Interactions among the Predatory Midge Aphidoletes aphidimyza (Diptera: Cecidomyiidae), the Fungal Pathogen Metarhizium brunneum (Ascomycota: Hypocreales), and Maize-Infesting Aphids in Greenhouse Mesocosms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Source and Maintenance of Insects
2.2. Source and Preparation of the Microbial Inoculum
2.3. Soil, Plant Material and Cages
2.4. Experimental Design
2.5. Presence of the Microbial Inoculum
2.6. Molecular Characterization Metarhizium Isolate
2.7. Data Analysis
3. Results
3.1. Presence of The Microbial Inoculum
3.2. Impact of M. brunneum on A. aphidimyza
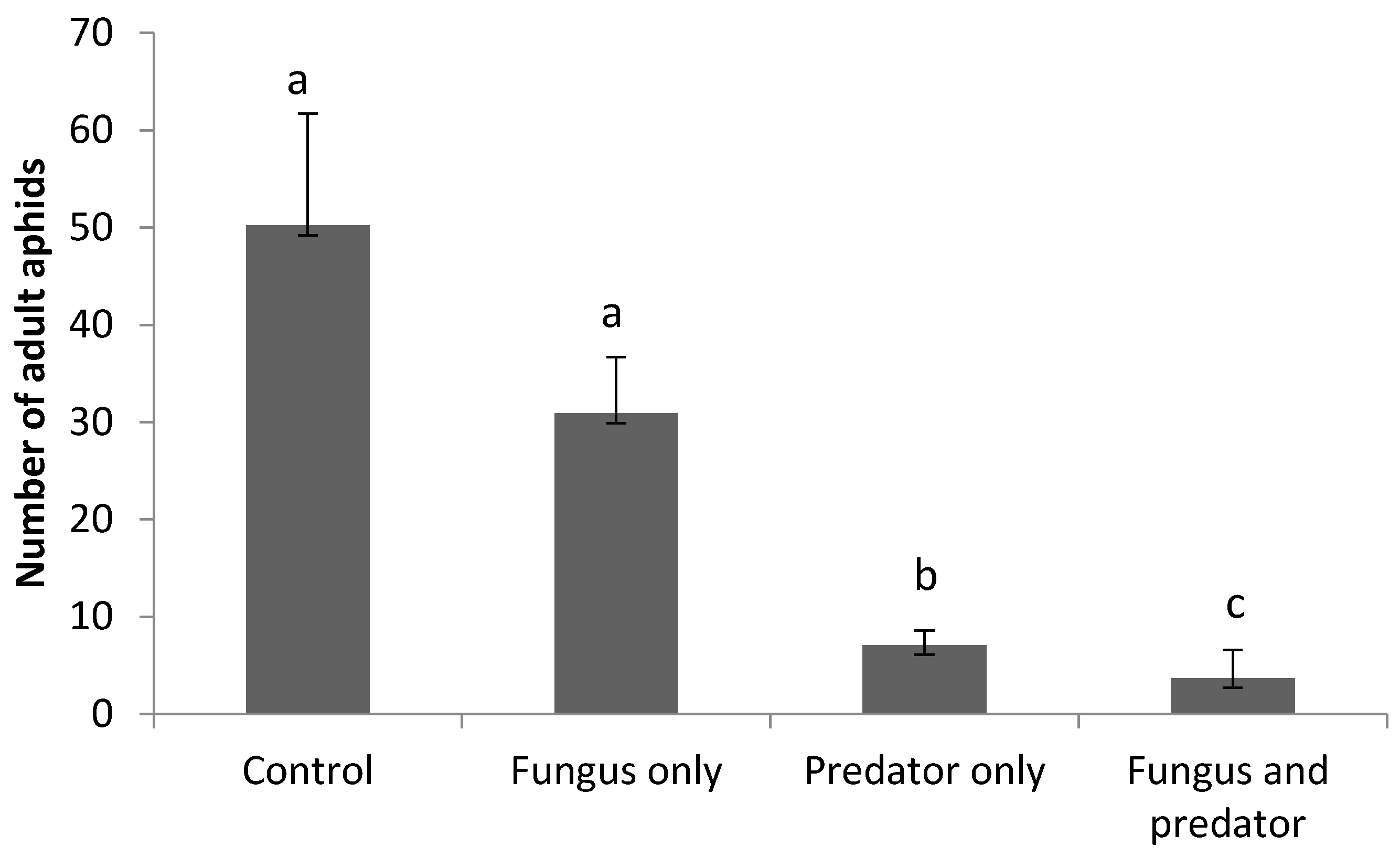
3.3. Impact of A. aphidimyza and M. brunneum on R. padi
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Smith, C.M.; Chuang, W.P. Plant resistance to aphid feeding: Behavioral, physiological, genetic and molecular cues regulate aphid host selection and feeding. Pest Manag. Sci. 2014, 70, 528–540. [Google Scholar] [CrossRef] [PubMed]
- Van Veen, F.J.F.; Müller, C.B.; Pell, J.K.; Godfray, H.C.J. Food web structure of three guilds of natural enemies: Predators, parasitoids and pathogens of aphids. J. Anim. Ecol. 2008, 77, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Sigsgaard, L. A survey of aphids and aphid parasitoids in cereal fields in Denmark, and the parasitoids’ role in biological control. J. Appl. Entomol. 2002, 126, 101–107. [Google Scholar] [CrossRef]
- Shang, L.T.; Feng, M.G. Evaluation of the biocontrol potential of various Metarhizium isolates against green peach aphid Myzus persicae (Homoptera: Aphididae). Pest Manag. Sci. 2010, 66, 669–675. [Google Scholar]
- Akmal, M.; Freed, S.; Malik, M.N.; Gul, H.T. Efficacy of Beauveria bassiana (Deuteromycotina: Hypomycetes) against different aphid species under laboratory conditions. Pak. J. Zool. 2013, 45, 71–78. [Google Scholar]
- Jandricic, S.E.; Filotas, M.; Sanderson, J.P.; Wraight, S.P. Pathogenicity of conidia-based preparations of entomopathogenic fungi against the greenhouse pest aphids Myzus persicae, Aphis gossypii, and Aulacorthum solani (Hemiptera: Aphididae). J. Invertebr. Pathol. 2014, 118, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Pilz, C.; Wegensteiner, R.; Keller, S. Natural occurrence of insect pathogenic fungi and insect parasitic nematodes in Diabrotica virgifera virgifera populations. Biocontrol 2008, 53, 353–359. [Google Scholar] [CrossRef]
- Pilz, C.; Enkerli, J.; Wegensteiner, R.; Keller, S. Establishment and persistence of the entomopathogenic fungus Metarhizium anisopliae in maize fields. J. Appl. Entomol. 2011, 135, 393–403. [Google Scholar] [CrossRef]
- Eckard, S.; Ansari, M.A.; Bacher, S.; Butt, T.M.; Enkerli, J.; Grabenweger, G. Virulence of in vivo and in vitro produced conidia of Metarhizium brunneum strains for control of wireworms. Crop Prot. 2014, 64, 137–142. [Google Scholar] [CrossRef]
- Husberg, G.B.; Heikki, M.T.H. Effects of Metarhizium anisopliae on the pollen beetle Meligethes aeneus and its parasitoids Phradis morionellus and Diospilus capito. BioControl 2001, 46, 261–273. [Google Scholar] [CrossRef]
