Electrophysiological Responses and Reproductive Behavior of Fall Webworm Moths (Hyphantria cunea Drury) are Influenced by Volatile Compounds from Its Mulberry Host (Morus alba L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Origin and Handling of Hyphantria cunea
2.2. Tested Volatiles
2.3. Assessing Effects of Morus alba Volatiles β-ocimene and cis-2-penten-1-ol on EAG Responses of Hyphantria cunea
2.4. Assessing Effects of Morus alba HIPV, MDV, β-ocimene and cis-2-penten-1-ol on Hyphantria cunea Mating and Oviposition
3. Results
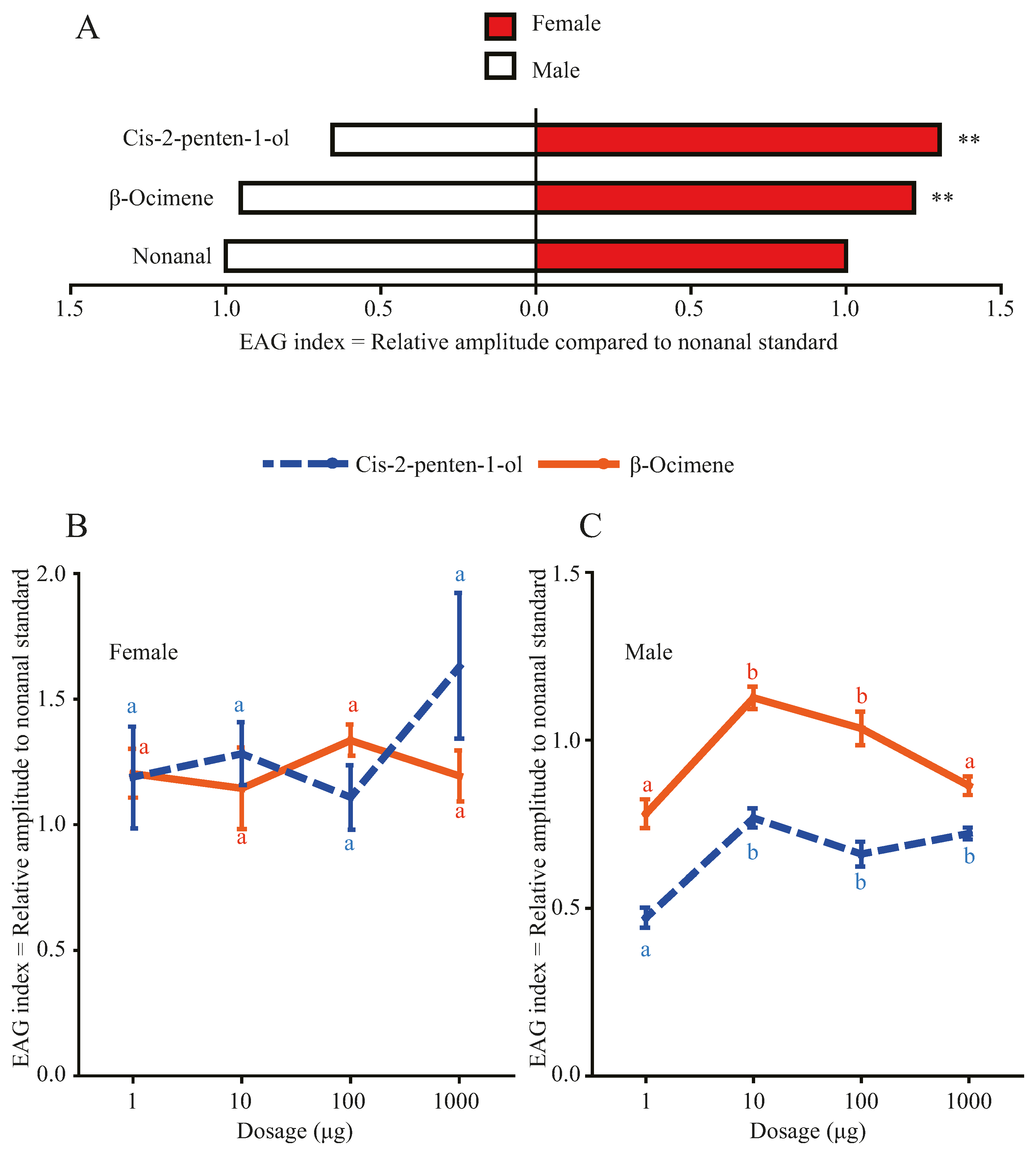
3.1. Effects of Morus alba Volatiles β-ocimene and cis-2-penten-1-ol on EAG Responses of Hyphantria cunea
3.2. Effects of Morus alba HIPV, MDV, β-ocimene and cis-2-penten-1-ol on Hyphantria cunea Mating and Oviposition
4. Discussion
5. Conclusions
Acknowledgments
Author contributions
Conflicts of Interest
References
- Tadić, M.D. Natural enemies of fall webworm (Hyphantria cunea Dr.) in North America. Entomophaga 1963, 8, 245–252. [Google Scholar] [CrossRef]
- Ito, Y.; Miyashita, K. Biology of Hyphantria cunea (Lepidoptera: Arctiidae) in Japan. V. Preliminary life tables and mortality data in urban areas. Res. Popul. Ecol. 1968, 10, 177–209. [Google Scholar] [CrossRef]
- Su, M.W.; Fang, Y.L.; Tao, W.Q.; Yan, G.Z.; Ma, W.E.; Zhang, Z.N. Identification and field evaluation of the sex pheromone of an invasive pest, the fall webworm Hyphantria cunea in China. Chin. Sci. Bull. 2008, 53, 555–560. [Google Scholar] [CrossRef]
- Yang, Z.Q.; Wei, J.R.; Wang, X.Y. Mass rearing and augmentative releases of the native parasitoid Chouioia cunea for biological control of the introduced fall webworm Hyphantria cunea in China. Biocontrol 2006, 51, 401–418. [Google Scholar] [CrossRef]
- Ono, M. Cultivation and breeding of mulberry in China. JPN J. Breed. 1983, 33, 337–340. (In Japanese) [Google Scholar] [CrossRef]
- Du, J.; Gao, B.J.; Zhou, G.N.; Miao, A.M. Genetic diversity and differentiation of fall webworm (Hyphantria cunea Drury) populations. For. Stud. China 2009, 11, 158–163. [Google Scholar] [CrossRef]
- Gomi, T. Seasonal adaptations of the fall webworm Hyphantria cunea (Drury) (Lepidoptera: Arctiidae) following its invasion of Japan. Ecol. Res. 2007, 22, 855–861. [Google Scholar] [CrossRef]
- Rehnberg, B. Temperature profiles inside webs of the fall webworm, Hyphantria cunea (Lepidoptera: Arctiidae): Influence of weather, compass orientation, and time of day. J. Therm. Biol. 2006, 31, 274–279. [Google Scholar] [CrossRef]
- Travis, H.J. The effect of eastern tent caterpillar (Malacasoma americanum) infestation on fall webworm (Hyphantria cunea) selection of black cherry (Prunus serotina) as a host tree. Am. Mid. Nat. 2005, 153, 270–275. [Google Scholar] [CrossRef]
