Improving Efficacy of Beauveria bassiana against Stored Grain Beetles with a Synergistic Co-Formulant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Insects
2.2. Test Items
2.3. Commodity
2.4. Bioassay 1: Initial Testing of O. surinamensis
2.5. Bioassay 2: Dose Response of O. surinamensis
2.6. Bioassay 3: Dose Response of T. confusum Adults and Larvae
2.7. Bioassay 4: Dose Response of S. granarius and the Effect of Temperature
2.8. Statistical Analysis
3. Results
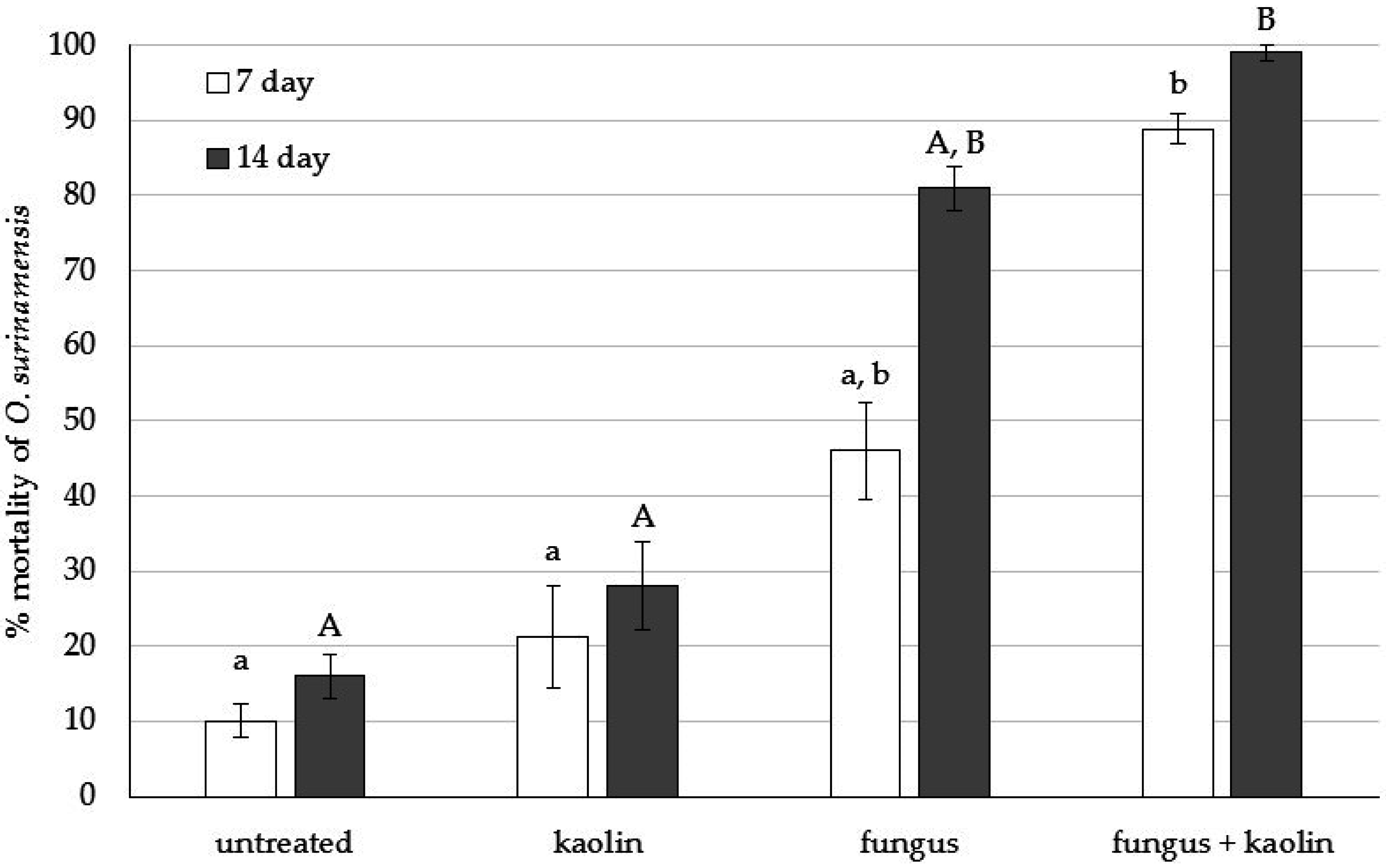
3.1. Bioassay 1: Initial Testing of O. surinamensis
3.2. Bioassay 2: Dose Response of O. surinamensis
3.3. Bioassay 3: Dose Response of T. confusum Adults and Larvae
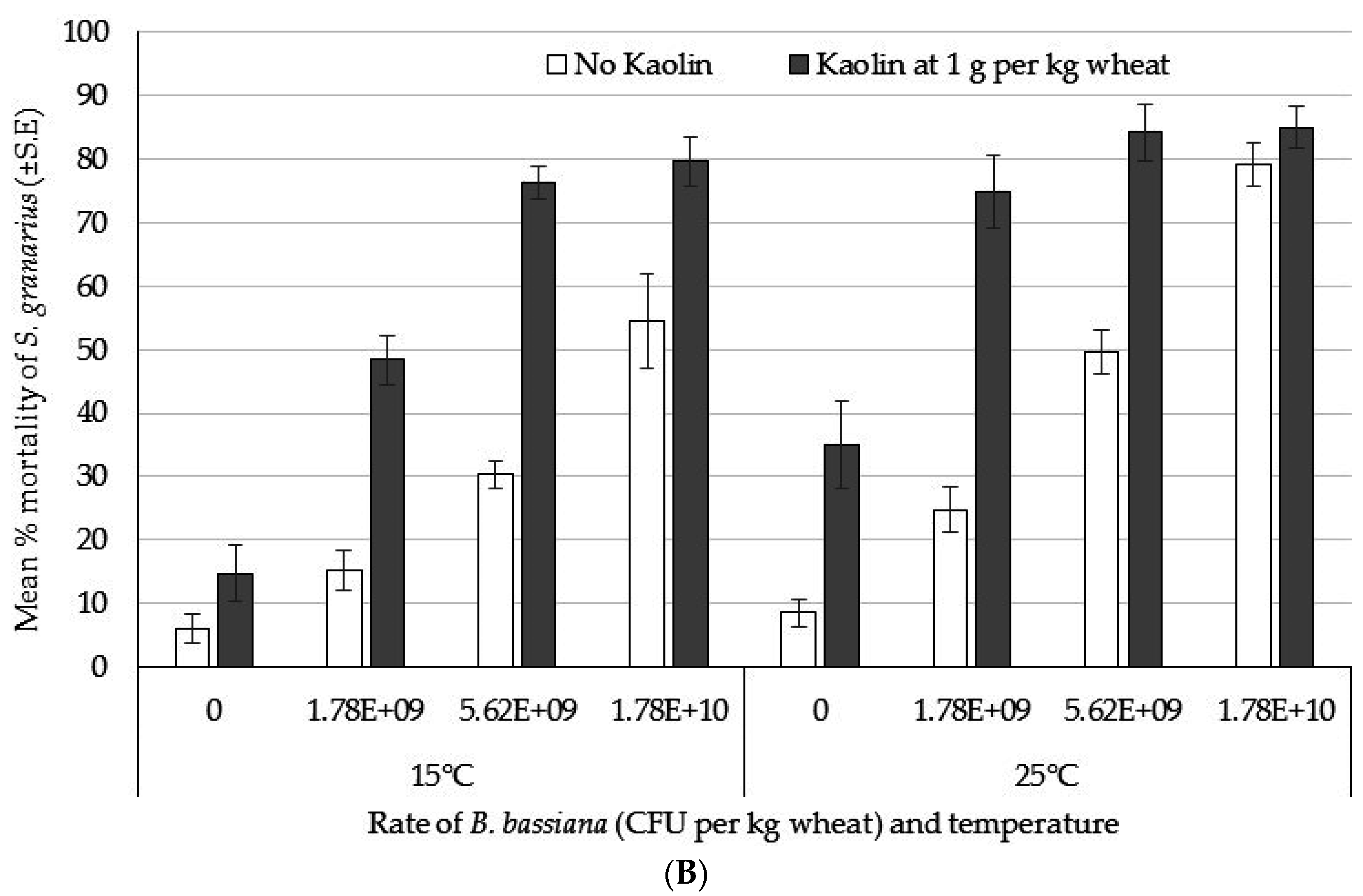
3.4. Bioassay 4: Dose Response of S. granarius and the Effect of Temperature
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pimentel, D. World resources and food losses to pests. In Ecology and Management of Food Industry Pests; Gorham, J.R., Ed.; Association of Official Analytical Chemists: Arlington, MA, USA, 1991; pp. 5–11. [Google Scholar]