- Jarrahi, A.; Safavi, S.A. Sublethal effects of Metarhizium anisopliae on life table parameters of Habrobracon hebetor parasitizing Helicoverpa armigera larvae at different time intervals. BioControl 2016, 6, 167–175. [Google Scholar] [CrossRef]
- Rännbäck, L.M.; Cotes, B.; Anderson, P.; Rämert, B.; Meyling, N.V. Mortality risk from entomopathogenic fungi affects oviposition behavior in the parasitoid wasp Trybliographa rapae. J. Invertebr. Pathol. 2015, 31, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Solomon, M.G.; Cross, J.V.; Fitzgerald, J.D.; Campbell, C.A.M.; Jolly, R.L.; Olszak, R.W.; Niemczyk, E.; Vogt, H. Biocontrol of pests of apples and pears in northern and central Europe-3. Predators. Biocontrol Sci. Technol. 2000, 10, 91–128. [Google Scholar] [CrossRef]
- Stara, J.; Ourednickova, J.; Kocourek, F. Laboratory evaluation of the side effects of insecticides on Aphidius colemani (Hymenoptera: Aphidiidae), Aphidoletes aphidimyza (Diptera: Cecidomyiidae), and Neoseiulus cucumeris (Acari: Phytoseidae). J. Pest. Sci. 2011, 84, 25–31. [Google Scholar] [CrossRef]
- Denoth, M.; Frid, L.; Myers, J.H. Multiple agents in biological control: Improving the odds? Biol. Control 2002, 24, 20–30. [Google Scholar] [CrossRef]
- Askary, H.; Brodeur, J. Susceptibility of Larval Stages of the Aphid Parasitoid Aphidius nigripes to the Entomopathogenic Fungus Verticillium lecanii. J. Invertebr. Pathol. 1999, 73, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, A.L.; Lacey, L.A. Interactions among the entomopathogenic fungus, Paecilomyces fumosoroseus (Deuteromycotina: Hyphomycetes), the parasitoid, Aphelinus asychis (Hymenoptera: Aphelinidae), and their aphid host. Biol. Control 2001, 22, 51–59. [Google Scholar] [CrossRef]
- Aqueel, M.A.; Leather, S.R. Virulence of Verticillium lecanii (Z.) against cereal aphids; does timing of infection affect the performance of parasitoids and predators? Pest Manag. Sci. 2013, 69, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Grasswitz, T.R.; Burts, E.C. Effect of native natural enemies and augmentative releases of Chrysoperla rufilabris Burmeister and Aphidoletes aphidimyza (Rondani) on the population dynamics of the green apple aphid, Aphis pomi De Geer. Int. J. Pest Manag. 1995, 41, 176–183. [Google Scholar] [CrossRef]
- Hindayana, D.; Meyhöfer, R.; Scholz, D.; Poehling, H.M. Intraguild predation among the hoverfly Episyrphus balteatus De Geer (Diptera: Syrphidae) and other aphidophagous predators. Biol. Control 2001, 20, 236–246. [Google Scholar] [CrossRef]
- Powell, J.R.; Webster, J.M. Interguild antagonism between biological controls: Impact of entomopathogenic nematode application on an aphid predator, Aphidoletes aphidimyza (Diptera: Cecidomyiidae). Biol. Control 2004, 30, 110–118. [Google Scholar] [CrossRef]
- Wu, S.; Gao, Y.; Smagghe, G.; Xu, X.; Lei, Z. Interactions between the entomopathogenic fungus Beauveria bassiana and the predatory mite Neoseiulus barkeri and biological control of their shared prey/host Frankliniella occidentalis. Biol. Control 2016, 98, 43–51. [Google Scholar] [CrossRef]
- Carvalho, V.F.P.; Vacari, A.M.; Pomari, A.F.; Bortoli, C.D.; Ramalho, D.G.; Bortoli, S.D. Interaction between the predator Podisus nigrispinus (Hemiptera: Pentatomidae) and the entomopathogenic bacteria Bacillus thuringiensis. Environ. Entomol. 2012, 41, 1454–1461. [Google Scholar] [CrossRef] [PubMed]