- Williams, K.S.; Myers, J.H. Previous herbivore attack of red alder may improve food quality for fall wabworm larvae. Oecologia 1984, 63, 166–170. [Google Scholar] [CrossRef]
- Hare, J.D. Ecological role of volatiles produced by plants in response to damage by herbivorous insects. Ann. Rev. Entomol. 2011, 56, 161–180. [Google Scholar] [CrossRef] [PubMed]
- Kessler, A.; Baldwin, I.T. Defensive function of herbivore-induced plant volatile emissions in nature. Science 2001, 291, 2141–2144. [Google Scholar] [CrossRef] [PubMed]
- Dicke, M.; Hilker, M. Induced plant defences: From molecular biology to evolutionary ecology. Basic Appl. Ecol. 2003, 4, 3–14. [Google Scholar] [CrossRef]
- Gouinguené, S.; Pickett, J.A.; Wadhams, L.J.; Birkett, M.A.; Turlings, T.C. Antennal electrophysiological responses of three parasitic wasps to caterpillar-induced volatiles from maize (Zea mays), cotton (Gossypium herbaceum), and cowpea (Vigna unguiculata). J. Chem. Ecol. 2005, 31, 1023–1038. [Google Scholar] [CrossRef] [PubMed]
- Dickens, J.C.; Smith, J.W.; Light, D.M. Green leaf volatiles enhance sex attractant pheromone of the tobacco budworm, Heliothis virescens (Lep. Noctuidae). Chemoecology 1993, 4, 175–177. [Google Scholar] [CrossRef]
- Dickens, J.C.; Visser, J.H.; van-der-Pers, J.C. Detection and deactivation of pheromone and plant odor components by the beet armyworm, Spodoptera exigua (Hübner) (Lepidoptera: Noctuidae). J. Insect Physiol. 1993, 39, 503–516. [Google Scholar] [CrossRef]
- Light, D.M.; Flath, R.A.; Buttery, R.G.; Zalom, F.G.; Rice, R.E.; Dickens, J.C.; Jang, E.B. Host-plant green-leaf volatiles synergize the synthetic sex pheromones of the corn earworm and codling moth (Lepidoptera). Chemoecology 1993, 4, 145–152. [Google Scholar] [CrossRef]
- Landolt, P.J. Host plant influences on sex pheromone behavior of phytophagous insects. Ann. Rev. Entomol. 1997, 42, 371–391. [Google Scholar] [CrossRef] [PubMed]
- Stelinski, L. Increased EAG responses of tortricid moths after prolonged exposure to plant volatiles: Evidence for octopamine-mediated sensitization. J. Insect Physiol. 2003, 49, 845–856. [Google Scholar] [CrossRef]
- Howard, R.W.; Blomquist, G.J. Ecological, behavioral, and biochemical aspects of insect hydrocarbons. Ann. Rev. Entomol. 2005, 50, 371–393. [Google Scholar] [CrossRef] [PubMed]
- Howe, G.A.; Jander, G. Plant immunity to insect herbivores. Ann. Rev. Plant Biol. 2008, 59, 41–66. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.Q.; Wang, H.Y.; Liu, S.S.; Jiang, N.X.; Shen, J.H. Analysis of volatile components of essential oil from fresh and air-dried mulberry leaves. Terr. Nat. Res. Study 2010, 2, 86–87. (In Chinese) [Google Scholar]
- Li, J.H.; Chen, D.W. Analysis of volatile chemical composition in mulberry leaves using microwave assisted extraction associated with solid phase microextraction. Chem. Ind. Forest Prod. 2007, 27, 107–110. (In Chinese) [Google Scholar]
