Enlightening Butterfly Conservation Efforts: The Importance of Natural Lighting for Butterfly Behavioral Ecology and Conservation
Abstract
1. Introduction
2. Nature and Extent of Light
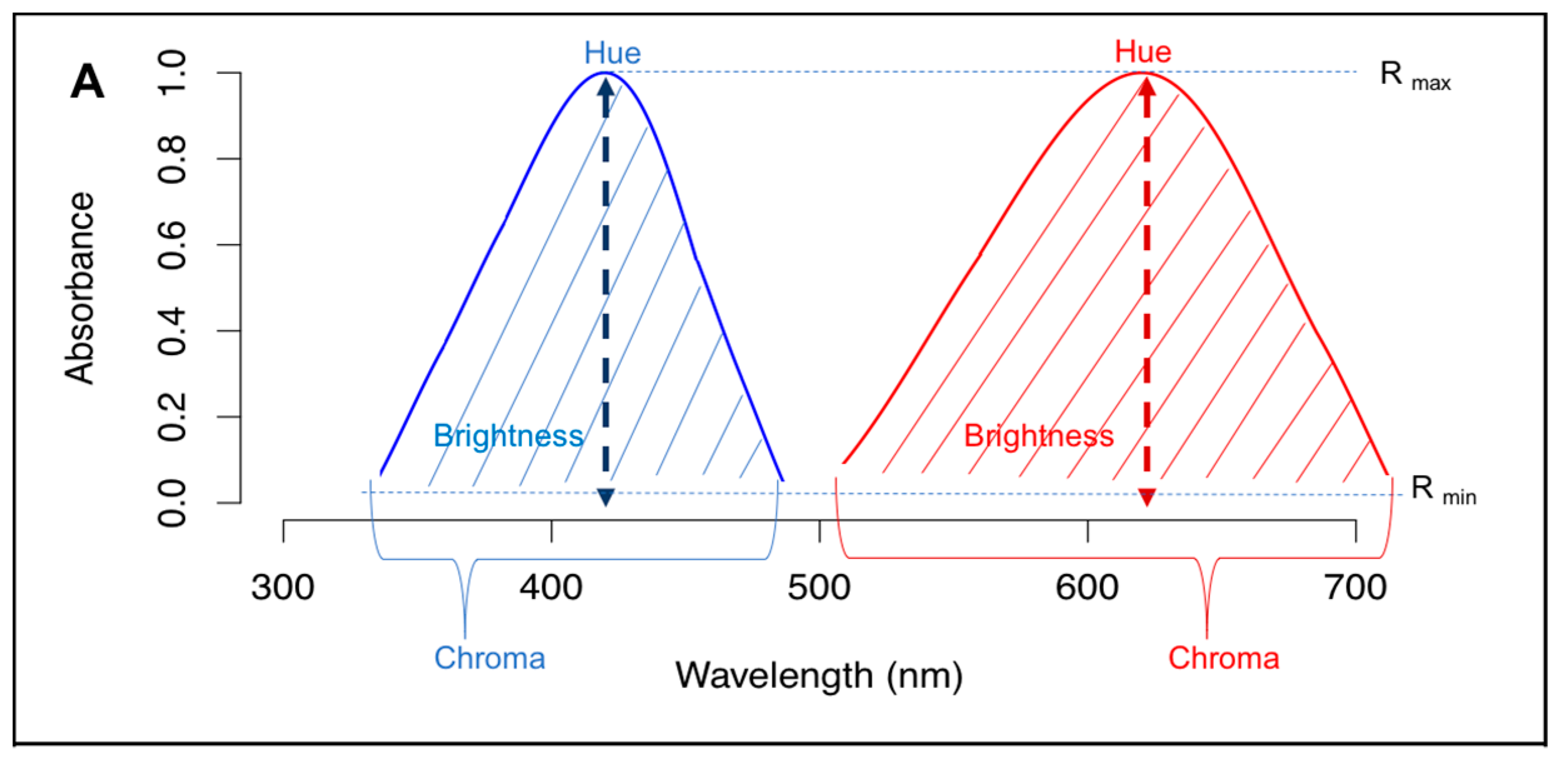
2.1. Physical Parameters of Light

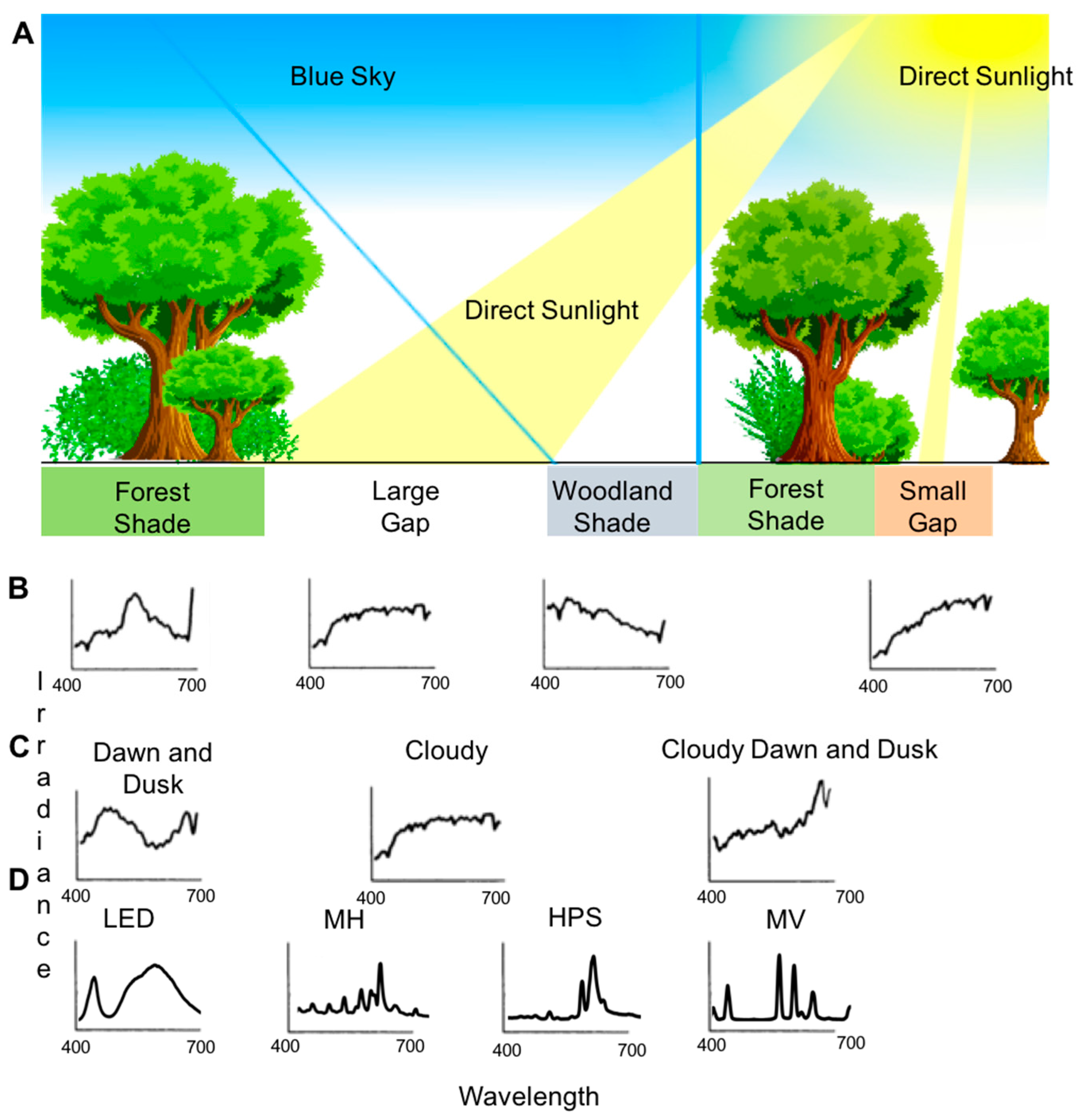
2.2. Natural Light Composition Is a Function of Several Environmental Factors
2.3. Anthropogenic Lighting and the Built Environment Produce Unnatural Light Conditions
2.4. A Problem with Studying Light—The Units
3. Light Reception in Butterflies
3.1. Butterfly Vision
3.2. Non-Visual Light Reception in Butterflies
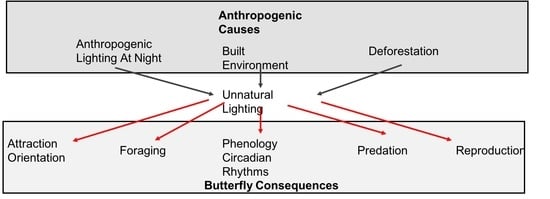
4. Unnatural Lighting Implications for Butterflies
4.1. Vulnerable Biological Functions and Underlying Mechanisms
4.2. Phenology and Circadian Rhythms
4.3. Attraction and Orientation
4.4. Foraging
4.5. Reproduction
4.6. Predation
4.7. Habitat Loss and Alteration
5. Moving Forward
5.1. Quantifying the Threat
5.2. Reducing Ecological Impacts of Unnatural Lighting on Butterflies
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cinzano, P.; Falchi, F.; Elvidge, C.D. The first World Atlas of the artificial night sky brightness. Mon. Not. R. Astron. Soc. 2001, 707, 689–707. [Google Scholar] [CrossRef]
- Falchi, F.; Cinzano, P.; Duriscoe, D.; Kyba, C.C.M.; Elvidge, C.D.; Baugh, K.; Portnov, B.A.; Rybnikova, N.A.; Furgoni, R. The new world atlas of artificial night sky brightness. Sci. Adv. 2016, 2, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Elvidge, C.D.; Keith, D.M.; Tuttle, B.T.; Baugh, K.E. Spectral identification of lighting type and character. Sensors 2010, 10, 3961–3988. [Google Scholar] [CrossRef] [PubMed]
- Elvidge, C.D.; Zhizhin, M.; Hsu, F.C.; Baugh, K.E. VIIRS nightfire: Satellite pyrometry at night. Remote Sens. 2013, 5, 4423–4449. [Google Scholar] [CrossRef]
- Endler, J.A. The color of light in forests and its implications. Ecol. Monogr. 1993, 63, 1–27. [Google Scholar] [CrossRef]
- Gaston, K.J.; Duffy, J.P.; Bennie, J. Quantifying the erosion of natural darkness in the global protected area system. Conserv. Biol. 2015, 29, 1132–1141. [Google Scholar] [CrossRef] [PubMed]
- Bennie, J.; Duffy, J.P.; Davies, T.W.; Correa-Cano, M.E.; Gaston, K.J. Global trends in exposure to light pollution in natural terrestrial ecosystems. Remote Sens. 2015, 7, 2715–2730. [Google Scholar] [CrossRef]
- Primack, R.B. Essentials of Conservation Biology, 6th ed.; Oxford University Press: Oxford, UK, 2014. [Google Scholar]
- Haddad, N.M.; Brudvig, L.A.; Clobert, J.; Davies, K.F.; Gonzalez, A.; Holt, R.D.; Lovejoy, T.E.; Sexton, J.O.; Austin, M.P.; Collins, C.D.; et al. Habitat fragmentation and its lasting impact on Earth’s ecosystems. Sci. Adv. 2015, 1, e1500052. [Google Scholar] [CrossRef] [PubMed]
- Gaston, K.J.; Bennie, J.; Davies, T.W.; Hopkins, J. The ecological impacts of nighttime light pollution: A mechanistic appraisal. Biol. Rev. 2013, 88, 912–927. [Google Scholar] [CrossRef] [PubMed]
- Gaston, K.J.; Gaston, S.; Bennie, J.; Hopkins, J. Benefits and costs of artificial nighttime lighting of the environment. Environ. Rev. 2014, 10, 1–10. [Google Scholar] [CrossRef]
- Gaston, K.J.; Visser, M.E.; Hölker, F. The biological impacts of artificial light at night: The research challenge. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140133. [Google Scholar] [CrossRef] [PubMed]
- Longcore, T.; Rich, C. Ecological Light Pollution; Islnd Press: Washington, DC, USA, 2004. [Google Scholar]
- Hoelker, F.; Wurzbacher, C.; Weissenborn, C.; Monaghan, M.T.; Holzhauer, S.I.J.; Premke, K. Microbial diversity and community respiration in freshwater sediments influenced by artificial light at night. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140130. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, R.G.; Bearhop, S.; Campbell, H.A.; Bryant, D.M. Shedding light on light: Benefits of anthropogenic illumination to a nocturnally foraging shorebird. J. Anim. Ecol. 2013, 82, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Dominoni, D. The effects of light pollution on biological rhythms of birds: An integrated, mechanistic perspective. J. Ornithol. 2015, 156, 409–418. [Google Scholar] [CrossRef]
- Kamrowski, R.L.; Limpus, C.; Pendoley, K.; Hamann, M. Influence of industrial light pollution on the sea- finding behaviour of flatback turtle hatchlings. Wildl. Res. 2014, 41, 421–434. [Google Scholar] [CrossRef]
- Lythgoe, J.N. The Ecology of Vision; Clarendon Press: Oxford, UK, 1979. [Google Scholar]
- Johnsen, S. The Optics of Life; Princeton University Press: Princeton, NJ, USA, 2012. [Google Scholar]
- Land, M.F.; Nilsson, D.E. Animal Eyes, 2nd ed.; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Montgomerie, R. Analyzing colors. In Bird Coloration. Volume 1 Mechanisms and Measurements; Harvard University Press: Cambridge, MA, USA, 2006; pp. 90–147. [Google Scholar]
