Micro-CT to Document the Coffee Bean Weevil, Araecerus fasciculatus (Coleoptera: Anthribidae), Inside Field-Collected Coffee Berries (Coffea canephora)
Abstract
:1. Introduction
2. Materials and Methods
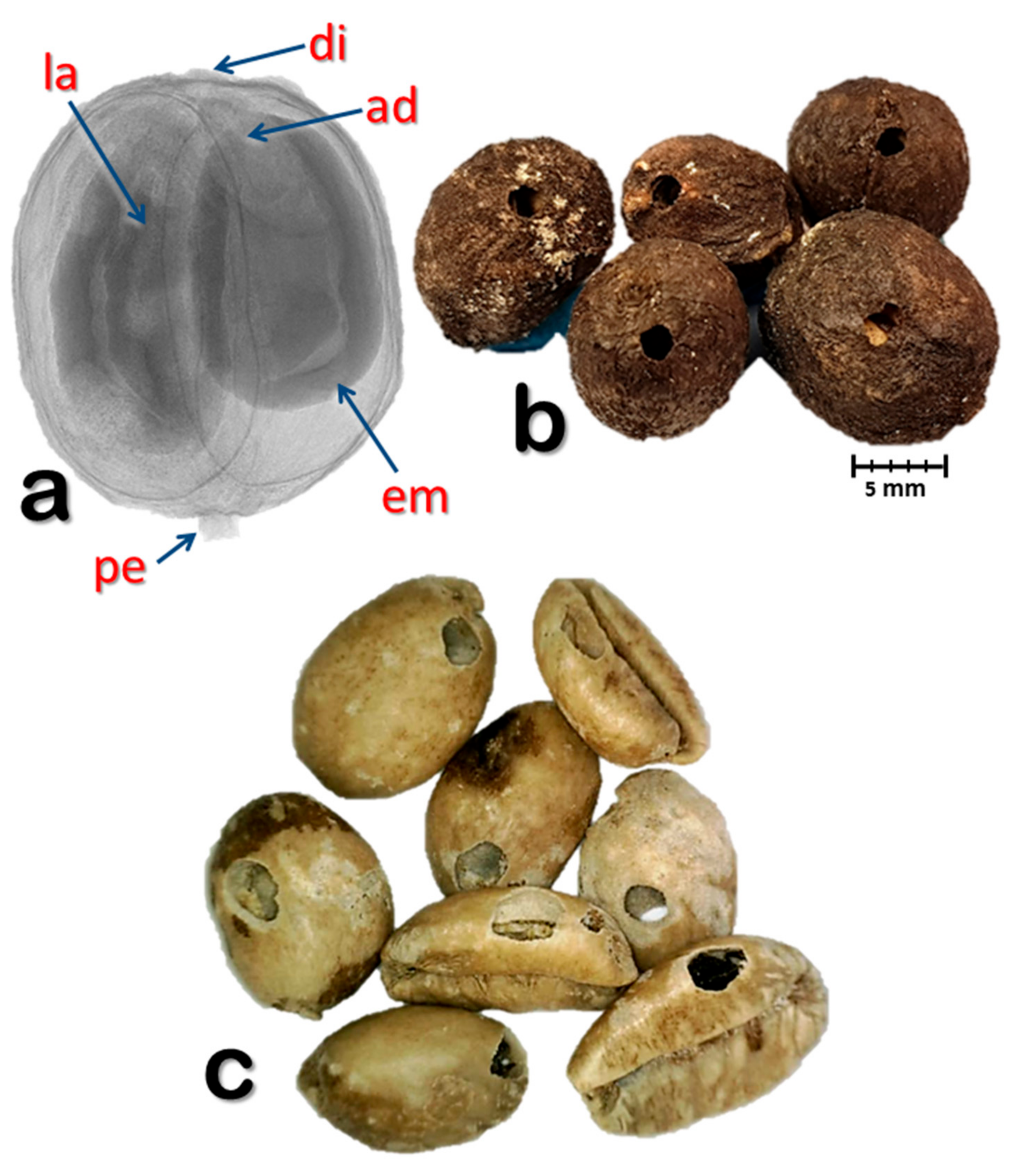
2.1. Coffee Berries
2.2. Micro-CT Scans
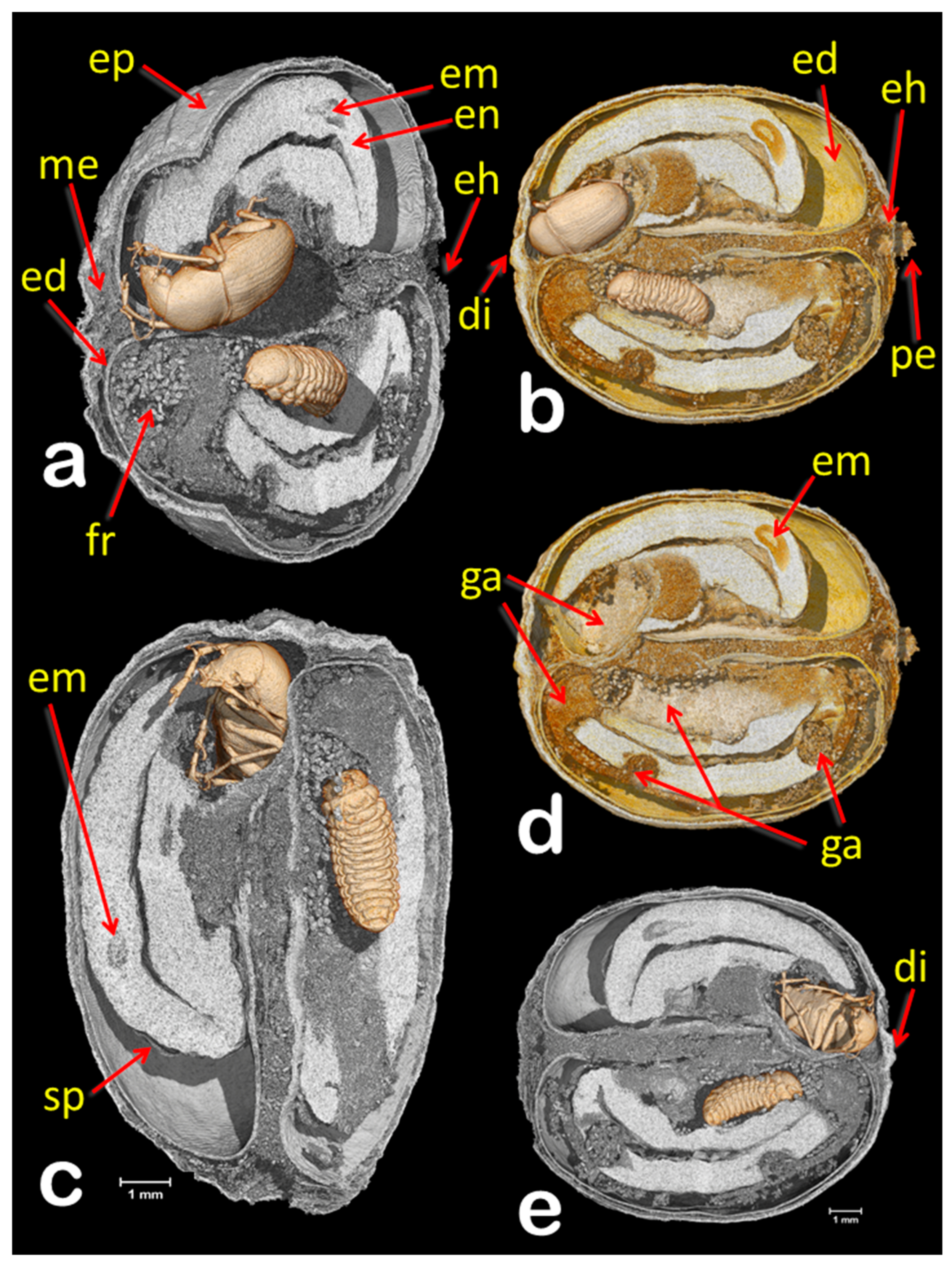
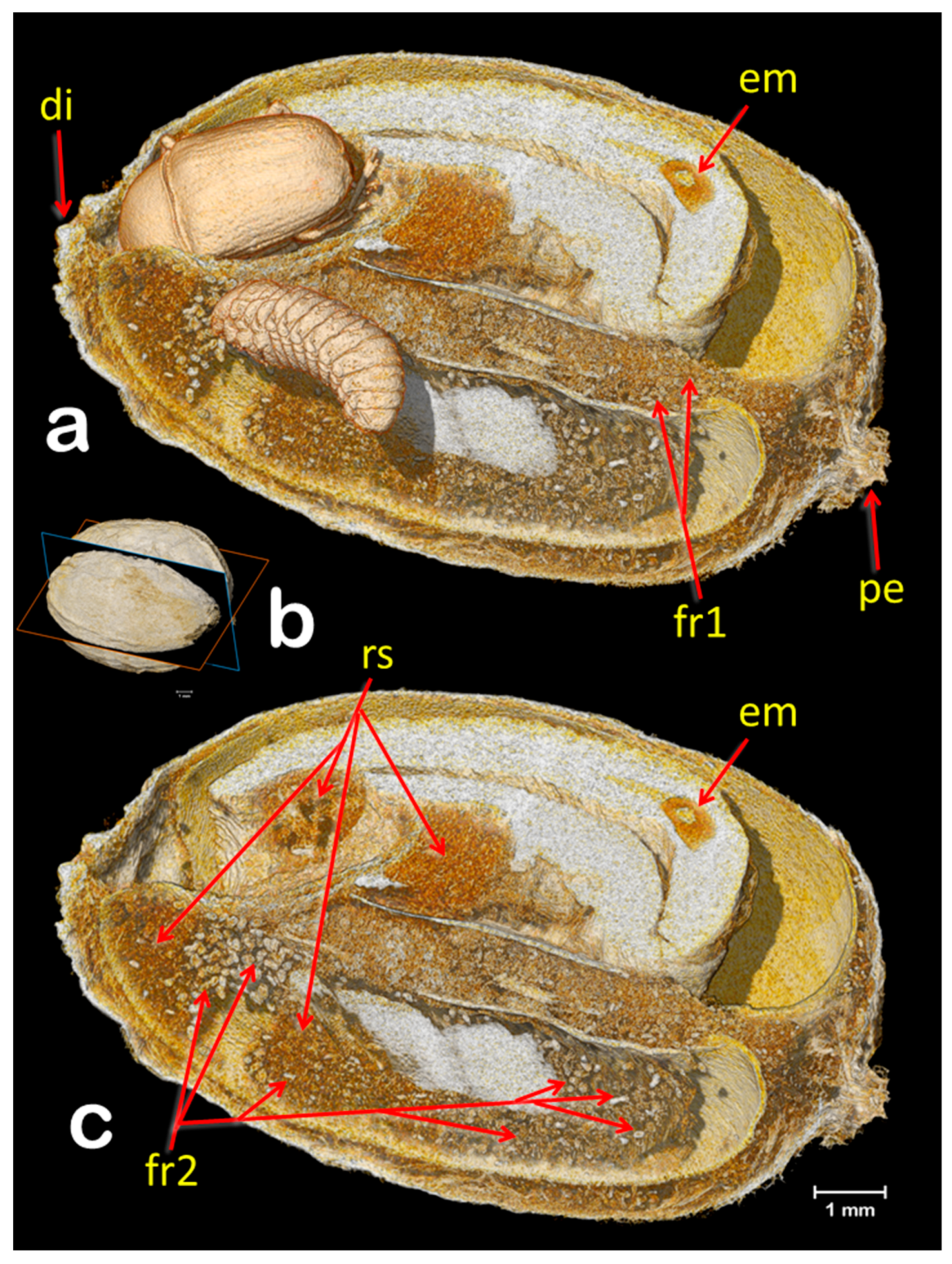