- Lord, J.C.; Campbell, J.F.; Sedlacek, J.D.; Vail, P.V. Application and evaluation of entomopathogens for managing insects in stored products. In Field Manual of Techniques in Invertebrate Pathology; Lacey, L.A., Kaya, H.K., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 677–693. [Google Scholar]
- Cherry, A.J.; Abalob, P.; Hell, K. A laboratory assessment of the potential of different strains of the entomopathogenic fungi Beauveria bassiana (Balsamo) Vuillemin and Metarhizium anisopliae (Metschnikoff) to control Callosobruchus maculatus (F.) (Coleoptera: Bruchidae) in stored cowpea. J. Stored Prod. Res. 2005, 41, 295–309. [Google Scholar] [CrossRef]
- Cherry, A.J.; Aba, P.; Hell, K.; Korie, S. Farm-scale trials to compare the entomopathogenic fungus Beauveria bassiana with pirimiphos methyl plus deltamethrin and essential oil of lemon grass for protection of stored cowpea against Callosobruchus maculatus (Coleoptera: Bruchidae). Ann. Appl. Biol. 2007, 151, 1–10. [Google Scholar] [CrossRef]
- Cox, P.D.; Wakefield, M.E.; Price, N.; Wildey, K.B.; Chambers, J.; Moore, D.; Aquino de Muro, M.; Bell, B.A. The Potential Use of Insect-Specific Fungi to Control Grain Storage Pests in Empty Grain Stores. Available online: https://cereals.ahdb.org.uk/media/363584/pr341-final-project-report.pdf (accessed on 19 July 2016).