- Shurcliff, W.A. Polarized Light: Production and Use; Harvard University Press: Cambridge, MA, USA, 1962. [Google Scholar]
- Pye, D. Polarised Light in Science and Nature; IOP Publishing: London, UK, 2001. [Google Scholar]
- Finkbeiner, S.D.; Fishman, D.A.; Osorio, D.; Briscoe, A.D. Ultraviolet and yellow reflectance but not fluorescence is important for visual discrimination of conspecifics by Heliconius erato. J. Exp. Biol. 2017, 220, 1267–1276. [Google Scholar] [CrossRef] [PubMed]
- Dacke, M.; Baird, E.; Byrne, M.; Scholtz, C.H.; Warrant, E.J. Dung beetles use the milky way for orientation. Curr. Biol. 2013, 23, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Bond, D.S.; Henderson, F.P. The Conquest of Darkness. Defense Documentation Center: Alexandria, VA, USA, 1963. [Google Scholar]
- Spitschan, M.; Aguirre, G.K.; Brainard, D.H.; Sweeney, A.M. Variation of outdoor illumination as a function of solar elevation and light pollution. Sci. Rep. 2016, 6, 26756. [Google Scholar] [CrossRef] [PubMed]
- Palmer, G.; Johnsen, S. Downwelling spectral irradiance during evening twilight as a function of the lunar phase. Appl. Opt. 2014, 54, B85. [Google Scholar] [CrossRef] [PubMed]
- Duriscoe, D.M. Photometric indicators of visual night sky quality derived from all-sky brightness maps. J. Quant. Spectrosc. Radiat. Transf. 2016, 1–13. [Google Scholar] [CrossRef]
- Endler, J.A.; Thery, M. Interacting effects of lek placement, Display behavior, Ambient light, and Color patterns in three neotropical forest-dwelling birds. Am. Nat. 1996, 148, 421–452. [Google Scholar] [CrossRef]
- Gaston, K.J.; Duffy, J.P.; Gaston, S.; Bennie, J.; Davies, T.W. Human alteration of natural light cycles: Causes and ecological consequences. Oecologia 2014, 176, 917–931. [Google Scholar] [CrossRef] [PubMed]
- Aube, M. Physical behaviour of anthropogenic light propagation into the nocturnal environment. Philos. Trans. R. Soc. B 2015, 370, 20140117. [Google Scholar] [CrossRef] [PubMed]
- Luginbuhl, C.B.; Boley, P.A.; Davis, D.R. The impact of light source spectral power distribution on sky glow. J. Quant. Spectrosc. Radiat. Transf. 2014, 139, 21–26. [Google Scholar] [CrossRef]
- Kyba, C.C.M.; Tong, K.P.; Bennie, J.; Birriel, I.; Birriel, J.J.; Cool, A.; Danielsen, A.; Davies, T.W.; Outer, P.N.; Edwards, W.; et al. Corrigendum: Worldwide variations in artificial skyglow. Sci. Rep. 2015, 5, 8409. [Google Scholar] [CrossRef] [PubMed]
- Davies, T.W.; Bennie, J.; Inger, R.; de Ibarra, N.H.; Gaston, K.J. Artificial light pollution: Are shifting spectral signatures changing the balance of species interactions? Glob. Chang. Biol. 2013, 19, 1417–1423. [Google Scholar] [CrossRef] [PubMed]
- Longcore, T.; Rich, C.; DelBusso, L. Artificial Night Lighting and Protected Lands Ecological Effects and Management Approaches. In Natural Resource Report NPS/NRSS/NSNS/NRR; National Park Service: Fort Collins, CO, USA, 2016; pp. 1–51. [Google Scholar]
- Horváth, G.; Kriska, G.; Malik, P.; Robertson, B. Polarized light pollution: A new kind of ecological photopollution. Front. Ecol. Environ. 2009, 7, 317–325. [Google Scholar] [CrossRef]
- Szaz, D.; Horvath, G.; Barta, A.; Robertson, B.A.; Farkas, A.; Egri, A.; Tarjanyi, N.; Racz, G.; Kriska, G. Lamp-lit bridges as dual light-traps for the night-swarming mayfly, Ephoron virgo: Interaction of polarized and unpolarized light pollution. PLoS ONE 2015, 10, e012194. [Google Scholar] [CrossRef] [PubMed]
- Horváth, G.; Varjú, D. Why Do Mayflies Lay Eggs on Dry Asphalt Roads? Water-Imitating Horizontally Polarized Light Reflected from Asphalt Attracts Ephemeroptera. Polariz. Light Anim. Vis. 2004, 201, 229–240. [Google Scholar] [CrossRef]
- Rutowski, R.L. Visual Ecology of Adult Butterflies. In Butterflies: Ecology and Evolution Taking Flight; University of Chicago Press: Chicago, IL, USA, 2003; pp. 9–25. [Google Scholar]
- Briscoe, A.D.; Chittka, L. The Evolution of Color Vision in Insects. Annu. Rev. Entomol. 2001, 46, 471–510. [Google Scholar] [CrossRef] [PubMed]
- Warrant, E.J.; Nilsson, D.E. Invertebrate Vision; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Seymoure, B.M.; McMillan, W.O.; Rutowski, R.L. Peripheral eye dimensions in Longwing (Heliconius) butterflies vary with body size and sex but not light environment nor mimicry ring. J. Res. Lepid. 2015, 48, 83–92. [Google Scholar]
- Frederiksen, R.; Warrant, E.J. Visual sensitivity in the crepuscular owl butterfly Caligo memnon and the diurnal blue morpho Morpho peleides: A clue to explain the evolution of nocturnal apposition eyes? J. Exp. Biol. 2008, 211, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Greiner, B.; Ribi, W.A.; Warrant, E.J. Retinal and optical adaptations for nocturnal vision in the halictid bee Megalopta genalis. Cell Tissue Res. 2004, 316, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Warrant, E.J.; Kelber, A.; Wallén, R.; Wcislo, W.T. Ocellar optics in nocturnal and diurnal bees and wasps. Arthropod Struct. Dev. 2006, 35, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Rutowski, R.L. Variation of eye size in butterflies: Inter- and intraspecific patterns. J. Zool. 2000, 252, 187–195. [Google Scholar] [CrossRef]
