Oviposition by Female Plodia interpunctella (Lepidoptera: Pyralidae): Description and Time Budget Analysis of Behaviors in Laboratory Studies
Abstract
:1. Introduction
2. Experimental Section
2.1. Insects
2.2. Study 1: Behaviors Exhibited by Female P. interpunctella during Oviposition
2.3. Study 2: Analysis of P. interpunctella Time Allocation Behavior during Oviposition
2.4. Study 3: Real-Time Analysis of the Visit Pattern of P. interpunctella Females toward a Food Source
2.5. Data Analyses
3. Results
3.1. Study 1: Behaviors Exhibited by P. interpunctella Females during Oviposition

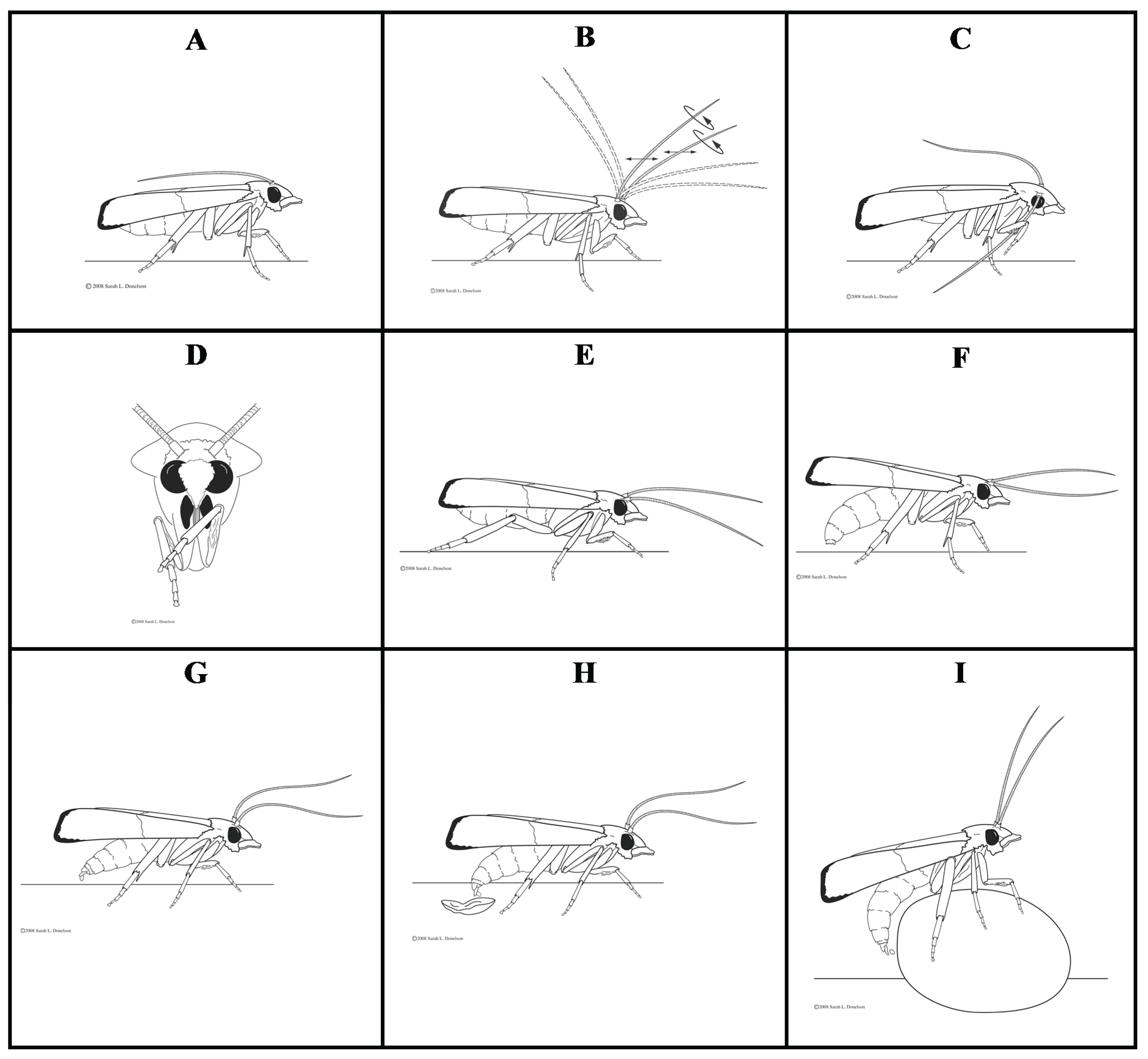
3.1.1. Resting
