Previous Interspecific Courtship Impairs Female Receptivity to Conspecifics in the Parasitoid Wasp Nasonia longicornis But Not in N. vitripennis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains, Rearing, and Preparation of Wasps
2.2. Behavioural Bioassays
2.3. Statistical Analysis
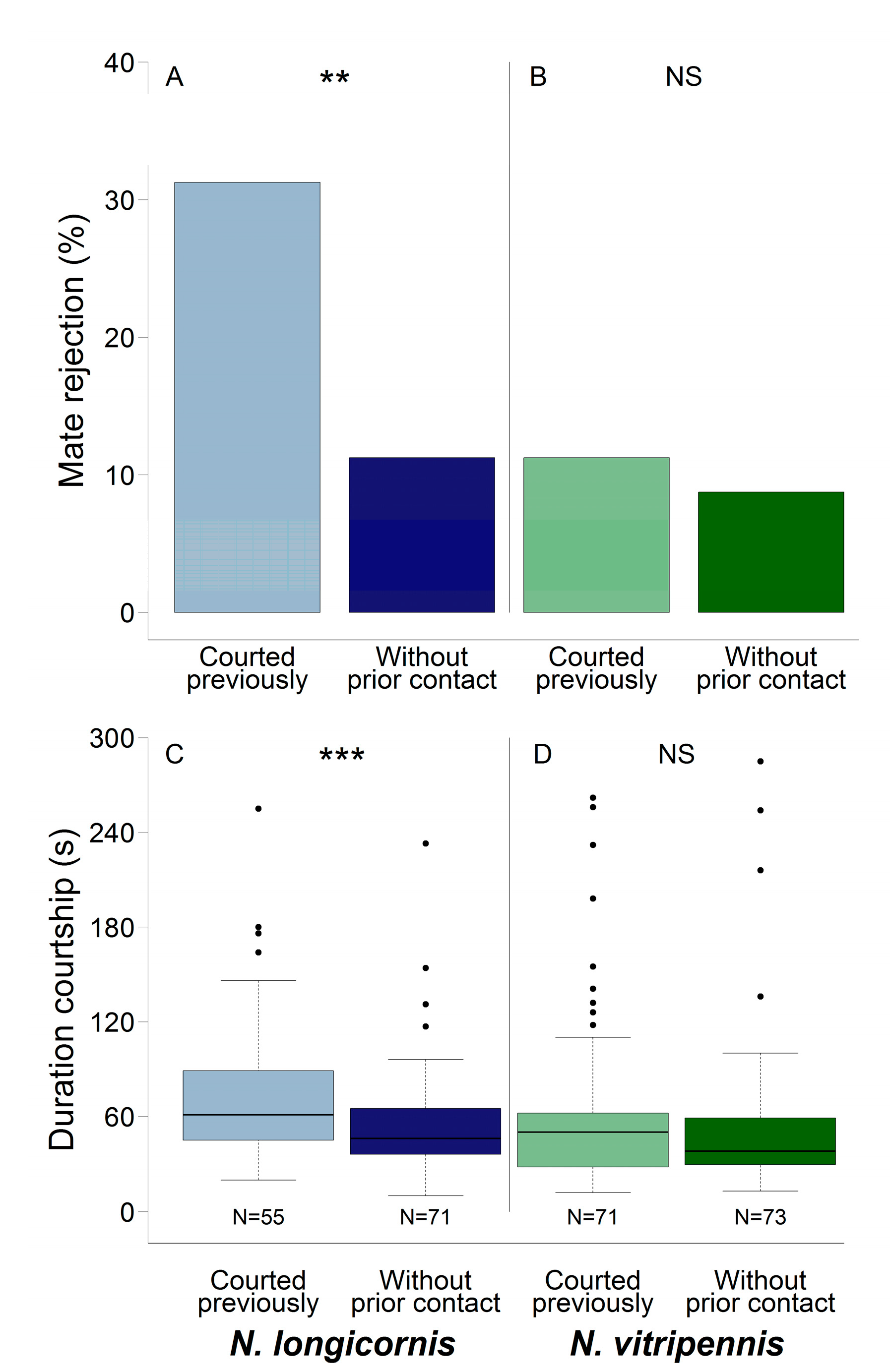
3. Results
3.1. Conspecific Mate Rejection
3.2. Duration of Courtship
3.3. Aggression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gröning, J.; Hochkirch, A. Reproductive interference between animal species. Q. Rev. Biol. 2008, 83, 257–282. [Google Scholar] [CrossRef] [PubMed]
- Groot, A.T.; Claßen, A.; Staudacher, H.; Schal, C.; Heckel, D.G. Phenotypic plasticity in sexual communication signal of a noctuid moth. J. Evol. Biol. 2010, 23, 2731–2738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doherty, J.A.; Howard, D.J. Lack of preference for conspecific calling songs in female crickets. Anim. Behav. 1996, 51, 981–990. [Google Scholar] [CrossRef]
- Andrews, R.H.; Petney, T.N.; Bull, C.M. Reproductive interference between three parapatric species of reptile tick. Oecologia 1982, 52, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Singer, F. Reproductive costs arising from incomplete habitat segregation among three species of Leucorrhinia dragonflies. Behaviour 1990, 115, 188–201. [Google Scholar] [CrossRef]
- Takafuji, A.; Kuno, E.; Fujimoto, H. Reproductive interference and its consequences for the competitive interactions between two closely related Panonychus spider mites. Exp. Appl. Acarol. 1997, 21, 379–391. [Google Scholar] [CrossRef]
- Wirtz, P. Mother species–father species: Unidirectional hybridization in animals with female choice. Anim. Behav. 1999, 58, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Doi, M.; Matsuda, M.; Tomaru, M.; Matsubayashi, H.; Oguma, Y. A locus for female discrimination behavior causing sexual isolation in Drosophila. Proc. Natl. Acad. Sci. USA 2001, 98, 6714–6719. [Google Scholar] [CrossRef] [PubMed]
- Noor, M.A.F. Reinforcement and other consequences of sympatry. Heredity 1999, 83, 503–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liou, L.W.; Price, T.D. Speciation by reinforcement of premating isolation. Evolution 1994, 48, 1451–1459. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, T.D. Pheromones and Animal Behavior, 2nd ed.; Cambridge University Press: Cambridge, UK, 2014; pp. 65–103. [Google Scholar]
- Talyn, B.C.; Dowse, H.B. The role of courtship song in sexual selection and species recognition by female Drosophila melanogaster. Anim. Behav. 2004, 68, 1165–1180. [Google Scholar] [CrossRef]