- Sabbour, M.M.; Abd-El-Aziz, S.E.; Sherief, M.A. Efficacy of three entomopathogenic fungi alone or in combination with diatomaceous earth. J. Plant Prot. Res. 2012, 52, 359–363. [Google Scholar] [CrossRef]
- Dal Bello, G.; Padin, S.; Lastra, C.L.; Fabrizio, M. Laboratory evaluation of chemical-biological control of the rice weevil (Sitophilus oryzae L.) in stored grains. J. Stored Prod. Res. 2001, 37, 77–84. [Google Scholar] [CrossRef]
- Moino, A.; Alves, S.B.; Pereira, R.M. Efficacy of Beauveria bassiana (Bal.) Vuilemin isolates for control of stored-grain pests. J. Appl. Entomol. 1998, 122, 301–305. [Google Scholar] [CrossRef]
- Ebeling, W. Sorptive dusts for pest control. Ann. Rev. Entomol. 1971, 16, 123–158. [Google Scholar] [CrossRef] [PubMed]
- Subramanyam, B.; Roesli, R. Inert Dusts. In Alternatives to Pesticides in Stored-Product IPM; Subramanyam, B., Hagstrum, D., Eds.; Springer: New York, NY, USA, 2000; pp. 321–380. [Google Scholar]
- Allen, S. Integration of inert dust into control of storage pests in bulk grain in storage in Australia. In Proceedings of the International Conference Controlled Atmosphere and Fumigation in Stored Products, Fresno, CA, USA, 29 October–3 November 2000; pp. 279–284.
