nematode Steinernema carpocapsae. Gene 2012, 500, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, N.; Simões, N. Cloning and molecular analysis of the aspartic protease Sc-ASP110 gene transcript in Steinernema carpocapsae. Parasitology 2013, 140, 1158–1167. [Google Scholar] [CrossRef] [PubMed]
- Toubarro, D.; Avila, M.M.; Hao, Y.; Balasubramanian, N.; Jing, Y.; Montiel, R.; Faria, T.Q.; Brito, R.M.; Simões, N. A serpin released by an entomopathogen impairs clot formation in insect defense system. PLoS ONE 2013, 8, e69161. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Frazer, J.; Banga, A.; Pruitt, K.; Harsh, S.; Jaenike, J.; Eleftherianos, I. Endosymbiont-based immunity in Drosophila melanogaster against parasitic nematode infection. PLoS ONE 2018, 13, e0192183. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Wu, G.; Lv, J.; Li, M. Eicosanoids mediate Galleria mellonella immune response to hemocoel injection of entomopathogenic nematode cuticles. Parasitol. Res. 2016, 115, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Walter, T.N.; Dunphy, G.B.; Mandato, C.A. Steinernema carpocapsae DD136: Metabolites limit the non-self adhesion responses of haemocytes of two lepidopteran larvae, Galleria mellonella (F. Pyralidae) and Malacosoma disstria (F. Lasiocampidae). Exp. Parasitol. 2008, 120, 2161–2174. [Google Scholar] [CrossRef] [PubMed]
- Brivio, M.F.; Toscano, A.; De Pasquale, S.M.; De Lerma Barbaro, A.; Giovannardi, S.; Finzi, G.; Mastore, M. Surface protein components from entomopathogenic nematodes and their symbiotic bacteria: Effects on immune responses of the greater wax moth, Galleria mellonella (Lepidoptera: Pyralidae). Pest Manag. Sci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Binda-Rossetti, S.; Mastore, M.; Protasoni, M.; Brivio, M.F. Effects of an entomopathogen nematode on the immune response of the insect pest red palm weevil: Focus on the host antimicrobial response. J. Invertebr. Pathol. 2016, 133, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Darsouei, R.; Karimi, J.; Ghadamyari, M.; Hosseini, M. Differential Change Patterns of Main Antimicrobial Peptide Genes during Infection of Entomopathogenic Nematodes and Their Symbiotic Bacteria. J. Parasitol. 2017, 103, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Ciche, T.A.; Darby, C.; Ehlers, R.U.; Forst, S.; Goodrich-Blaire, H. Dangerous liaisons: The symbiosis of entomopathogenic nematodes and bacteria. Biol. Control 2006, 38, 22–46. [Google Scholar] [CrossRef]
- Givaudan, A.S.; Baghdiguian, S.; Lanois, A.; Boemare, N. Swarming and swimming changes concomitant with phase variation in Xenorhabdus nematophilus. Appl. Environ. Microbiol. 1995, 61, 1408–1413. [Google Scholar] [PubMed]
- Volgyi, A.; Fodor, A.; Szentirmai, A.; Forst, S. Phase Variation in Xenorhabdus nematophilus. Appl. Environ. Microbiol. 1998, 64, 1188–1193. [Google Scholar] [PubMed]
- Sugar, D.R.; Murfin, K.E.; Chaston, J.M.; Andersen, A.W.; Richards, G.R.; deLéon, L.; Baum, J.A.; Clinton, W.P.; Forst, S.; Goldman, B.S.; et al. Phenotypic variation and host interactions of Xenorhabdus bovienii SS-2004, the entomopathogenic symbiont of Steinernema jollieti nematodes. Environ. Microbiol. 2012, 14, 924–939. [Google Scholar] [CrossRef] [PubMed]
- Akhurst, R.J.; Boemare, N.E. Biology and Taxonomy of Xenorhabdus. In Entomopathogenic Nematodes in Biological Control; Gaugler, R., Kaya, H.K., Eds.; CRC Press: Boca Raton, FL, USA, 1990; pp. 75–90. [Google Scholar]
- Abdel-Razek, A.S. Pathogenicity of Bacteria Symbiotically Associated with Insect Pathogenic Nematodes against the Greater Wax Moth, Galleria mellonella. Arch. Phytopathol. Plant Prot. 2002, 35, 53–60. [Google Scholar] [CrossRef]
- Herbert, E.E.; Goodrich-Blair, H. Friend and foe: The two faces of Xenorhabdus nematophila. Nat. Rev. Microbiol. 2007, 5, 634–646. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, A.; Bhatnagar, N.B.; Bhatnagar, R. Bacterial Insecticidal Toxins. Crit. Rev. Microbiol. 2004, 30, 33–54. [Google Scholar] [CrossRef] [PubMed]
- Dunphy, G.B.; Webster, J.M. Interaction of Xenorhabdus nematophilus subsp. Nematophilus with the hemolymph of Galleria mellonella. J. Insect Physiol. 1984, 30, 883–889. [Google Scholar] [CrossRef]
- Dunphy, G.B.; Webster, J.M. Influence of the Mexican strain of Steinernema feltiae and its associated bacterium Xenorhabdus nematophilus on Galleria mellonella. J. Parasitol. 1986, 72, 130–135. [Google Scholar] [CrossRef]