- Rutowski, R.L.; Gislén, L.; Warrant, E.J. Visual acuity and sensitivity increase allometrically with body size in butterflies. Arthropod Struct. Dev. 2009, 38, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Kelber, A.; Thunell, C.; Arikawa, K. Polarisation-dependent colour vision in Papilio butterflies. J. Exp. Biol. 2001, 204, 2469–2480. [Google Scholar] [PubMed]
- Hammerle, B.; Kolb, G. Retinal ultrastructure of the dorsal eye region of Pararge aegeria (Linne) (Lepidoptera: Satyridae). Int. J. Insect Morphol. Embryol. 1996, 25, 305–315. [Google Scholar] [CrossRef]
- Stavenga, D.G.; Hardie, R.C. Facets of Vision; Springer: Berlin, Germany, 1989; ISBN 9783642740824. [Google Scholar]
- Sweeney, A.; Jiggins, C.; Johnsen, S.; Greene, M.J.; Gordon, D.M. Polarized light as a butterfly mating signal. Nature 2002, 17, 2002–2003. [Google Scholar] [CrossRef] [PubMed]
- Douglas, J.M.; Cronin, T.W.; Chiou, T.-H.; Dominy, N.J. Light habitats and the role of polarized iridescence in the sensory ecology of neotropical nymphalid butterflies (Lepidoptera: Nymphalidae). J. Exp. Biol. 2007, 210, 788–799. [Google Scholar] [CrossRef] [PubMed]
- Reppert, S.M.; Zhu, H.; White, R.H. Polarized Light Helps Monarch Butterflies Navigate. Curr. Biol. 2004, 14, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Heinze, S.; Reppert, S.M. Sun compass integration of skylight cues in migratory monarch butterflies. Neuron 2011, 69, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Zwicky, K.T. A light response in the tail of Urodacus, a scorpion. Life Sci. 1968, 7, 257–262. [Google Scholar] [CrossRef]
- Wilkens, L.A. The crayfish caudal photoreceptor: Advances and questions after the first half century. Comp. Biochem. Physiol. 1988, 91C, 61–68. [Google Scholar] [CrossRef]
- Arikawa, K.; Eguchi, E.; Yoshida, A.; Aoki, K. Multiple extraocular photoreceptive areas on genitalia of butterfly Papilio xuthus. Nature 1980, 288, 700–702. [Google Scholar] [CrossRef]
- Arikawa, K.; Aoki, K. Response characteristics and occurence of extraocular photoreceptors on leipdopteran genitalia. J. Comp. Physiol. A 1982, 148, 483–489. [Google Scholar] [CrossRef]
- Merlin, C.; Gegear, R.J.; Reppert, S.M. Antennal circadian clocks coordinate sun compass orientation in migratory monarch butterflies. Science 2009, 325, 1700–1704. [Google Scholar] [CrossRef] [PubMed]
- Arikawa, K. Valva-Opening Response Induced by the Light Stimulation of the Genital Photoreceptors of Male Butterflies. Naturwissenschaften 1993, 80, 326–328. [Google Scholar] [CrossRef]
- Arikawa, K.; Takagi, N. Genital Photoreceptors Have Crucial Role in Oviposition in Japanese Yellow Swallowtail Butterfly, Papilio xuthus. Zool. Sci. 2001, 18, 175–179. [Google Scholar] [CrossRef]
- Arikawa, K. Hindsight of Butterflies. Bioscience 2001, 51, 219–225. [Google Scholar] [CrossRef]
- Zhu, H.; Sauman, I.; Yuan, Q.; Casselman, A.; Emery-Le, M.; Emery, P.; Reppert, S.M. Cryptochromes define a novel circadian clock mechanism in monarch butterflies that may underlie sun compass navigation. PLoS Biol. 2008, 6, 0138–0155. [Google Scholar] [CrossRef] [PubMed]
- Francis, C.D.; Barber, J.R. A framework for understanding noise impacts on wildlife: An urgent conservation priority. Front. Ecol. Environ. 2013, 11, 305–313. [Google Scholar] [CrossRef]
- Swaddle, J.P.; Francis, C.; Barber, J.R.; Cooper, C.B.; Kyba, C.C.M.; Dominoni, D.M.; Shannon, G.; Aschehoug, E.; Goodwin, S.E.; Kawahara, A.Y.; et al. A framework to asses evolutionary responses to anthropogenic light and sound. Trends Ecol. Evol. 2016, 30, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Shannon, G.; Mckenna, M.F.; Angeloni, L.M.; Crooks, K.R.; Fristrup, K.M.; Brown, E.; Warner, K.A.; Nelson, M.D.; White, C.; Briggs, J.; et al. A synthesis of two decades of research documenting the effects of noise on wildlife. Biol. Rev. 2015, 24. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, A.Y.; Plotkin, D.; Hamilton, C.; Gough, H.; St Laurent, R.; Owens, H.; Homziak, N.T.; Barber, J.R. Diel behavior in moths and butterflies: A synthesis of data illuminates the evolution of temporal activity. Org. Divers. Evol. 2018. [Google Scholar] [CrossRef]
- Davies, T.W.; Duffy, J.P.; Bennie, J.; Gaston, K.J. The nature, extent, and ecological implications of marine light pollution. Front. Ecol. Environ. 2014, 12, 347–355. [Google Scholar] [CrossRef]
- Gaston, K.J.; Davies, T.W.; Nedelec, S.L.; Holt, L.A. Impacts of Artificial Light at Night on Biological Timings. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 49–68. [Google Scholar] [CrossRef]