3.1.2. Antennal Movement
3.1.3. Grooming
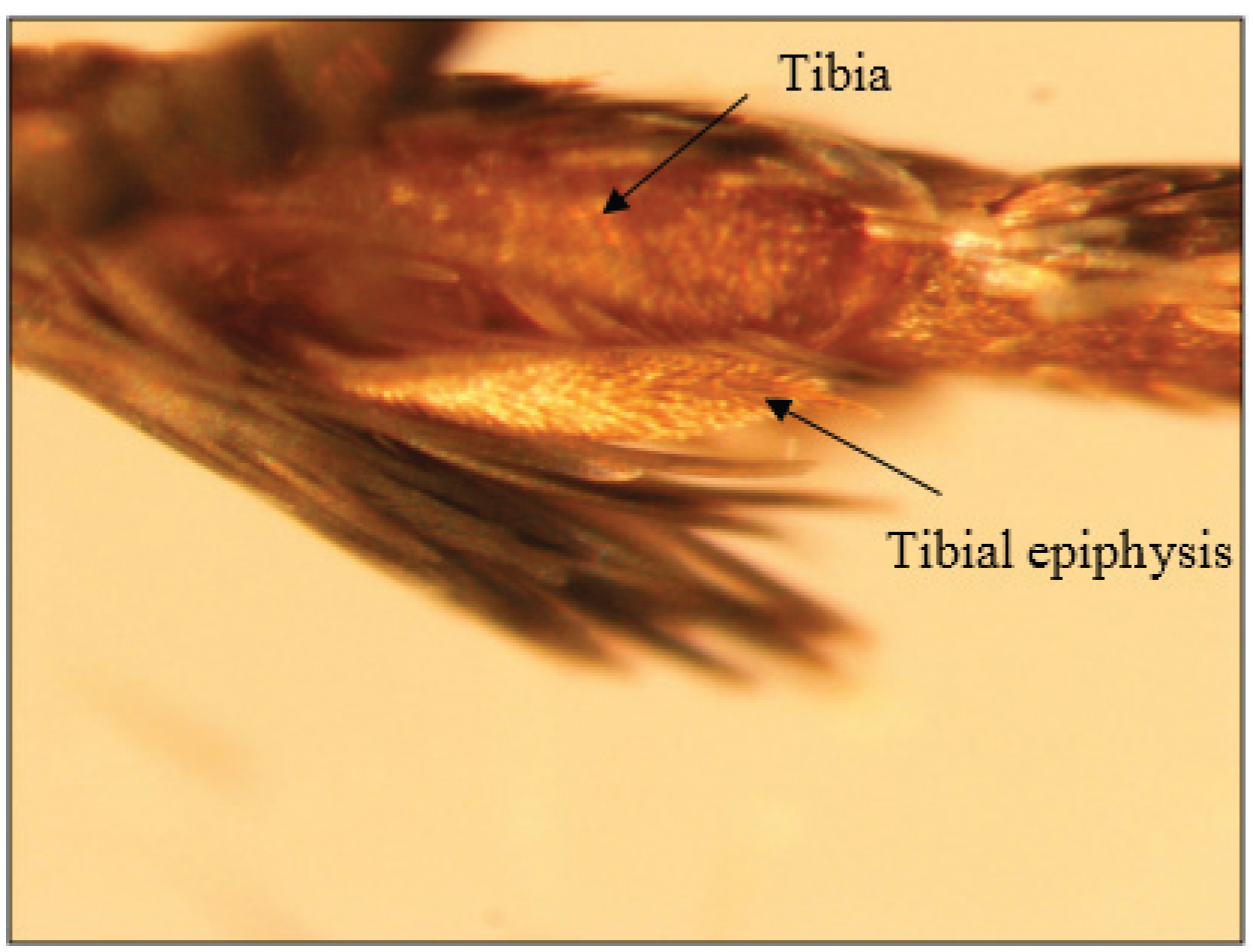
3.1.4. Walking and Flying
3.1.5. Abdomen Bending and Dragging
3.1.6. Oviposition
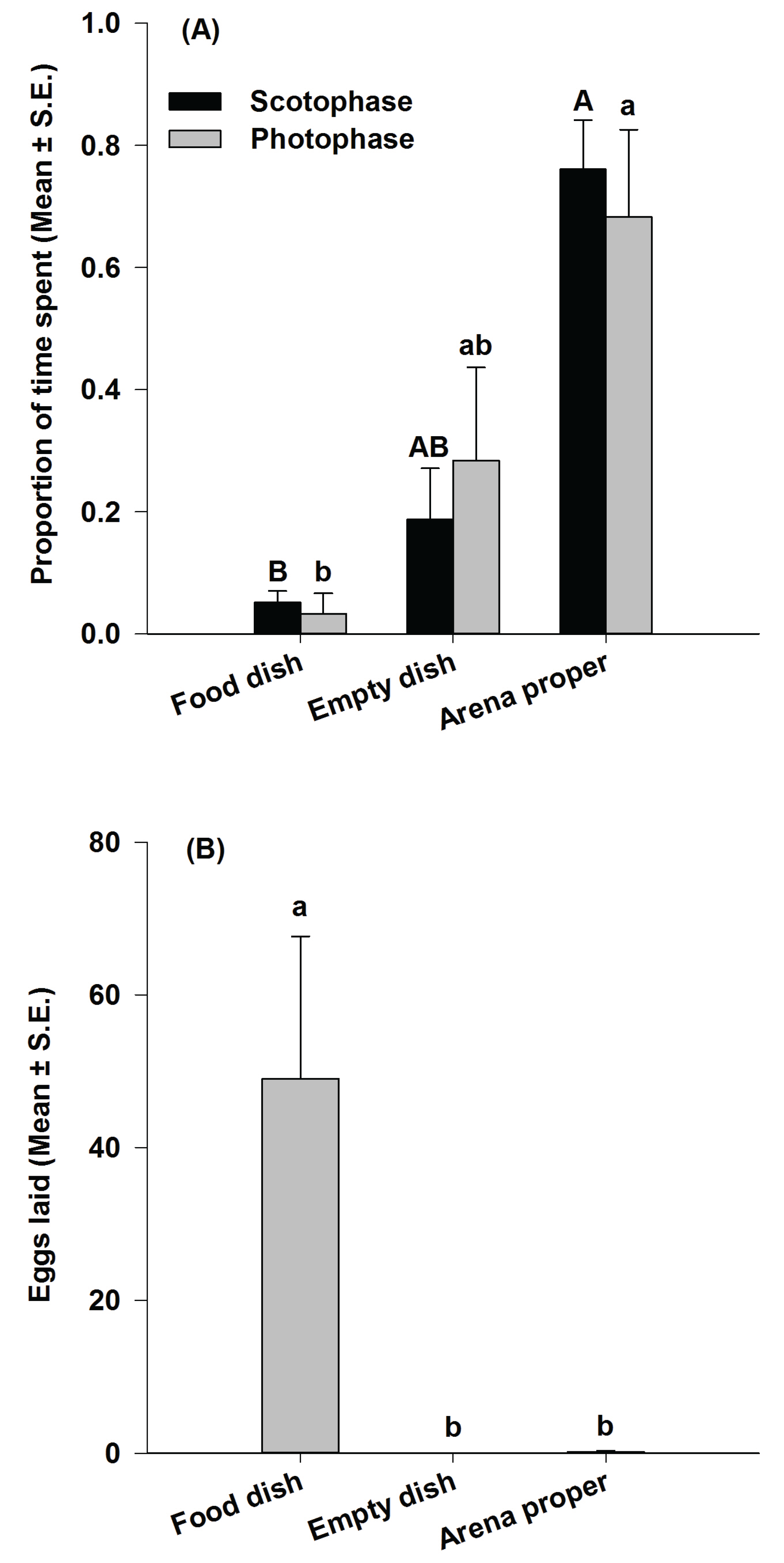
3.2. Study 2: Analysis of P. interpunctella Time Allocation Behavior during Oviposition
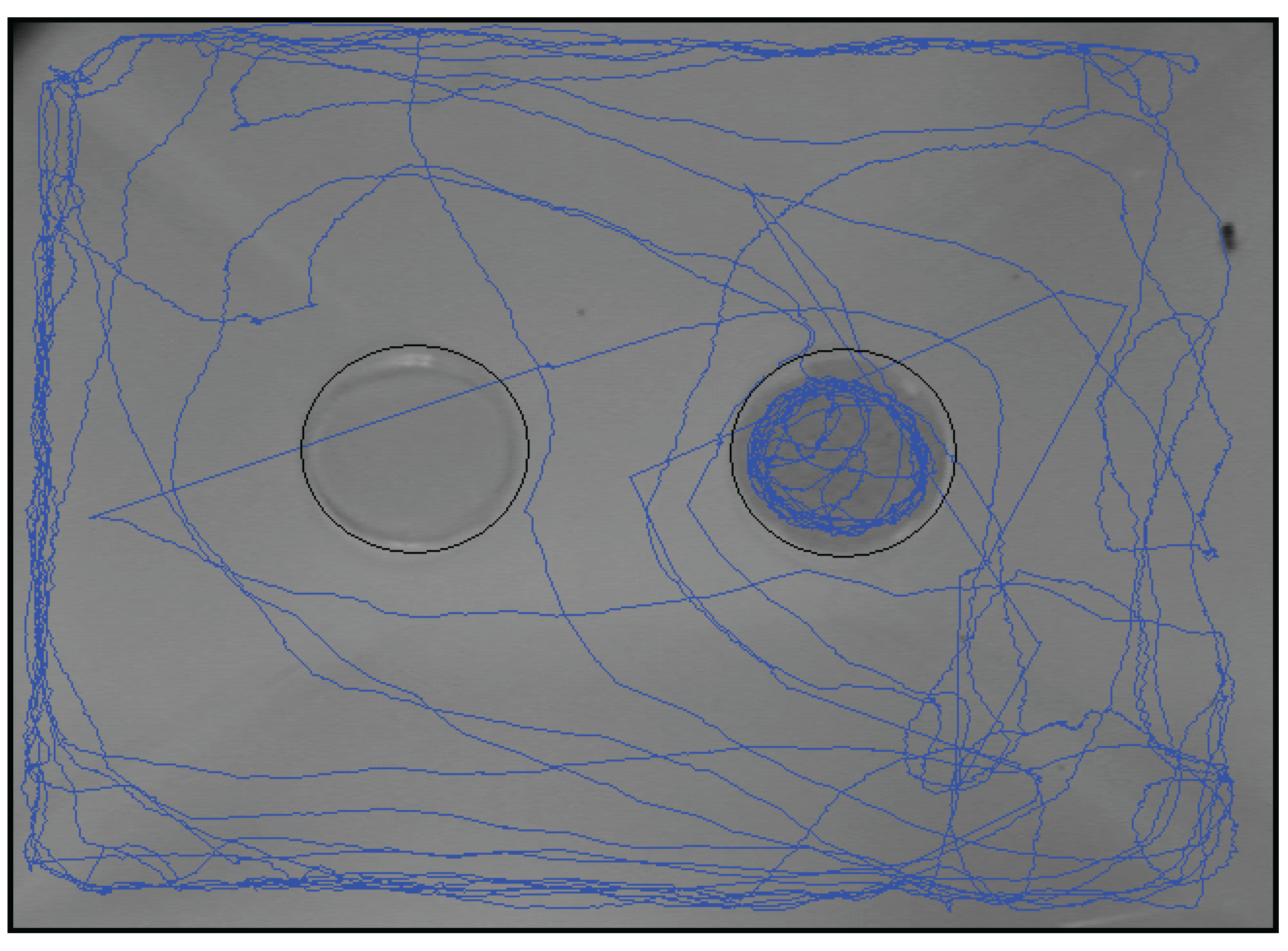
3.3. Study 3: Real-Time Analysis of the Visit Pattern of P. interpunctella Females Toward a Food Source
| Observation † | Day 1 | Day 2 | Total |
|---|---|---|---|
| Number of visits | 6.5 ± 1.2 | 11.5 ± 1.9 | 18.0 ± 1.6 |
| Total time spent in food dish (min) | 28.0 ± 9.3 | 53.9 ± 10.5 | 81.9 ± 11.9 |
| Observations | |
|---|---|