- Li, W.G.; Zhang, L.W.; Wang, C.; Wang, Y.W.; Mu, Z.M.; Ji, X.L. Analysis of mulberry leaf volatile components by static headspace-gas chromatography-mass spectrometry. Sci. Seric. 2009, 35, 355–361. (In Chinese) [Google Scholar]
- Tanaka, K.; Uda, Y.; Ono, Y.; Nakagawa, T.; Suwa, M.; Yamaoka, R.; Touhara, K. Highly selective tuning of a silkworm olfactory receptor to a key mulberry leaf volatile. Curr. Biol. 2009, 19, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Su, M.W.; Zhang, Z.N. Electroantennogram responses of an invasive species fall webworm (Hyphantria cunea) to host volatile compounds. Chin. Sci. Bull. 2012, 57, 4560–4568. [Google Scholar] [CrossRef]
- Tang, R.; Zhang, J.P.; Zhang, Z.N. Electrophysiological and behavioral responses of male fall webworm moths (Hyphantria cunea) to herbivory-induced mulberry (Morus alba) leaf volatiles. PLoS ONE 2012, 7, e49256. [Google Scholar] [CrossRef] [PubMed]
- Reddy, G.V.; Guerrero, A. Interactions of insect pheromones and plant semiochemicals. Trends Plant Sci. 2004, 9, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.L.; Sun, J.H.; Zhang, Z.N. Response of Brontispa longissima to coconut palm (Cocos nucifera) leaf volatiles. Physiol. Entomol. 2011, 36, 321–326. [Google Scholar] [CrossRef]
- Allmann, S.; Spathe, A.; Bisch-Knaden, S.; Kallenbach, M.; Reinecke, A.; Sachse, S.; Baldwin, I.T.; Hansson, B.S. Feeding-induced rearrangement of green leaf volatiles reduces moth oviposition. Elife 2013. [Google Scholar] [CrossRef]
- Visser, J.H. Electroantennogram responses of the Colorado beetle, Leptinotarsa decemlineata, to plant volatiles. Entomol. Exp. Appl. 1979, 25, 86–97. [Google Scholar] [CrossRef]
- Burguiere, L.; Marion-Poll, F.; Cork, A. Electrophysiological responses of female Helicoverpa armigera (Hübner) (Lepidopetera; Noctuidae) to synthetic host odours. J. Insect Physiol. 2001, 47, 509–514. [Google Scholar] [CrossRef]
- De Moraes, C.M.; Mescher, M.C.; Tumlinson, J.H. Caterpillar-induced nocturnal plant volatiles repel conspecific females. Nature 2001, 410, 577–580. [Google Scholar] [CrossRef] [PubMed]
- Dudareva, N.; Martin, D.; Kish, C.M.; Kolosova, N.; Gorenstein, N.; Fäldt, J.; Miller, B.; Bohlmann, J. (E)-beta-Ocimene and myrcene synthase genes of floral scent biosynthesis in snapdragon: Function and expression of three terpene synthase genes of a new terpene synthase subfamily. Plant Cell 2003, 15, 1227–1241. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, K.; Matsui, K.; Ozawa, R.; Takabayashi, J. Volatile C6-aldehydes and Allo-ocimene activate defense genes and induce resistance against Botrytis cinerea in Arabidopsis thaliana. Plant Cell Physiol. 2005, 46, 1093–1102. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, K.; Matsui, K.; Ozawa, R.; Takabayashi, J. Analysis of defensive responses activated by volatile allo-ocimene treatment in Arabidopsis thaliana. Phytochemistry 2006, 67, 1520–1529. [Google Scholar] [CrossRef] [PubMed]