- Davies, T.W.; Smyth, T. Why artificial light at night should be a focus for global change research in the 21st century. Glob. Chang. Biol. 2017, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Delhey, K.; Peters, A. Conservation implications of anthropogenic impacts on visual communication and camouflage. Conserv. Biol. 2017, 31, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, M.; Shimada, N.; Arikawa, K. Colour vision of the foraging swallowtail butterfly Papilio xuthus. J. Exp. Biol. 1999, 202, 95–102. [Google Scholar] [PubMed]
- Koshitaka, H.; Arikawa, K.; Kinoshita, M. Intensity contrast as a crucial cue for butterfly landing. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2011, 197, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Cory, J.S.; Goulson, D. Flower constancy and learning in foraging preferences of the green veined butterfly Pieris napi. Ecol. Entomol. 1993, 18, 315–320. [Google Scholar]
- Van Asch, M.; Visser, M.E. Phenology of Forest Caterpillars and Their Host Trees: The Importance of Synchrony. Annu. Rev. Entomol. 2007, 52, 37–55. [Google Scholar] [CrossRef] [PubMed]
- Aide, T.M.; Londoño, E.C. The Effects of Rapid Leaf Expansion on the Growth and Suvivorship of a Lepidopteran Herbivore. Oikos 1989, 55, 66–70. [Google Scholar] [CrossRef]
- Singer, M.C.; Parmesan, C. Phenological asynchrony between herbivorous insects and their hosts: Signal of climate change or pre-existing adaptive strategy? Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 3161–3176. [Google Scholar] [CrossRef] [PubMed]
- Posledovich, D.; Toftegaard, T.; Wiklund, C.; Ehrlén, J.; Gotthard, K. The developmental race between maturing host plants and their butterfly herbivore—The influence of phenological matching and temperature. J. Anim. Ecol. 2015, 84, 1690–1699. [Google Scholar] [CrossRef] [PubMed]
- Sandre, S.-L.; Stevens, M.; Mappes, J. The effect of predator appetite, prey warning coloration and luminance on predator foraging decisions. Behaviour 2010, 147, 1121–1143. [Google Scholar] [CrossRef]
- Olofsson, M.; Vallin, A.; Jakobsson, S.; Wiklund, C. Marginal eyespots on butterfly wings deflect bird attacks under low light intensities with UV wavelengths. PLoS ONE 2010, 5. [Google Scholar] [CrossRef] [PubMed]
- Pegram, K.V.; Han, H.A.; Rutowski, R.L. Overnight perching aggregations of the aposematic Pipevine Swallowtail (Battus philenor: Lepidoptera: Papilionidae): Implications for predation risk and warning signal use. J. Res. Lepid. 2012, 45, 9–16. [Google Scholar]
- Douglas, J.M. Ambient Light Environment and the Evolution of Brigthness, Chroma, and Perceived Chromaticity in the Warning Signals of Butterflies; Arizona State University: Tempe, AZ, USA, 2013. [Google Scholar]
- Bergman, M.; Gotthard, K.; Berger, D.; Olofsson, M.; Kemp, D.J.; Wiklund, C. Mating success of resident versus non-resident males in a territorial butterfly. Proc. R. Soc. B Biol. Sci. 2007, 274, 1659–1665. [Google Scholar] [CrossRef] [PubMed]
- Bergman, M.; Lessios, N.; Seymoure, B.M.; Rutowski, R.L. Mate detection in a territorial butterfly—The effect of background and luminance contrast. Behav. Ecol. 2015, 26, 851–860. [Google Scholar] [CrossRef]
- Gilbert, L.E. Ecological consequences of a coevolved mutualism between butterflies and plants. In Coevolution of Animals and Plants; Gilbert, L.E., Raven, P., Eds.; University of Texas Pres: Austin, TX, USA, 1975; pp. 210–240. [Google Scholar]
- Rutowski, R.L.; Rajyaguru, P.K. Male-specific Iridescent Coloration in the Pipevine Swallowtail (Battus philenor) is Used in Mate Choice by Females but not Sexual Discrimination by Males. J. Insect Behav. 2012. [Google Scholar] [CrossRef]
- White, T.E.; Zeil, J.; Kemp, D.J. Signal design and courtship presentation coincide for highly biased delivery of an iridescent butterfly mating signal. Evolution 2015, 69, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Soren, R. Light attracted butterflies: A review from the Indian sub-region with an inventory from West Bengal, India. J. Threat. Taxa 2011, 3, 1868–1871. [Google Scholar] [CrossRef]
- Kolligs, D. Ecological effects of artificial light sources on nocturnally active insects, in particular on butterflies (Lepidoptera). Faunist. Oekol. Mitt. Suppl. 2000, 28, 1–136. [Google Scholar]
- Beshkov, S. Butterflies and day-flying moths in light traps (Lepidoptera). Phegea 1998, 3, 118–120. [Google Scholar]
- Minnaar, C.; Boyles, J.G.; Minnaar, I.A.; Sole, C.L.; Mckechnie, A.E. Stacking the odds: Light pollution may shift the balance in an ancient predator-prey arms race. J. Appl. Ecol. 2015, 52, 522–531. [Google Scholar] [CrossRef]
- Wakefield, A.; Stone, E.L.; Jones, G.; Harris, S. Light-emitting diode street lights reduce last-ditch evasive manoeuvres by moths to bat echolocation. R. Soc. Open Sci. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Macgregor, C.J.; Pocock, M.J.O.; Fox, R.; Evans, D.M. Pollination by nocturnal Lepidoptera, and the effects of light pollution: A review. Ecol. Entomol. 2015, 40, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Seymoure, B.M. Heliconius in a New Light: The Effects of Light Environments on Mimetic Coloration, Behavior, and Visual Systems; Arizona State University: Tempe, AZ, USA, 2016. [Google Scholar]