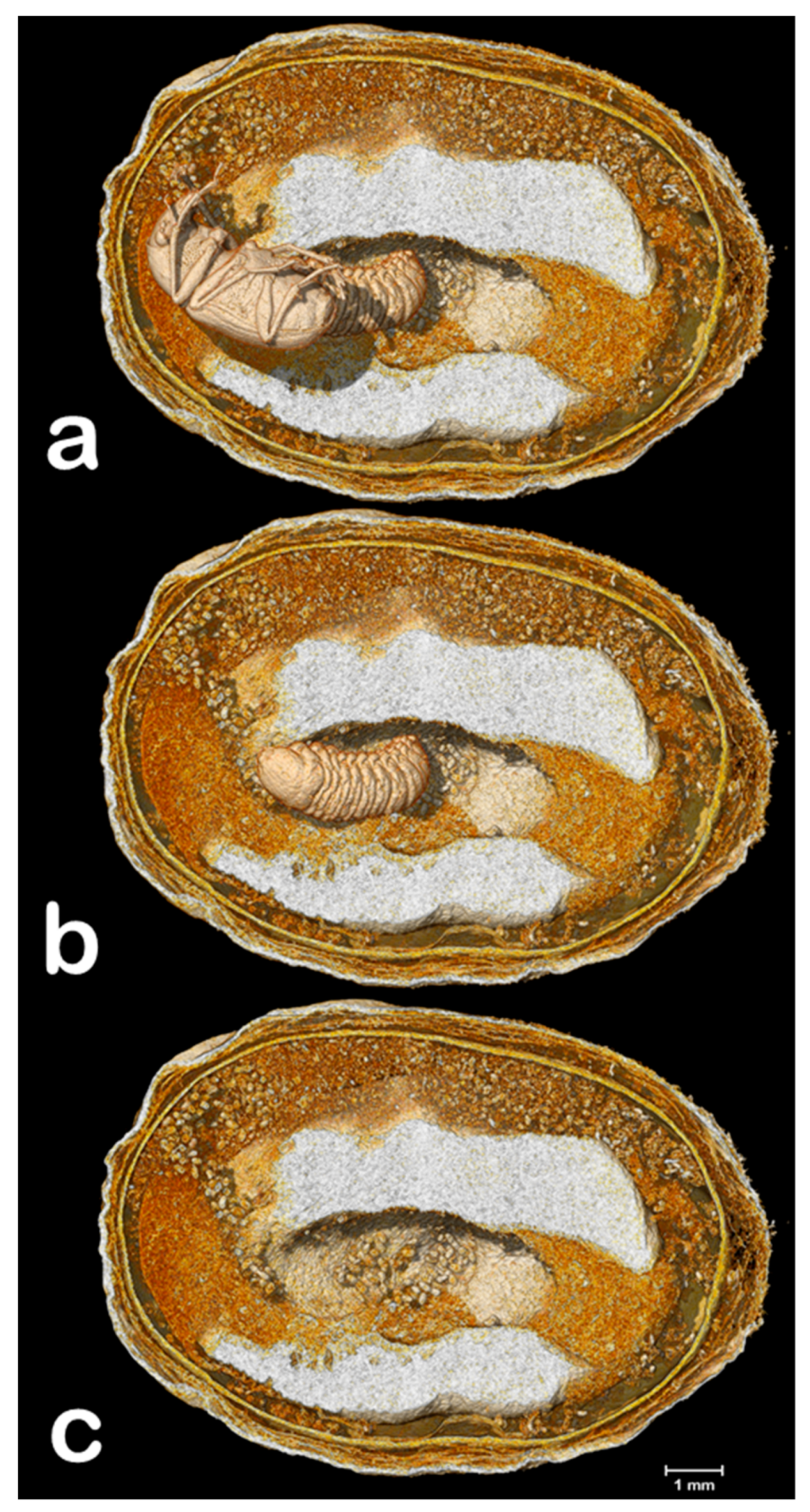
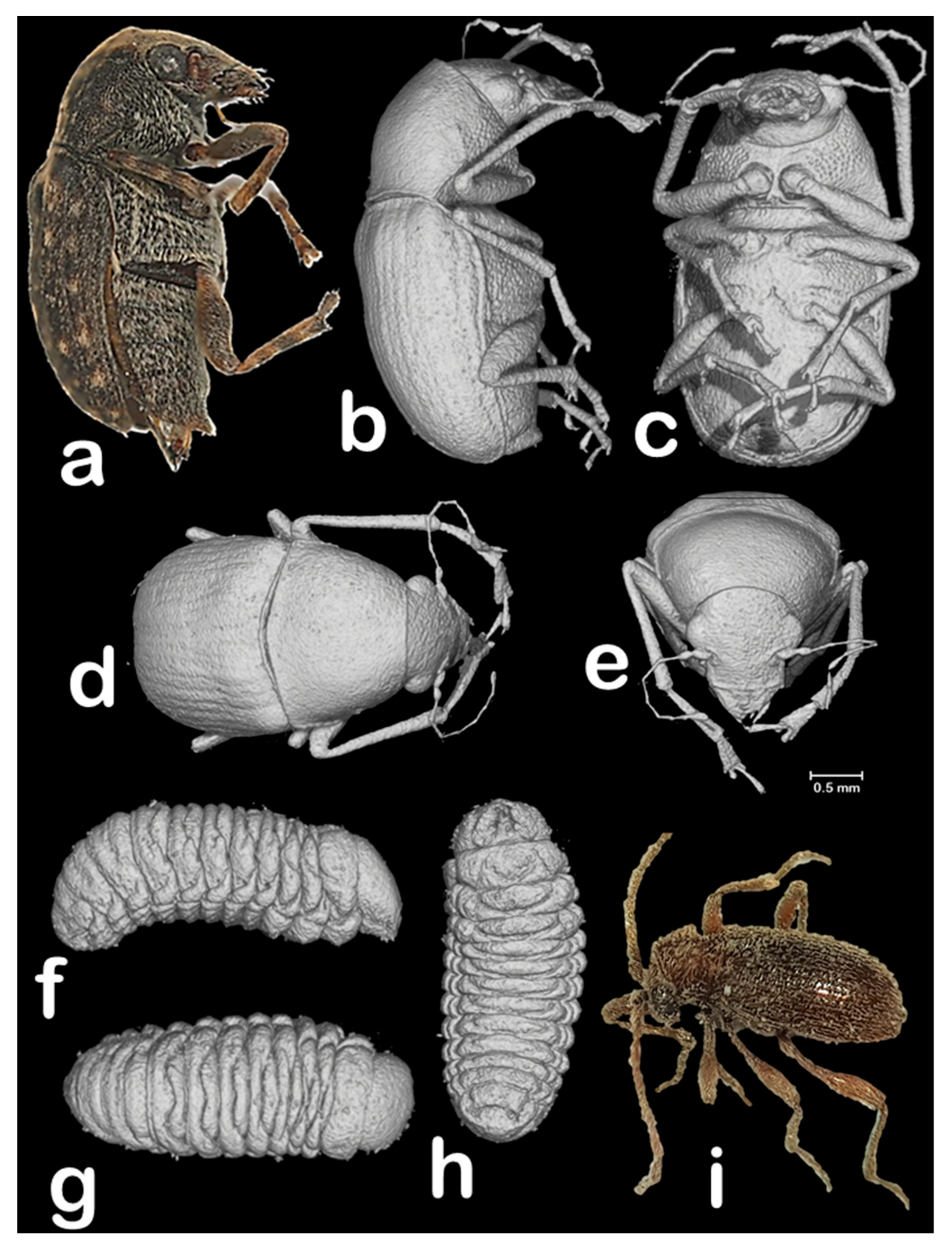
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Valentine, B.D. The scientific name of the coffee bean weevil and some additional bibliography (Coleoptera: Anthribidae: Araecerus Schönherr). Insecta Mundi 2005, 19, 247–253. [Google Scholar]
- Chittenden, F.H. Insects Affecting Cereals and Other Dry Vegetable Foods; U.S. Department of Agriculture, Division of Entomology, Bulletin No. 4—New Series; Government Printing Office: Washington, DC, USA, 1896; pp. 112–130.