- Vallet-Gely, I.; Lemaitre, B.; Boccard, F. Bacterial strategies to overcome insect defences. Nat. Rev. Microbiol. 2008, 6, 302–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richards, G.R.; Goodrich-Blair, H. Masters of conquest and pillage: Xenorhabdus nematophila global regulators control transitions from virulence to nutrient acquisition. Cell. Microbiol. 2009, 11, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Khandelwal, P.; Banerjee-Bhatnagar, N. Insecticidal activity associated with the outer membrane vesicles of Xenorhabdus nematophilus. Appl. Environ. Microbiol. 2003, 69, 2032–2037. [Google Scholar] [CrossRef] [PubMed]
- Herbert Tran, E.E.; Goodrich-Blair, H. CpxRA contributes to Xenorhabdus nematophila virulence through regulation of LRHA and modulation of insect immunity. Appl. Environ. Microbiol. 2009, 75, 3998–4006. [Google Scholar] [CrossRef] [PubMed]
- Ellis, T.N.; Kuehn, M.J. Virulence and immunomodulatory roles of bacterial outer membrane vesicles. Microbiol. Mol. Biol. Rev. 2010, 74, 81–94. [Google Scholar] [CrossRef] [PubMed]
- McMullen, J.G.; Peterson, B.F.; Forst, S.; Blair, H.G.; Stock, S.P. Fitness costs of symbiont switching using entomopathogenic nematodes as a model. BMC Evol. Biol. 2017, 17, 100. [Google Scholar] [CrossRef] [PubMed]
- McMullen, J.; McQuade, R.; Ogier, J.; Pagès, S.; Gaudriault, S.; Patricia Stock, S. Variable virulence phenotype of Xenorhabdus bovienii (γ-Proteobacteria: Enterobacteriaceae) in the absence of their vector hosts. Microbiology 2017, 163, 510–522. [Google Scholar] [CrossRef] [PubMed]
- McQuade, R.; Stock, S.P. Secretion Systems and Secreted Proteins in Gram-Negative Entomopathogenic Bacteria: Their Roles in Insect Virulence and Beyond. Insects 2018, 9, 68. [Google Scholar] [CrossRef] [PubMed]
- Brillard, J.; Duchaud, E.; Boemare, N.; Kunst, F.; Givaudan, A. The PhlA hemolysin from the entomopathogenic bacterium Photorhabdus luminescens belongs to the two-partner secretion family of hemolysins. J. Bacteriol. 2002, 184, 3871–3878. [Google Scholar] [CrossRef] [PubMed]
- Cowles, K.N.; Goodrich-Blair, H. Expression and activity of a Xenorhabdus nematophila haemolysin required for full virulence towards Manduca sexta insects. Cell. Microbiol. 2005, 7, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Murfin, K.E.; Whooley, A.C.; Klassen, J.L.; Goodrich-Blair, H. Comparison of Xenorhabdus bovienii bacterial strain genomes reveals diversity in symbiotic functions. BMC Genom. 2015, 16, 889. [Google Scholar] [CrossRef] [PubMed]
- Chapuis, E.; Emelianoff, V.; Paulmier, V.; Le Brun, N.; Pages, S.; Sicard, M.; Ferdy, J.B. Manifold aspects of specificity in a nematode-bacterium mutualism. J. Evol. Biol. 2009, 22, 2104–2117. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Amphipathic Linear Peptides | |||
| Name | Source | Size (aa) | Activity |
| Cecropin | Lepidoptera, Diptera | 31–39 | Gram negative/positive |
| Moricin | Lepidoptera | 42 | Gram negative/positive |
| Mellitin | Hymenoptera | 26 | Gram negative/positive |
| Cyclic Cysteine Rich Peptides | |||
| Defensin | Diptera, Hemiptera, Coleoptera, Lepidoptera | 32–43 | Gram positive/negative |
| Drosomycin | Diptera | 44 | Fungi |
| Peptides Rich in Specific Amino Acids | |||
| Drosocin | Diptera | 19 | Gram negative |
| Diptericin | Diptera | 100–110 | Gram negative |
| Attacin | Lepidoptera | 214–224 | Gram negative |
| Coleptericin | Coleoptera | 74 | Gram negative |
| Gloverin | Lepidoptera | 36–261 * | Gram negative |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brivio, M.F.; Mastore, M. Nematobacterial Complexes and Insect Hosts: Different Weapons for the Same War. Insects 2018, 9, 117. https://doi.org/10.3390/insects9030117
Brivio MF, Mastore M. Nematobacterial Complexes and Insect Hosts: Different Weapons for the Same War. Insects. 2018; 9(3):117. https://doi.org/10.3390/insects9030117
Chicago/Turabian StyleBrivio, Maurizio Francesco, and Maristella Mastore. 2018. "Nematobacterial Complexes and Insect Hosts: Different Weapons for the Same War" Insects 9, no. 3: 117. https://doi.org/10.3390/insects9030117
APA StyleBrivio, M. F., & Mastore, M. (2018). Nematobacterial Complexes and Insect Hosts: Different Weapons for the Same War. Insects, 9(3), 117. https://doi.org/10.3390/insects9030117