- Bergman, M.; Wiklund, C. Visual mate detection and mate flight pursuit in relation to sunspot size in a woodland territorial butterfly. Anim. Behav. 2009, 78, 17–23. [Google Scholar] [CrossRef]
- Wu, S.; Refinetti, R.; Kok, L.T.; Youngman, R.R.; Reddy, V.P.; Xue, F.; Wu, S.; Refinetti, R.; Kok, L.T.; Youngman, R.R. Photoperiod and Temperature Effects on the Adult Eclosion and Mating Rhythms in Pseudopidorus fasciata (Lepidoptera: Zygaenidae). Environ. Entomol. 2014, 43, 1650–1655. [Google Scholar] [CrossRef] [PubMed]
- Sencio, K. Daily Eclosion Patterns in Nymphalid Butterflies and Their Causes, Ph.D. Thesis, Arizona State University, Tempe, AZ, USA, 2017. [Google Scholar]
- Sauman, I.; Briscoe, A.D.; Zhu, H.; Shi, D.; Froy, O.; Stalleicken, J.; Yuan, Q.; Casselman, A.; Reppert, S.M. Connecting the navigational clock to sun compass input in monarch butterfly brain. Neuron 2005, 46, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Grenis, K.; Tjossem, B.; Murphy, S.M. Predation of larval Lepidoptera in habitat fragments varies spatially and temporally but is not affected by light pollution. J. Insect Conserv. 2015, 19, 559–566. [Google Scholar] [CrossRef]
- Gotthard, K. Increased risk of predation as a cost of high growth rate: An experimental test in a butterfly. J. Anim. Ecol. 2000, 69, 896–902. [Google Scholar] [CrossRef] [PubMed]
- Devries, P.J. The Butterflies of Costa Rica and Their Natural History, Volume I: Papilionidae, Pieridae, Nymphalidae; Princeton University Press: Princeton, NJ, USA, 1987. [Google Scholar]
- Takeuchi, T. Mate-locating behavior of the butterfly Lethe diana (Lepidoptera: Satyridae): Do males diurnally or seasonally change their mating strategy? Zool. Sci. 2010, 27, 821–825. [Google Scholar] [CrossRef] [PubMed]
- Niesenbaum, R.A.; Kluger, E.C. When studying the effects of light on herbivory, should one consider temperature? The case of Epimecis hortaria F. (Lepidoptera: Geometridae) feeding on Lindera benzoin L. (Lauraceae). Environ. Entomol. 2006, 35, 600–606. [Google Scholar] [CrossRef]
- Bennie, J.; Davies, T.W.; Cruse, D.; Inger, R.; Gaston, K.J. Cascading effects of artificial light at night: Resource-mediated control of herbivores in a grassland ecosystem. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140131. [Google Scholar] [CrossRef] [PubMed]
- Longcore, T. Sensory ecology: Night lights alter reproductive behavior of blue tits. Curr. Biol. 2010, 20, R893–R895. [Google Scholar] [CrossRef] [PubMed]
- Dominoni, D.M.; Partecke, J. Does light pollution alter daylength? A test using light loggers on free-ranging European blackbirds (Turdus merula). Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2015, 370. [Google Scholar] [CrossRef] [PubMed]
- Arikawa, K.; Uchiumi, K.; Eguchi, E. Extraocular photoreceptors in the last abdominal ganglion of a swallowtail butterfly, Papilio xuthus. Naturwissenschaften 1991, 78, 82–84. [Google Scholar] [CrossRef]
- Saunders, D.S.; Lewis, R.D.; Warman, G.R. Photoperiodic induction of diapause: Opening the black box. Physiol. Entomol. 2004, 29, 1–15. [Google Scholar] [CrossRef]
- Paranjpe, D.A.; Sharma, V.K. Evolution of temporal order in living organisms. J. Circ. Rhythm. 2005, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, P.M.; Turner, J.R.G.; Brown, K.S.; Benson, W.W.; Singer, M.S. Genetics and the Evolution of Muellerian Mimicry in Heliconius Butterflies. Philos. Trans. R. Soc. B. Biol. Sci. 1985, 308, 433–610. [Google Scholar] [CrossRef]
- Russell, F.L.; Louda, S.M. Phenological synchrony affects interaction strength of an exotic weevil with Platte thistle, a native host plant. Oecologia 2004, 139, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Forkner, R.E.; Marquis, R.J.; Lill, J.T.; Corff, J. Le Timing is everything? Phenological synchrony and population variability in leaf-chewing herbivores of Quercus. Ecol. Entomol. 2008, 33, 276–285. [Google Scholar] [CrossRef]
- Rafferty, N.E.; Caradonna, P.J.; Burkle, L.A.; Iler, A.M.; Bronstein, J.L. Phenological overlap of interacting species in a changing climate: An assessment of available approaches. Ecol. Evol. 2013, 3, 3183–3193. [Google Scholar] [CrossRef] [PubMed]
- Richard, H.; Somers-yeates, R.; Bennie, J.; Economou, T.; Hodgson, D.; Spalding, A.; Mcgregor, P.K.; Bennie, J.; Economou, T.; Hodgson, D.; et al. Light pollution is associated with earlier tree budburst across the United Kingdom. Proc. R. Soc. B 2016. [Google Scholar] [CrossRef]
- Gotthard, K.; Nylin, S.; Wiklund, C. Seasonal plasticity in two satyrine butterflies: State-dependent decision making in relation to daylength. Oikos 1999, 84, 453–462. [Google Scholar] [CrossRef]
- Niepoth, N.; Ke, G.; de Roode, J.C.; Groot, A.T. Comparing Behavior and Clock Gene Expression between Caterpillars, Butterflies, and Moths. J. Biol. Rhythms 2017. [Google Scholar] [CrossRef] [PubMed]