- Chittenden, F.H. An invasion of the coffee-bean weevil. In Some Little-Known Insects Affecting Stored Vegetable Products: A Collection of Articles Detailing Certain Original Observations Made Upon Insects of This Class; U.S. Department of Agriculture, Division of Entomology, Bulletin No. 8—New Series; Government Printing Office: Washington, DC, USA, 1897; pp. 36–38. [Google Scholar]
- Autuori, M. Dados biologicos sobre o Araecerus fasciculatus (De Geer) (Col. Anthribiidae). Rev. Entomol. 1931, 1, 52–61. [Google Scholar]
- Mphuru, A.N. Araecerus fasciculatus De Geer (Coleoptera: Anthribidae): A review. Trop. Stored Prod. Inf. 1974, 26, 7–15. [Google Scholar]
- Waller, J.M.; Bigger, M.; Hillocks, R.J. Coffee Pests, Diseases and Their Management; CABI Publishing: Wallingford, UK, 2007; ISBN 13 978-1-84593-129-2. [Google Scholar]
- Cabal Concha, A. Biología y control del gorgojo del café: Araecerus fasciculatus De Geer Fam: (Anthribiidae), en Barranquilla—Colombia. Rev. Facul. Nacional Agron. 1952, 18, 49–72. [Google Scholar]
- De Figuereido, E.R., Jr. O contrôle do “caruncho” das tulhas. O Biol. 1957, 23, 197–200. [Google Scholar]
- Abrahão, J.; Bitran, E.A. Caruncho das tulhas atacando lavouras de café. O Biol. 1973, 39, 245–247. [Google Scholar]
- Caspersen, B.A. A focus on flaws. Do you use the SCAA’s green arabica coffee classification system? Roast 2016, 2016, 24–28, 30–32, 34, 36–40. [Google Scholar]
- Locatelli, D.P.; Viganò, C.A. Contaminazioni entomatiche in caffe crudo e tostato. Ind. Aliment. 1991, 30, 977–991. [Google Scholar]
- Bemelmans, J. Les ennemis du caféier. Ann. Gembloux 1930, 36, 418–424. [Google Scholar]
- Cárdenas Murillo, R.; Posada Flórez, F.J. Los Insectos y Otros Habitantes de Cafetales y Platanales; Comité Departamental de Cafeteros del Quindío: Armenia, Colombia, 2001; ISBN 958-95930-1-0. [Google Scholar]
- Da Costa Lima, A. Insetos do Brasil, Volume 10, Coleópteros, Fourth and Last Part; Serie Didática #12; Escola Nacional de Agronomia: Rio de Janeiro, Brazil, 1956; pp. 13–15. [Google Scholar]
- Mancion, J.; Alibert, H. La production du café au Togo et quelques insectes déprédateurs du caféier. L’Agron. Coloniale 1936, 224, 33–43. [Google Scholar]
- Padi, B. Insects associated with coffee berries in Ghana. In Proceedings of the 18th International Scientific Colloquium on Coffee, Helsinki, Finland, 2–6 August 1999; Association Scientifique Internationale du Café (ASIC): Paris, France, 1999; pp. 524–528. [Google Scholar]
- Sekhar, P.S. Entomology in India; Entomological Society of India: Delhi, India, 1964. [Google Scholar]
- Directoria de Agricultura. Praga do café. Bol. Agric. 1923, 24, 83–88. [Google Scholar]
- Alba-Tercedor, J.; Alba-Alejandre, I.; Vega, F.E. Micro-CT unveils the secret life of the coffee berry borer (Hypothenemus hampei; Coleoptera, Curculionidae: Scolytinae) inside coffee berries. In Proceedings of the Bruker Micro-CT User Meeting, Ghent, Belgium, 16–19 April 2018; pp. 165–173. Available online: http://bruker-microct.com/company/UM2018/2018_30.pdf (accessed on 18 June 2018).