- Boake, C.R.B.; Andreadis, D.K.; Witzel, A. Behavioural isolation between two closely related Hawaiian Drosophila species: The role of courtship. Anim. Behav. 2000, 60, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Gray, D.A. Does courtship behavior contribute to species-level reproductive isolation in field crickets? Behav. Ecol. 2005, 16, 201–206. [Google Scholar] [CrossRef]
- Chaplin, S.J. Reproductive isolation between two sympatric species of Oncopeltus (Hemiptera: Lygaeidae) in the tropics. Ann. Entomol. Soc. Am. 1973, 66, 997–1000. [Google Scholar] [CrossRef]
- Crowder, D.W.; Sitvarin, M.I.; Carrière, Y. Plasticity in mating behaviour drives asymmetric reproductive interference in whiteflies. Anim. Behav. 2010, 79, 579–587. [Google Scholar] [CrossRef]
- Kozak, G.M.; Boughman, J.W. Learned conspecific mate preference in a species pair of sticklebacks. Behav. Ecol. 2009, 20, 1282–1288. [Google Scholar] [CrossRef] [Green Version]
- Irwin, D.E.; Price, T. Sexual imprinting, learning and speciation. Heredity 1999, 82, 347–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whiting, A.R. The biology of the parasitic wasp Mormoniella vitripennis [=Nasonia vitripennis] (Walker). Q. Rev. Biol. 1967, 42, 333–406. [Google Scholar] [CrossRef]
- Darling, D.; Werren, J.H. Biosystematics of Nasonia (Hymenoptera: Pteromalidae): Two new species reared from birds’ nests in North America. Ann. Entomol. Soc. Am. 1990, 83, 352–370. [Google Scholar] [CrossRef]
- Grillenberger, B.K.; van de Zande, L.; Bijlsma, R.; Gadau, J.; Beukeboom, L.W. Reproductive strategies under multiparasitism in natural populations of the parasitoid wasp Nasonia (Hymenoptera). J. Evol. Biol. 2009, 22, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Raychoudhury, R.; Grillenberger, B.K.; Gadau, J.; Bijlsma, R.; van de Zande, L.; Werren, J.H.; Beukeboom, L.W. Phylogeography of Nasonia vitripennis (Hymenoptera) indicates a mitochondrial-Wolbachia sweep in North America. Heredity 2010, 104, 318–326. [Google Scholar] [CrossRef] [PubMed]
- King, B.H.; Grimm, K.; Reno, H. Effects of mating on female locomotor activity in the parasitoid wasp Nasonia vitripennis (Hymenoptera: Pteromalidae). Environ. Entomol. 2000, 29, 927–933. [Google Scholar] [CrossRef]
- Grillenberger, B.K.; Koevoets, T.; Burton-Chellew, M.N.; Sykes, E.M.; Shuker, D.M.; van de Zande, L.; Bijlsma, R.; Gadau, J.; Beukeboom, L.W. Genetic structure of natural Nasonia vitripennis populations: Validating assumptions of sex-ratio theory. Mol. Ecol. 2008, 17, 2854–2864. [Google Scholar] [CrossRef] [PubMed]
- Ruther, J.; McCaw, J.; Böcher, L.; Pothmann, D.; Putz, I. Pheromone diversification and age-dependent behavioural plasticity decrease interspecific mating costs in Nasonia. PLoS ONE 2014, 9, e89214. [Google Scholar] [CrossRef] [PubMed]
- Buellesbach, J.; Greim, C.; Raychoudhury, R.; Schmitt, T. Asymmetric assortative mating behaviour reflects incomplete pre-zygotic isolation in the Nasonia species complex. Ethology 2014, 120, 1–10. [Google Scholar] [CrossRef]