- Kyba, C.C.M.; Kuester, T.; Sánchez De Miguel, A.; Baugh, K.; Jechow, A.; Hölker, F.; Bennie, J.; Elvidge, C.D.; Gaston, K.J.; Guanter, L. Artificially lit surface of Earth at night increasing in radiance and extent. Sci. Adv. 2017, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tomioka, K.; Matsumoto, A. Circadian molecular clockworks in non-model insects. Curr. Opin. Insect Sci. 2015, 7, 58–64. [Google Scholar] [CrossRef]
- Danks, H.V. How similar are daily and seasonal biological clocks? J. Insect Physiol. 2005, 51, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.A.; Matter, S.F.; Roland, J. Edge avoidance and movement of the butterfly Parnassius smintheus in matrix and non-matrix habitat. Landsc. Ecol. 2005, 20, 127–135. [Google Scholar] [CrossRef]
- Dover, J.; Settele, J. The influences of landscape structure on butterfly distribution and movement: A review. J. Insect Conserv. 2009, 13, 3–27. [Google Scholar] [CrossRef]
- Papageorgis, C. Mimicry in Neotropical Butterflies: Why are there so many different wing-coloration complexes in one place? Am. Sci. 1975, 63, 522–532. [Google Scholar] [CrossRef]
- Mallet, J.; Gilbert, L.E. Why are there so many mimicry rings? Correlations between habitat, behaviour and mimicry in Heliconius butterflies. Biol. J. Linn. Soc. 1995, 55, 159–180. [Google Scholar] [CrossRef]
- Ockinger, E.; Dyck, H. Van Landscape Structure Shapes Habitat Finding Ability in a Butterfly. PLoS ONE 2012, 7, e41517. [Google Scholar] [CrossRef] [PubMed]
- Turlure, C.; Schtickzelle, N.; Van Dyck, H.; Seymoure, B.; Rutowski, R. Flight Morphology, Compound Eye Structure and Dispersal in the Bog and the Cranberry Fritillary Butterflies: An Inter- and Intraspecific Comparison. PLoS ONE 2016, 11, e0158073. [Google Scholar] [CrossRef] [PubMed]
- Luck, G.W.; Daily, G.C.; Ehrlich, P.R. Population diversity and ecosystem services. Trends Ecol. Evol. 2003, 18, 331–336. [Google Scholar] [CrossRef]
- Rutowski, R.L.; Macedonia, J.M.; Merry, J.W.; Morehouse, N.I.; Yturralde, K.; Taylor-Taft, L.; Gaalema, D.; Kemp, D.J.; Papke, R.S. Iridescent ultraviolet signal in the orange sulphur butterfly (Colias eurytheme): Spatial, temporal and spectral properties. Biol. J. Linn. Soc. 2007, 90, 349–364. [Google Scholar] [CrossRef]
- Rutowski, R.L.; Nahm, A.C.; Macedonia, J.M. Iridescent hindwing patches in the Pipevine Swallowtail: Differences in dorsal and ventral surfaces relate to signal function and context. Funct. Ecol. 2010. [Google Scholar] [CrossRef]
- Stevenson, D.E.; Harris, M.K. Determining circadian response of adult male Acrobasis nuxvorella (Lepidoptera: Pyralidae) to synthetic sex attractant pheromone through time-segregated trapping with a new clockwork timing trap. Environ. Entomol. 2009, 38, 1690–1696. [Google Scholar] [CrossRef] [PubMed]
- Ide, J.Y. Weather factors affecting the male mate-locating tactics of the small copper butterfly (Lepidoptera: Lycaenidae). Eur. J. Entomol. 2010, 107, 369–376. [Google Scholar] [CrossRef]
- Kinoshita, M.; Yamazato, K.; Arikawa, K. Polarization-based brightness discrimination in the foraging butterfly, Papilio xuthus. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Cott, H.B. Adaptive Coloration in Animals; Methuen and Co: London, UK, 1940. [Google Scholar]
- Edmunds, M. Defence in Animals; Prentice Hall Press: Upper Saddle River, NJ, USA, 1974. [Google Scholar]
- Ruxton, G.D.; Sherratt, T.N.; Speed, M.P. Avoiding Attack: The Evolutionary Ecology of Crypsis, Warning Signals & Mimicry; Oxford University Press: Oxford, UK, 2004. [Google Scholar]
- Bohlin, T.; Tullberg, B.S.; Merilaita, S. The effect of signal appearance and distance on detection risk in an aposematic butterfly larva (Parnassius apollo). Anim. Behav. 2008, 76, 577–584. [Google Scholar] [CrossRef]
- Seymoure, B.M.; Aiello, A. Keeping the band together: Evidence for false boundary disruptive coloration in a butterfly. J. Evol. Biol. 2015, 28, 1618–1624. [Google Scholar] [CrossRef] [PubMed]
- Seymoure, B.M.; Raymundo, A.; McGraw, K.J.; Owen McMillan, W.; Rutowski, R.L. Environment-dependent attack rates of cryptic and aposematic butterflies. Curr. Zool. 2017, 1–7. [Google Scholar] [CrossRef]
- Brower, L.P. Ecological chemistry. Sci. Am. 1969, 220, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, C.E.G. Palatability and escaping ability in neotropical butterflies: Tests with wild kingbirds (Tyrannus melancholicus, Tyrannidae). Biol. J. Linn. Soc. 1996, 59, 351–365. [Google Scholar] [CrossRef]
- Chai, P. Field observations and feeding experiments on the responses of rufous-tailed jacamars (Galbula ruficauda) to free-flying butterflies in a tropical rainforest. Biol. J. Linn. Soc. 1986, 29, 161–189. [Google Scholar] [CrossRef]
- Thurman, T.; Seymoure, B.M. A Bird’s Eye View of Two Mimetic Tropical Butterflies: Coloration Matches Predator’s Sensitivity. J. Zool. 2016, 298, 159–168. [Google Scholar] [CrossRef]