- Maisonnasse, A.; Lenoir, J.C.; Beslay, D.; Crauser, D.; Le Conte, Y. E-beta-ocimene, a volatile brood pheromone involved in social regulation in the honey bee colony (Apis mellifera). PLoS ONE 2010, 5, e13531. [Google Scholar] [CrossRef] [PubMed]
- Arimura, G.; Huber, D.P.; Bohlmann, J. Forest tent caterpillars (Malacosoma disstria) induce local and systemic diurnal emissions of terpenoid volatiles in hybrid poplar (Populus trichocarpa × deltoides): cDNA cloning, functional characterization, and patterns of gene expression of (−)-germacrene D synthase, PtdTPS1. Plant J. 2004, 37, 603–616. [Google Scholar] [PubMed]
- Arimura, G.; Ozawa, R.; Kugimiya, S.; Takabayashi, J.; Bohlmann, J. Herbivore-induced defense response in a model legume. Two-spotted spider mites induce emission of (E)-beta-ocimene and transcript accumulation of (E)-beta-ocimene synthase in Lotus japonicus. Plant Physiol. 2004, 135, 1976–1983. [Google Scholar] [CrossRef] [PubMed]
- Navia-Giné, W.G.; Yuan, J.S.; Mauromoustakos, A.; Murphy, J.B.; Chen, F.; Korth, K.L. Medicago truncatula (E)-β-ocimene synthase is induced by insect herbivory with corresponding increases in emission of volatile ocimene. Plant Physiol. Biochem. 2009, 47, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Meiners, T.; Wäckers, F.; Lewis, W.J. Associative learning of complex odours in parasitoid host location. Chem. Senses 2003, 28, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Lu, M.; Gillette, N.E.; Wingfield, M.J. Red turpentine beetle: Innocuous native becomes invasive tree killer in China. Annu. Rev. Entomol. 2013, 58, 293–311. [Google Scholar] [CrossRef] [PubMed]
- Dickens, J.C. Green leaf volatiles enhance aggregation pheromone of the boll weevil Anthonomus grandis. Entomol. Exp. Appl. 1989, 52, 191–203. [Google Scholar] [CrossRef]
- Zhang, J.P.; Salcedo, C.; Fang, Y.L.; Zhang, R.J.; Zhang, Z.N. An overlooked component: (Z)-9-tetradecenal as a sex pheromone in Helicoverpa armigera. J. Insect Physiol. 2012, 58, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.Z.; Trinnaman, L.; Bardsley, K.; St Hilaire, C.J.; Da Costa, N.C. Volatile compounds and sensory analysis of both harvests of double-cut Yakima peppermint (Mentha piperita L.). J. Food Sci. 2011. [Google Scholar] [CrossRef]
- Kobayashi, A.; Sato, H.; Yamanishi, T. Cis-2-Penten-1-ol in the essential oil from freshly plucked tea-leaves and black tea. Agric. Biol. Chem. 1965, 29, 488–489. [Google Scholar] [CrossRef]
- Crop Protection Compendium. Available online: http://www.cabi.org/cpc/datasheet/28302 (accessed on 18 April 2016).