- Giesbers, M.C.W.G.; Gerritsma, S.; Buellesbach, J.; Diao, W.; Pannebakker, B.A.; van de Zande, L.; Schmitt, T.; Beukeboom, L.W. Prezygotic isolation in the parasitoid wasp genus Nasonia. In Speciation: Natural Processes, Genetics and Biodiversity; Pawel, M., Ed.; Nova Science Publishers: New York, NY, USA, 2013; pp. 165–191. [Google Scholar]
- Mair, M.M.; Kmezic, V.; Huber, S.; Pannebakker, B.A.; Ruther, J. The chemical basis of mate recognition in two parasitoid wasp species of the genus Nasonia. Entomol. Exp. Appl. 2017, 164, 1–15. [Google Scholar] [CrossRef]
- Van den Assem, J.; Jachmann, F.; Simbolotti, P. Courtship behaviour of Nasonia vitripennis (Hym., Pteromalidae): Some qualitative, experimental evidence for the role of pheromones. Behaviour 1980, 75, 301–307. [Google Scholar] [CrossRef]
- Ruther, J.; Thal, K.; Blaul, B.; Steiner, S. Behavioural switch in the sex pheromone response of Nasonia vitripennis females is linked to receptivity signalling. Anim. Behav. 2010, 80, 1035–1040. [Google Scholar] [CrossRef]
- Van den Assem, J.; Werren, J.H. A comparison of the courtship and mating behavior of three species of Nasonia (Hymenoptera: Pteromalidae). J. Insect Behav. 1994, 7, 53–66. [Google Scholar] [CrossRef]
- Van den Assem, J.; Jachmann, F.; de Jong, K.A.G. Courtship behaviour of Nasonia vitripennis: Head nodding, mouth-part extrusion and pheromone discharge by abdomectomized males. Entomol. Exp. Appl. 1981, 30, 215–218. [Google Scholar] [CrossRef]
- Van den Assem, J.; Vernel, C. Courtship behaviour of Nasonia vitripennis (Hym.: Pteromalidae): Observations and experiments on male readiness to assume copulatory behaviour. Behaviour 1979, 68, 118–135. [Google Scholar] [CrossRef]
- Bordenstein, S.R.; Werren, J.H. Effects of A and B Wolbachia and host genotype on interspecies cytoplasmic incompatibility in Nasonia. Genetics 1998, 148, 1833–1844. [Google Scholar] [PubMed]
- Bordenstein, S.R.; O’Hara, F.P.; Werren, J.H. Wolbachia-induced incompatibility precedes other hybrid incompatibilities in Nasonia. Nature 2001, 409, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Tram, U.; Fredrick, K.; Werren, J.H.; Sullivan, W. Paternal chromosome segregation during the first mitotic division determines Wolbachia-induced cytoplasmic incompatibility phenotype. J. Cell Sci. 2006, 119, 3655–3663. [Google Scholar] [CrossRef] [PubMed]
- Van den Assem, J.; Visser, J. Aspects of sexual receptivity in female Nasonia vitripennis. Biol. Behav. 1976, 1, 37–56. [Google Scholar]
- Van de Zande, L.; Ferber, S.; de Haan, A.; Beukeboom, L.W.; van Heerwaarden, J.; Pannebakker, B.A. Development of a Nasonia vitripennis outbred laboratory population for genetic analysis. Mol. Ecol. Resour. 2014, 14, 578–587. [Google Scholar] [CrossRef] [PubMed]