- Banks, J.; Fields, P.G. Physical Methods for Insect Control in Stored-Grain Ecosystems. In Stored-Grain Ecosystems; Jayas, D.S., White, N.D.G., Muir, W.E., Eds.; Marcel Dekker: New York, NY, USA, 1995; pp. 353–409. [Google Scholar]
- Golob, P. Current status and future perspectives for inert dusts for control of stored product insects. J. Stored Prod. Res. 1997, 33, 69–79. [Google Scholar] [CrossRef]
- Arthur, F.H. Toxicity of diatomaceous earth to red flour beetles and confused flour beetles (Coleoptera: Tenebrionidae): Effects of temperature and relative humidity. J. Econ. Entomol. 2000, 93, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Fields, P.; Korunic, Z. The effect of grain moisture content and temperature on the efficacy of diatomaceous earths from different geographical locations against stored-product beetles. J. Stored Prod. Res. 2000, 36, 1–13. [Google Scholar] [CrossRef]
- Subramanyam, B.; Swanson, C.L.; Madamanchi, N.; Norwood, S. Effectiveness of Insecto®, a new diatomaceous earth formulation in suppressing several stored grain insect species. In Proceedings of the 6th International Working Conference on Stored Product Protection, Canberra, Australia, 17–23 April 1994; Volume 2, pp. 650–659.
- Timlick, B.; Fields, P.G. A comparison of the effect of two diatomaceous earth formulations on Plodia interpunctella (Hübner) and the effect of different commodities on diatomaceous earth efficacy. In Proceedings of the 10th International Working Conference on Stored Product Protection, Estoril, Portugal, 27 June–2 July 2010.
- Vayias, B.J.; Athanassiou, C.G. Factors affecting efficacy of the diatomaceous earth formulation SilicoSec® against adults and larvae of the confused beetle Tribolium confusum Du Val (Coleoptera: Tenebrionidae). Crop Prot. 2004, 23, 565–573. [Google Scholar] [CrossRef]
- Korunic, Z.; Fields, P.G.; Kovacs, M.I.P.; Noll, J.S.; Lukow, O.M.; Demianyk, C.J.; Shibley, K.J. The effect of diatomaceous earth on grain quality. Postharvest Biol. Technol. 1996, 9, 373–387. [Google Scholar] [CrossRef]
- Korunic, Z.; Cenkowski, S.; Fields, P. Grain bulk density as affected by diatomaceous earth and application method. Postharvest Biol. Technol. 1998, 13, 81–89. [Google Scholar] [CrossRef]
- Murray, H.H. Traditional and new applications for kaolin, smectite, and palygorskite: A general overview. Appl. Clay Sci. 2000, 17, 207–221. [Google Scholar] [CrossRef]
- Arthur, F.H.; Puterka, G.J. Evaluation of kaolinite-based particle films to control Tribolium species (Coleoptera: Tenebrionidae). J. Stored Prod. Res. 2002, 38, 341–348. [Google Scholar] [CrossRef]
- Mahmoud, A.E.M.; El-Sebai, O.A.; Shahen, A.A.; Marzouk, A.A. Impact of kaolin-based particle film dusts on Callosobruchus maculatus (F.) and C. chinenesis (L.) after different storage periods of treated broad bean seeds. Julius Kühn-Institut 2010, 425, 638–646. [Google Scholar]