- Cory, J.S.; Ericsson, J.D. Fungal entomopathogens in a tritrophic context. BioControl 2010, 55, 75–88. [Google Scholar] [CrossRef]
- Van Lenteren, J.C. (Ed.) Quality Control and Production of Biological Control Agents: Theory and Testing Procedures; CABI Publishing: Wallingford, UK, 2003; Available online: http://users.ugent.be/~padclerc/AMRQC/proceedings/QCflyer.doc (accessed on 15 January 2016).
- Sigsgaard, L.; Steinwender, B.M.; Dimitrova, D.; Hansen, E.W.; Eilenberg, J. Technical Protocol for Tests of Direct and Indirect Side-Effects of New BCA Formulations on Non-Target Invertebrates. Available online: http://inbiosoil.uni-goettingen.de/fileadmin/INBIOSOIL/Deliverables/3.1%20Defined%20technical%20protocol%20for%20test%20of%20side%20effects%20....pdf (accessed on 10 November 2016).
- Lucas, É.; Coderre, D.; Brodeur, J. Intraguild predation among aphid predators: Characterization and influence of extraguild prey density. Ecology 1998, 79, 1084–1092. [Google Scholar] [CrossRef]
- Parsa, S.; Ortiz, V.; Vega, F.E. Establishing fungal entomopathogens as endophytes: Towards endophytic biological control. JoVE J. Vis. Exp. 2013, 74, e50360. [Google Scholar] [CrossRef] [PubMed]
- Meyling, N.V.; Pilz, C.; Keller, S.; Widmer, F.; Enkerli, J. Diversity of Beauveria spp. isolates from pollen beetles Meligethes aeneus in Switzerland. J. Invertebr. Pathol. 2012, 109, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Meyling, N.V.; Pell, J.K.; Eilenberg, J. Dispersal of Beauveria bassiana by the activity of nettle insects. J. Invertebr. Pathol. 2006, 93, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Ganassi, S.; Grazioso, P.; Moretti, A.; Sabatini, M.A. Effects of the fungus Lecanicillium lecanii on survival and reproduction of the aphid Schizaphis graminum. Biocontrol 2010, 55, 299–312. [Google Scholar] [CrossRef]
- Rashki, M.; Shirvani, A. The effect of entomopathogenic fungus, Beauveria bassiana on life table parameters and behavioural response of Aphis gossypii. Bull. Insectol. 2013, 66, 85–91. [Google Scholar]
- Crowder, D.W.; Jabbour, R. Relationships between biodiversity and biological control in agroecosystems: Current status and future challenges. Biol. Control 2014, 75, 8–17. [Google Scholar] [CrossRef]
- Rashki, M.; Kharazi-Pakdel, A.; Allahyari, H.; Van Alphen, J.J.M. Interactions among the entomopathogenic fungus, Beauveria bassiana (Ascomycota: Hypocreales), the parasitoid, Aphidius matricariae (Hymenoptera: Braconidae), and its host, Myzus persicae (Homoptera: Aphididae). Biol. Control 2009, 50, 324–328. [Google Scholar] [CrossRef]
- Emami, F.; Alichi, M.; Minaei, K. Interaction between the entomopathogenic fungus, Beauveria bassiana (Ascomycota: Hypocreales) and the parasitoid wasp, Aphidius colemani Viereck (Hymenoptera: Braconidae). J. Entomol. Acarol. Res. 2013, 45, 4. [Google Scholar] [CrossRef]
- Danks, H.V. The roles of insect cocoons in cold conditions. Eur. J. Entomol. 2004, 101, 433–438. [Google Scholar] [CrossRef]