- Ruther, J.; Homann, M.; Steidle, J.L.M. Female-derived sex pheromone mediates courtship behaviour in the parasitoid Lariophagus distinguendus. Entomol. Exp. Appl. 2000, 96, 265–274. [Google Scholar] [CrossRef]
- Dukas, R. Learning decreases heterospecific courtship and mating in fruit flies. Biol. Lett. 2008, 4, 645–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magurran, A.E.; Ramnarine, I.W. Learned mate recognition and reproductive isolation in guppies. Anim. Behav. 2003, 67, 1077–1082. [Google Scholar] [CrossRef]
- Giesbers, M.C.W.G.; Pannebakker, B.A.; van de Zande, L.; Beukeboom, L. Within-Host-Mating in the Nasonia Genus is Largely Dependent on Male Behaviour. Ph.D. Thesis, University of Groningen, Groningen, The Netherlands, 2016. [Google Scholar]
- Leonard, J.E.; Boake, C.R.B. Site-dependent aggression and mating behaviour in three species of Nasonia (Hymenoptera: Pteromalidae). Anim. Behav. 2006, 71, 641–647. [Google Scholar] [CrossRef]
- Leonard, J.E.; Boake, C.R.B. Associations between male courtship and female polyandry in three species of wasp, Nasonia (Hymenoptera: Pteromalidae). Anim. Behav. 2008, 76, 637–647. [Google Scholar] [CrossRef]
- Niehuis, O.; Buellesbach, J.; Gibson, J.D.; Pothmann, D.; Hanner, C.; Mutti, N.S.; Judson, A.K.; Gadau, J.; Ruther, J.; Schmitt, T. Behavioural and genetic analyses of Nasonia shed light on the evolution of sex pheromones. Nature 2013, 494, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Mair, M.M.; Ruther, J. Territoriality and behavioural strategies at the natal host patch differ in two microsympatric Nasonia species. Anim. Behav. 2018, 143, 113–129. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mair, M.M.; Seifert, N.; Ruther, J. Previous Interspecific Courtship Impairs Female Receptivity to Conspecifics in the Parasitoid Wasp Nasonia longicornis But Not in N. vitripennis. Insects 2018, 9, 112. https://doi.org/10.3390/insects9030112
Mair MM, Seifert N, Ruther J. Previous Interspecific Courtship Impairs Female Receptivity to Conspecifics in the Parasitoid Wasp Nasonia longicornis But Not in N. vitripennis. Insects. 2018; 9(3):112. https://doi.org/10.3390/insects9030112
Chicago/Turabian StyleMair, Magdalena M., Nicole Seifert, and Joachim Ruther. 2018. "Previous Interspecific Courtship Impairs Female Receptivity to Conspecifics in the Parasitoid Wasp Nasonia longicornis But Not in N. vitripennis" Insects 9, no. 3: 112. https://doi.org/10.3390/insects9030112
APA StyleMair, M. M., Seifert, N., & Ruther, J. (2018). Previous Interspecific Courtship Impairs Female Receptivity to Conspecifics in the Parasitoid Wasp Nasonia longicornis But Not in N. vitripennis. Insects, 9(3), 112. https://doi.org/10.3390/insects9030112