- Golob, P.; Webley, D.J. The Use of Plants and Minerals as Traditional Protectants of Stored Products; Tropical Products Institute: London, UK, 1980. [Google Scholar]
- Athanassiou, C.G.; Steenberg, T. Insecticidal effect of Beauveria bassiana (Balsamo) Vuillemin (Ascomycota: Hypocreales) in combination with three diatomaceous earth formulations against Sitophilus granarius (L.) (Coleoptera: Curculionidae). Biol. Control 2007, 40, 411–416. [Google Scholar] [CrossRef]
- Michalaki, M.P.; Athanassiou, C.G.; Kavallieratos, N.G.; Batta, Y.A.; Balotis, G.N. Effectiveness of Metarhizium anisopliae (Metschinkoff) Sorokin applied alone or in combination with diatomaceous earth against Tribolium confusum Du Val larvae: Influence of temperature, relative humidity and type of commodity. Crop Prot. 2006, 25, 418–425. [Google Scholar] [CrossRef]
- Kavallieratos, N.G.; Athanassiou, C.G.; Michalaki, M.P.; Batta, Y.A.; Rigatos, H.A.; Pashalidou, F.G.; Balotis, G.N.; Tomanović, Ž.; Vayias, B.J. Effect of the combined use of Metarhizium anisopliae (Metschinkoff) Sorokin and diatomaceous earth for the control of three stored-product beetle species. Crop Prot. 2006, 25, 1087–1094. [Google Scholar] [CrossRef]
- Michalaki, M.P.; Athanassiou, C.G.; Steenberg, T.; Buchelos, C.T. Effect of Paecilomyces fumosoroseus (Wise) Brown and Smith (Ascomycota: Hypocreales) alone or in combination with diatomaceous earth against Tribolium confusum Jacquelin du Val (Coleoptera: Tenebrionidae) and Ephestia kuehniella Zeller (Lepidoptera: Pyralidae). Biol. Control 2007, 40, 280–286. [Google Scholar]
- Lord, J.C. Dessicant dusts synergise the effect of Beauveria bassiana (Hyphomycetes: Moniliales) on stored-grain beetles. J. Econ. Entomol. 2001, 94, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Akbar, W.; Lord, J.C.; Nechols, J.R.; Howard, R.W. Diatomaceous earth increases the efficacy of Beauveria bassiana against Tribolium castaneum larvae and increased conidial attachment. J. Econ. Entomol. 2004, 97, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Samodra, H.; Ibrahim, Y. Effectiveness of selected entomopathogenic fungi in packed rice grain at room temperature against Corcyra cephalonica Stainton. ASEAN J. Sci. Technol. Dev. 2006, 23, 183–192. [Google Scholar]
- Samodra, H.; Ibrahim, Y. Effects of dust formulation of three entomopathogenic fungal isolates against Sitophilus oryzae (Coleopteran: Curculionidae) in rice grain. J. Biosains 2006, 17, 1–7. [Google Scholar]
- Rees, D. Insects of Stored Grain: A Pocket Reference; CSIRO Publishing: Collingwood, Australia, 2007. [Google Scholar]
- Wakefield, M.E. Factors affecting storage insect susceptibility to the entomopathogenic fungus Beauveria bassiana. In Proceedings of the 9th International Working Conference on Stored Product Protection, Campinas, São Paulo, Brazil, 15–18 October 2006.