- Van Lenteren, J.C.; Schettino, M.; Isidoro, N.; Romani, R.; Schelt, J. Morphology of putative female sex pheromone glands and mating behavior in Aphidoletes aphidimyza. Entomol. Exp. Appl. 2002, 102, 199–209. [Google Scholar] [CrossRef]
- Ansari, M.A.; Carpenter, S.; Butt, T.M. Susceptibility of Culicoides biting midge larvae to the insect-pathogenic fungus, Metarhizium anisopliae: Prospects for bluetongue vector control. Acta Trop. 2010, 113, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.A.; Pope, E.C.; Carpenter, S.; Scholte, E.J.; Butt, T.M. Entomopathogenic fungus as a biological control for an important vector of livestock disease: The Culicoides biting midge. PLoS ONE 2011, 6, e16108. [Google Scholar] [CrossRef] [PubMed]
- Higashida, K.; Yano, E.; Toyonishi, H.; Nakauchi, M.; Abe, J. Reproduction of Aphidoletes aphidimyza (Diptera: Cecidomyiidae) on a banker plant system of sorghum with Melanaphis sacchari (Hemiptera: Aphididae) and its oviposition selection between this system and eggplant with Aphis gossypii (Hemiptera: Aphididae). Appl. Entomol. Zool. 2017, 1–9. [Google Scholar] [CrossRef]
- Hosseini, M.; Ashouri, A.; Enkegaard, A.; Weisser, W.W.; Goldansaz, S.H.; Mahalati, M.N.; Sarraf Moayeri, H.R. Plant quality effects on intraguild predation between Orius laevigatus and Aphidoletes aphidimyza. Entomol. Exp. Appl. 2010, 135, 208–216. [Google Scholar] [CrossRef]
- Goettel, M.S.; Alma, C.; Jaramillo, P.; Kim, J.J.; Gillespie, D.; Roitberg, B. Entomopathogenic fungi in greenhouse ecosystems: Present and future roles. J. Anhui Agric. Univ. 2007, 2, 157–161. [Google Scholar]
- Pilz, C.; Wegensteiner, R.; Keller, S. Selection of entomopathogenic fungi for the control of the western corn rootworm Diabrotica virgifera virgifera. J. Appl. Entomol. 2007, 131, 426–431. [Google Scholar] [CrossRef]



| Organism | Release Date | Corresponding Week | Number of Organisms Released |
|---|---|---|---|
| M. brunneum (suspension) | 18 March 2015 | 0 | 8 × 109 conidia/pot |
| R. padi (adults) | 10 April 2015 | 3 | 5/pot |
| 13 April 2015 | 4 | 10/pot | |
| A. aphidimyza (pupae) | 16 April 2015 | 4 | 20/pot |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azevedo, A.G.C.d.; Steinwender, B.M.; Eilenberg, J.; Sigsgaard, L. Interactions among the Predatory Midge Aphidoletes aphidimyza (Diptera: Cecidomyiidae), the Fungal Pathogen Metarhizium brunneum (Ascomycota: Hypocreales), and Maize-Infesting Aphids in Greenhouse Mesocosms. Insects 2017, 8, 44. https://doi.org/10.3390/insects8020044
Azevedo AGCd, Steinwender BM, Eilenberg J, Sigsgaard L. Interactions among the Predatory Midge Aphidoletes aphidimyza (Diptera: Cecidomyiidae), the Fungal Pathogen Metarhizium brunneum (Ascomycota: Hypocreales), and Maize-Infesting Aphids in Greenhouse Mesocosms. Insects. 2017; 8(2):44. https://doi.org/10.3390/insects8020044
Chicago/Turabian StyleAzevedo, Ana Gorete Campos de, Bernhardt Michael Steinwender, Jørgen Eilenberg, and Lene Sigsgaard. 2017. "Interactions among the Predatory Midge Aphidoletes aphidimyza (Diptera: Cecidomyiidae), the Fungal Pathogen Metarhizium brunneum (Ascomycota: Hypocreales), and Maize-Infesting Aphids in Greenhouse Mesocosms" Insects 8, no. 2: 44. https://doi.org/10.3390/insects8020044