- Fox, C.W.; Mousseau, T.A. Larval host plant affects fitness consequences of egg size variation in the seed beetle Stator limbatus. Oecologia 1996, 107, 541–548. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Takahashi, T.; Yoshioka, T.; Nakasuji, F. Changes in egg size of the diamondback moth Plutella xylostella (Lepidoptera: Yponomeutidae) treated with fenvalerate at sublethal doses and viability of the eggs. Appl. Entomol. Zool. 2002, 37, 103–109. [Google Scholar] [CrossRef]
- Berrigan, D. The allometry of egg size and number in insects. Oikos 1991, 60, 313–321. [Google Scholar] [CrossRef]

| Chemical | Type | Test | Tested insects | Purity | Source |
|---|---|---|---|---|---|
| Pentane 1 & ether 2 | Solvent | EAG & bioassay | sample size 5 independent pairs, 5 replicates | Analytically pure ** | 1 Shantou Xilong Chemical Factory, Guangdong, China 2 Beijing Chemical Factory, Beijing, China |
| Acetone | Washing | - | - | Analytically pure ** | Beijing Chemical Factory, Beijing, China |
| Nonanal | Standard compound | EAG | All tested antennae | GC pure | Sigma-Aldrich, St. Louis, MO, USA |
| β-ocimene | Volatile from HIPVs | EAG & bioassay | sample size 5 independent pairs, 5 replicates | 95% | Sigma-Aldrich, St. Louis, MO, USA |
| cis-2-penten-1-ol | Volatile from MDVs | EAG & bioassay | sample size 5 independent pairs, 5 replicates | 95% | Tokyo Chemical Industry Co., Tokyo, Japan |
| HIPVs | Volatile from larvae-damaged Morus alba | bioassay | sample size 5 independent pairs, 5 replicates | Produced by head space absorption method | |
| MDVs | Volatile from mechanically-damaged Morus alba | bioassay | sample size 5 independent pairs, 5 replicates | Produced by head space absorption method |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, R.; Zhang, F.; Zhang, Z.-N. Electrophysiological Responses and Reproductive Behavior of Fall Webworm Moths (Hyphantria cunea Drury) are Influenced by Volatile Compounds from Its Mulberry Host (Morus alba L.). Insects 2016, 7, 19. https://doi.org/10.3390/insects7020019
Tang R, Zhang F, Zhang Z-N. Electrophysiological Responses and Reproductive Behavior of Fall Webworm Moths (Hyphantria cunea Drury) are Influenced by Volatile Compounds from Its Mulberry Host (Morus alba L.). Insects. 2016; 7(2):19. https://doi.org/10.3390/insects7020019
Chicago/Turabian StyleTang, Rui, Feng Zhang, and Zhong-Ning Zhang. 2016. "Electrophysiological Responses and Reproductive Behavior of Fall Webworm Moths (Hyphantria cunea Drury) are Influenced by Volatile Compounds from Its Mulberry Host (Morus alba L.)" Insects 7, no. 2: 19. https://doi.org/10.3390/insects7020019