- Wakefield, M.E.; Moore, D.; Luke, B.; Taylor, B.; Storm, C.G.; Collins, D.A.; Grammare, P.; Potin, O. Progress in the development of a biopesticide for the structural treatment of grain stores. Julius Kühn-Institut 2010, 425, 760–765. [Google Scholar]
- Vassilakos, T.N.; Athanassiou, C.G.; Kavallieratos, N.G.; Vayias, B.J. Influence of temperature on the insecticidal effect of Beauveria bassiana in combination with diatomaceous earth against Rhyzopertha dominica and Sitophilus oryzae on stored wheat. Biol. Control 2006, 38, 270–281. [Google Scholar] [CrossRef]
- Ekesi, S.; Maniania, N.K.; Ampong-Nyarko, K. Effect of Temperature on Germination, Radial Growth and Virulence of Metarhizium anisopliae and Beauveria bassiana on Megalurothrips sjostedti. Biocontrol Sci. Technol. 1999, 9, 177–185. [Google Scholar] [CrossRef]
- Luz, C.; Fargues, J. Temperature and moisture requirements for conidial germination of an isolate of Beauveria bassiana, pathogenic to Rhodnius prolixus. Mycopathologia 1997, 138, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Lord, J.C. Low humidity, moderate temperature, and desiccant dust favor efficacy of Beauveria bassiana (Hyphomycetes: Moniliales) for the lesser grain borer, Rhyzopertha dominica (Coleoptera: Bruchidae). Biol. Control 2005, 34, 180–186. [Google Scholar] [CrossRef]
- Lord, J.C. Enhanced efficacy of Beauveria bassiana for red flour beetle with reduced moisture. J. Econ. Entomol. 2007, 100, 1071–1074. [Google Scholar] [CrossRef]
- Huafeng, L.; Meizhen, F.; Zengzhi, L.; Cui, H. Pathogenic effect of Beauveria bassiana infected on Dendrolimus under different temperature and humidity. Chin. J. Appl. Ecol. 1998, 9, 195–200. [Google Scholar]
- Luz, C.; Fargues, J. Dependence of the entomopathogenic fungus, Beauveria bassiana, on high humidity for infection of Rhodnius prolixus. Mycopathologia 1999, 146, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Padin, S.; Dal Bello, G.; Fabrizio, M. Grain loss caused by Tribolium castaneum, Sitophilus oryzae and Acanthoscelides obtectus in stored durum wheat and bean treated with Beauveria bassiana. J. Stored Prod. Res. 2002, 38, 69–74. [Google Scholar] [CrossRef]
- Rice, W.C.; Cogburn, R.R. Activity of the entomopathogenic fungus Beauveria bassiana (Deuteromycota: Hypomycetes) against three coleopteran pests of stored grain. J. Econ. Entomol. 1999, 92, 691–694. [Google Scholar] [CrossRef]
- Lord, J.C. Desiccation increases the efficacy of Beauveria bassiana for stored-grain pest insect control. J. Stored Prod. Res. 2007, 43, 535–539. [Google Scholar] [CrossRef]
- Shayestah, N.; Ziaee, M.; Safaralizadeh, M.H. Insecticidal efficacy of diatomaceous earth against Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). In Proceedings of the 9th International Working Conference on Stored Product Protection, Passo Fundo, RS, Brazil, 15–18 October 2006; pp. 1212–1217.
- Glenn, D.M.; Puterka, G.; Vanderzwet, T.; Bryers, T.; Feldhake, C. Hydrophobic particle films: A new paradigm for the suppression of arthropod pests and plant diseases. J. Econ. Entomol. 1999, 92, 751–771. [Google Scholar] [CrossRef]