- Alba-Tercedor, J.; Alba-Alejandre, I. Comparing micro-CT results of insects with classical anatomical studies: The European honey bee (Apis mellifera Linnaeus, 1758) as a benchmark (Insecta: Hymenoptera, Apidae). In Proceedings of the Bruker Micro-CT Users Meeting, Brussels, Belgium, 12–15 June 2017; pp. 147–167. Available online: http://bruker-microct.com/company/UM2017/AbstractBook2017.pdf (accessed on 18 June 2018).
- Alba-Tercedor, J. From the sample preparation to the volume rendering images of small animals: A step by step example of a procedure to carry out the micro-CT study of the leafhopper insect Homalodisca vitripennis (Hemiptera: Cicadellidae). In Proceedings of the Bruker Micro-CT Users Meeting, Ostend, Belgium, 5–8 May 2014; pp. 260–288. Available online: http://www.skyscan.be/company/UM2014/008_Javier_Alba_Tercedor.pdf (accessed on 18 June 2018).
- Amira. Amira for Life Sciences: 3D Visualization and Analysis Software. ThermoFisher Scientific. 2016. Available online: https://www.fei.com/software/amira-for-life-sciences (accessed on 18 June 2018).
- Cotton, R.T. Four Rhynchophora attacking corn in storage. J. Agric. Res. 1921, 20, 605–614. [Google Scholar]
- Archibald, R.D.; Chalmers, I. Stored product Coleoptera in New Zealand. N. Z. Entomol. 1983, 7, 371–397. [Google Scholar] [CrossRef]
- Varón, E.H.; Hanson, P.; Borbón, O.; Carballo, M.; Hilje, L. Potencial de hormigas como depredadores de la broca del café (Hypothenemus hampei) en Costa Rica. Manejo Integr. Plagas Agroecol. 2004, 73, 42–50. [Google Scholar]
- Wilkinson, H. The Coffee Berry Borer Beetle Stephanoderes hampei (Ferr.); Printed by the Government Printer; Colony and Protectorate of Kenya: Nairobi, Kenya, 1928; 10p. [Google Scholar]
- De Geer, C. Memoires pour Servir a l’Histoire des Insectes; Pierre Hesselberg: Stockholm, Sweden, 1775; Volume 5. [Google Scholar]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alba-Alejandre, I.; Alba-Tercedor, J.; Vega, F.E. Micro-CT to Document the Coffee Bean Weevil, Araecerus fasciculatus (Coleoptera: Anthribidae), Inside Field-Collected Coffee Berries (Coffea canephora). Insects 2018, 9, 100. https://doi.org/10.3390/insects9030100
Alba-Alejandre I, Alba-Tercedor J, Vega FE. Micro-CT to Document the Coffee Bean Weevil, Araecerus fasciculatus (Coleoptera: Anthribidae), Inside Field-Collected Coffee Berries (Coffea canephora). Insects. 2018; 9(3):100. https://doi.org/10.3390/insects9030100
Chicago/Turabian StyleAlba-Alejandre, Ignacio, Javier Alba-Tercedor, and Fernando E. Vega. 2018. "Micro-CT to Document the Coffee Bean Weevil, Araecerus fasciculatus (Coleoptera: Anthribidae), Inside Field-Collected Coffee Berries (Coffea canephora)" Insects 9, no. 3: 100. https://doi.org/10.3390/insects9030100
APA StyleAlba-Alejandre, I., Alba-Tercedor, J., & Vega, F. E. (2018). Micro-CT to Document the Coffee Bean Weevil, Araecerus fasciculatus (Coleoptera: Anthribidae), Inside Field-Collected Coffee Berries (Coffea canephora). Insects, 9(3), 100. https://doi.org/10.3390/insects9030100