- Kodandaramaiah, U. The evolutionary significance of butterfly eyespots. Behav. Ecol. 2011, 22, 1264–1271. [Google Scholar] [CrossRef]
- Olofsson, M.; Jakobsson, S.; Wiklund, C. Bird attacks on a butterfly with marginal eyespots and the role of prey concealment against the background. Biol. J. Linn. Soc. 2013, 109, 290–297. [Google Scholar] [CrossRef]
- Merilaita, S.; Vallin, A.; Kodandaramaiah, U.; Dimitrova, M.; Ruuskanen, S.; Laaksonen, T. Number of eyespots and their intimidating effect on naive predators in the peacock butterfly. Behav. Ecol. 2011, 22, 1326–1331. [Google Scholar] [CrossRef]
- Nylin, S.; Wickman, P.-O.; Wiklund, C. Seasonal plasticity in growth and development of the speckled wood butterfly, Pararge aegeria (Satyrinae). Biol. J. Linn. Soc. 1989, 38, 155–171. [Google Scholar] [CrossRef]
- Letallec, T.; Théry, M.; Perret, M. Effects of light pollution on seasonal estrus and daily rhythms in a nocturnal primate. J. Mammal. 2015, 96, 438–445. [Google Scholar] [CrossRef]
- Bell, R.A.; Rasul, C.G.; Joachim, F.G. Photoperiodic induction of the pupal diapause in the tobacco hornworm, Manduca sexta. J. Insect Physiol. 1975, 21, 1471–1480. [Google Scholar] [CrossRef]
- van Langevelde, F.; Ettema, J.A.; Donners, M.; WallisDeVries, M.F.; Groenendijk, D. Effect of spectral composition of artificial light on the attraction of moths. Biol. Conserv. 2011, 144, 2274–2281. [Google Scholar] [CrossRef]
- Somers-Yeates, R.; Hodgson, D.; McGregor, P.K.; Spalding, A.; Ffrench-Constant, R.H. Shedding light on moths: Shorter wavelengths attract noctuids more than geometrids. Biol. Lett. 2013, 9. [Google Scholar] [CrossRef] [PubMed]
- Lacoeuilhe, A.; Machon, N.; Julien, J.F.; Le Bocq, A.; Kerbiriou, C. The influence of low intensities of light pollution on bat communities in a semi-natural context. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Stone, E.L.; Wakefield, A.; Harris, S.; Jones, G.; Stone, E.L. The impacts of new street light technologies: Experimentally testing the effects on bats of changing from low-pressure sodium to white metal halide. Philos. Trans. R. Soc. B 2015, 370, 20140127. [Google Scholar] [CrossRef] [PubMed]
- Schoeman, M.C. Light pollution at stadiums favors urban exploiter bats. Anim. Conserv. 2016, 19, 120–130. [Google Scholar] [CrossRef]
- Brooks, T.M.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Rylands, A.B.; Konstant, W.R.; Flick, P.; Pilgrim, J.; Oldfield, S.; Magin, G.; et al. Society for Conservation Biology Habitat Loss and Extinction in the Hotspots of Biodiversity Habitat Loss and Extinction in the Hotspots of Biodiversity. Source Conserv. Biol. 2002, 16, 909–923. [Google Scholar] [CrossRef]
- Crooks, K.R.; Burdett, C.L.; Theobald, D.M.; King, S.R.B.; Di, M.; Rondinini, C. Quantification of habitat fragmentation reveals extinction risk in terrestrial mammals. Proc. Natl. Acad. Sci. USA 2017, 114, 7635–7640. [Google Scholar] [CrossRef] [PubMed]
- Heinrichs, J.A.; Bender, D.J.; Schumaker, N.H. Habitat degradation and loss as key drivers of regional population extinction. Ecol. Model. 2016, 335, 64–73. [Google Scholar] [CrossRef]
- Hambler, C.; Henderson, P.A.; Speight, M.R. Extinction rates, extinction-prone habitats, and indicator groups in Britain and at larger scales. Biol. Conserv. 2011, 144, 713–721. [Google Scholar] [CrossRef]
- Krauss, J.; Bommarco, R.; Guardiola, M.; Heikkinen, R.K.; Helm, A.; Kuussaari, M.; Lindborg, R.; Öckinger, E.; Pärtel, M.; Pino, J.; et al. Habitat fragmentation causes immediate and time-delayed biodiversity loss at different trophic levels. Ecol. Lett. 2010, 13, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.A.; Simcox, D.J.; Clarke, R.T. Successful Conservation of a Threatened Maculinea Butterfly. Science 2009, 325, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Endler, J.A. Light, behavior, and conservation of forest-dwelling organisms. In Behavioral Approaches to Conservation in the Wild; Cambridge University Press: Cambridge, UK, 1997; pp. 329–356. [Google Scholar]
- Leal, M.; Fleishman, L.J. Evidence for habitat partitioning based on adaptation to environmental light in a pair of sympatric lizard species. Proc. Biol. Sci. 2001, 269, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Hutton, P.; Ligon, R.A.; McGraw, K.J.; Seymoure, B.M.; Simpson, R.K. Dynamic color communication. Curr. Opin. Behav. Sci. 2015, 6, 41–49. [Google Scholar] [CrossRef]
- Cronin, T.W.; Shashar, N.; Caldwell, R.L.; Marshall, J.; Cheroske, A.G.; Chiou, T.-H. Polarization vision and its role in biological signaling. Integr. Comp. Biol. 2003, 43, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Cronin, T.W.; Johnsen, S.; Marshall, N.J.; Warrant, E.J. Visual Ecology; Princeton University Press: Princeton, NJ, USA, 2014. [Google Scholar]
- Kricher, J. Tropical Ecology; Princeton University Press: Princeton, NJ, USA, 2011. [Google Scholar]