- Puterka, G.J.; Glenn, D.M.; Sekatowski, D.G.; Unruh, T.R.; Jones, S.K. Progress towards liquid formulations of particle films for insect and disease control in pear. Environ. Entomol. 2000, 29, 329–339. [Google Scholar] [CrossRef]

| Formulation Applied to Wheat (g per kg) | Rate of Conidia Applied to Wheat (CFU per kg) | Amount in Formulation (g per kg) | |||
|---|---|---|---|---|---|
| Fungal Conidia | Kaolin | Entostat | Sipernat d17 | ||
| 0.0507 | 1.78E+9 | 350 | 0 | 645 | 5 |
| 0.1606 | 5.62E+10 | 350 | 0 | 645 | 5 |
| 0.5086 | 1.78E+10 | 350 | 0 | 645 | 5 |
| 1.0509 | 1.78E+9 | 16.9 | 951.6 | 26.5 | 5 |
| 1.1605 | 5.62E+10 | 48.4 | 861.6 | 84.9 | 5 |
| 1.5086 | 1.78E+10 | 118 | 662.9 | 214.1 | 5 |
| Rate of Kaolin (g per kg of Wheat) | LC50 (CFU per kg of Wheat) | Lower Bound 95% | Upper Bound 95% | R2 | χ2 | p-Value |
|---|---|---|---|---|---|---|
| 0 | 1.582E+10 | 1.008E+10 | 3.230E+10 | 0.099 | 47.541 | <0.0001 |
| 0.0963 | 7.47E+09 | 5.43E+09 | 1.13E+10 | 0.129 | 67.925 | <0.0001 |
| 0.9626 | 1.228E+09 | 8.752E+08 | 1.606E+09 | 0.202 | 96.657 | <0.0001 |
| 1.9253 | 3.586E+08 | 2.045E+08 | 5.174E+08 | 0.230 | 78.411 | <0.0001 |
| Rate of Kaolin (g per kg of Wheat) | LC50 (CFU per kg of Wheat) | Lower Bound 95% | Upper Bound 95% | R2 | χ2 | p-Value |
|---|---|---|---|---|---|---|
| 0 | 1.99E+11 | 8.86E+10 | 3.48E+15 | 0.013 | 7.008 | 0.008 |
| 0.178 | 1.07E+11 | 6.72E+10 | 5.04E+11 | 0.020 | 10.885 | 0.001 |
| 0.316 | 8.97E+10 | 5.83E+10 | 3.28E+11 | 0.020 | 10.612 | 0.001 |
| 0.562 | 8.60E+10 | 5.65E+10 | 2.92E+11 | 0.021 | 11.352 | 0.001 |
| 1.000 | 7.23E+10 | 5.07E+10 | 1.58E+11 | 0.025 | 13.459 | >0.001 |
| Days after Treatment | Temperature (°C) | Kaolin | LC50 (CFU per kg of Wheat) | Lower Bound 95% | Upper Bound 95% | R2 | χ2 | p-Value |
|---|---|---|---|---|---|---|---|---|
| 14 | 15 | No | 4.21E+10 | 2.48E+10 | 2.54E+11 | 0.180 | 33.154 | <0.0001 |
| Yes | 1.12E+10 | 8.22E+09 | 1.73E+10 | 0.081 | 40.373 | <0.0001 | ||
| 25 | No | 1.44E+10 | 1.11E+10 | 2.08E+10 | 0.117 | 54.743 | <0.0001 | |
| Yes | 4.28E+09 | 3.24E+09 | 5.45E+09 | 0.087 | 62.332 | <0.0001 | ||
| 28 | 15 | No | 1.49E+10 | 1.13E+10 | 2.22E+10 | 0.123 | 54.862 | <0.0001 |
| Yes | 1.51E+09 | 7.09E+08 | 2.32E+09 | 0.036 | 33.985 | <0.0001 | ||
| 25 | No | 5.49E+09 | 4.46E+09 | 6.62E+09 | 0.149 | 103.483 | <0.0001 | |
| Yes | No analysis possible, all responses >50% | |||||||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Storm, C.; Scoates, F.; Nunn, A.; Potin, O.; Dillon, A. Improving Efficacy of Beauveria bassiana against Stored Grain Beetles with a Synergistic Co-Formulant. Insects 2016, 7, 42. https://doi.org/10.3390/insects7030042
Storm C, Scoates F, Nunn A, Potin O, Dillon A. Improving Efficacy of Beauveria bassiana against Stored Grain Beetles with a Synergistic Co-Formulant. Insects. 2016; 7(3):42. https://doi.org/10.3390/insects7030042
Chicago/Turabian StyleStorm, Clare, Freya Scoates, Adam Nunn, Olivier Potin, and Aoife Dillon. 2016. "Improving Efficacy of Beauveria bassiana against Stored Grain Beetles with a Synergistic Co-Formulant" Insects 7, no. 3: 42. https://doi.org/10.3390/insects7030042