- Gaston, K.J.; Davies, T.W.; Bennie, J.; Hopkins, J. Reducing the ecological consequences of night-time light pollution: Options and developments. J. Appl. Ecol. 2012, 49, 1256–1266. [Google Scholar] [CrossRef] [PubMed]
- Falchi, F.; Cinzano, P.; Elvidge, C.D.; Keith, D.M.; Haim, A. Limiting the impact of light pollution on human health, environment and stellar visibility. J. Environ. Manag. 2011, 92, 2714–2722. [Google Scholar] [CrossRef] [PubMed]
- Witherington, B.E.; Martin, R.E. Understanding, Assessing, and Resolving Light-Pollution Problems on Sea Turtle Nesting Beaches. Chelonian Conserv. Biol. 2000, 2, 463. [Google Scholar]
- Bogard, P. The End of Night: Searching for Natural Darkness in an Age of Artificial Light; Back Bay Books: New York, NY, USA, 2013. [Google Scholar]


| Mechanism | Attraction and Orientation | Foraging | Phenology and Circadian Rhythms | Predation | Reproduction |
|---|---|---|---|---|---|
| Masking | The built environment could mask polarized light cues that Monarch butterflies use for migration [37,54,55]. | Butterflies rely upon visual cues to identify nectar resources and hostplants. Altering light environments will change these visual cues as they rely upon ambient illumination [73,74,75]. | Butterflies rely upon the natural light regimes of their habitats for the timing of daily and seasonal activity patterns. Through habitat destruction and anthropogenic lighting, these regimes are masked with unnatural light conditions [76,77,78,79]. | Butterflies rely on visual defenses such as deimatic, warning, and cryptic coloration. Through altered light environments, these signals are altered and can increase predation risk [80,81,82,83]. | Sexual signals have evolved under specific light conditions and unnatural lighting will mask the visual signal between males and females [84,85,86,87,88]. |
| Distraction | Anthropogenic lights attract, and thus distract, butterflies from normal nocturnal behaviors [89,90,91]. | As butterflies are distracted and attracted to anthropogenic lighting, they are more vulnerable to predation [92,93,94]. | |||
| Misleading | Altering habitat structure through deforestation and anthropogenic lighting at night changes light environments that mislead butterfly orientation [55,84,85,91,95,96] | As with masking, altering the light environment will change the perceived visual cues of nectar sources and hostplants, which could mislead butterflies into attempting to forage upon the wrong species of plant [73,74,75]. | Butterflies use day length as an environmental cue for timing of pupation, eclosion, migration, and diapause. Anthropogenic lighting is increasing day length, which is likely misleading butterflies on when to pupate, eclose, migrate, and begin diapause [76,77,78,79,97,98,99]. | Butterflies rely on light environments as a cue for correct habitat and unnatural light environments could mislead butterflies into occupying habitats where survival is decreased due to predation [95,100]. Also, butterflies use natural light regimes for development and when butterflies increase developmental rate due to heightened light levels, predation increases [101]. | Butterflies rely on visual cues for courtship and mate detection. Through unnatural lighting and the built environment, both color and polarized light signals could become misleading and butterflies may be courting inappropriate objects [37,52,53]. |
| Temporal Niche | Anthropogenic lighting at night is likely to extend the butterfly activity into dawn and dusk and thus butterflies could be feeding earlier and later in the day [102,103,104,105]. | Both butterfly and predator daily temporal patterns are increased by anthropogenic lighting. This increased behavior by butterflies makes them more vulnerable to novel predators (e.g., bats) and their natural predators are also able to hunt earlier and later in the day, increasing predation [106,107] | Butterflies have genital photoreceptors that enable copulation and thus anthropogenic lighting could increase the available time that butterflies are able to copulate [58,59,61,108]. |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seymoure, B.M. Enlightening Butterfly Conservation Efforts: The Importance of Natural Lighting for Butterfly Behavioral Ecology and Conservation. Insects 2018, 9, 22. https://doi.org/10.3390/insects9010022
Seymoure BM. Enlightening Butterfly Conservation Efforts: The Importance of Natural Lighting for Butterfly Behavioral Ecology and Conservation. Insects. 2018; 9(1):22. https://doi.org/10.3390/insects9010022
Chicago/Turabian StyleSeymoure, Brett M. 2018. "Enlightening Butterfly Conservation Efforts: The Importance of Natural Lighting for Butterfly Behavioral Ecology and Conservation" Insects 9, no. 1: 22. https://doi.org/10.3390/insects9010022
APA StyleSeymoure, B. M. (2018). Enlightening Butterfly Conservation Efforts: The Importance of Natural Lighting for Butterfly Behavioral Ecology and Conservation. Insects, 9(1), 22. https://doi.org/10.3390/insects9010022