| Duration of visit (range) | 0.02–27.40 min |
| Duration of visit latency (range) | 0.02–277.10 min |
| Average number of eggs laid in the dish † | 166.3 ± 70.2 |
| Average total number of eggs † | 168.5 ± 70.8 |

4. Discussion
5. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rees, D.P. Insects of Stored Products; CSIRO Publishing: Collingwood, Victoria, Australia, 2004. [Google Scholar]
- Doud, C.W.; Phillips, T.W. Activity of Plodia interpunctella (Lepidoptera:Pyralidae) in and around flour mills. J. Econ. Entomol. 2000, 93, 1842–1847. [Google Scholar] [CrossRef] [PubMed]
- Vick, K.W.; Koehler, P.G.; Neal, J.J. Incidence of stored-product Phycitinae moths in food distribution warehouses as determined by sex pheromone-baited traps. J. Econ. Entomol. 1986, 79, 936–939. [Google Scholar] [CrossRef]
- Campbell, J.F.; Mullen, M.A.; Dowdy, A.K. Monitoring stored-product pests in food processing plants with pheromone trapping, contour mapping, and mark-recapture. J. Econ. Entomol. 2002, 95, 1089–1101. [Google Scholar] [CrossRef] [PubMed]
- Arbogast, R.T.; Kendra, P.E.; Mankin, R.W.; McGovern, J.E. Monitoring insect pests in retail stores by trapping and spatial analysis. J. Econ. Entomol. 2000, 93, 1531–1542. [Google Scholar] [CrossRef] [PubMed]
- Grant, G.; Brady, U. Courtship behavior of phycitid moths. I. Comparison of Plodia interpunctella and Cadra cautella and role of male scent glands. Can. J. Zool. 1975, 53, 813–826. [Google Scholar] [CrossRef]
- Phelan, P.L.; Baker, T.C. Comparative study of courtship in twelve phycitine moths (Lepidoptera: Pyralidae). J. Insect Behav. 1990, 3, 303–326. [Google Scholar] [CrossRef]
- Cox, P.D.; Bell, C.H. Biology and ecology of moth pests of stored foods. In Ecology and Management of Food-Industry Pests; FDA Tech. Bull. 4; Gorham, J.R., Ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1991; pp. 181–194. [Google Scholar]
- Mohandass, S.; Arthur, F.H.; Zhu, K.Y.; Throne, J.E. Biology and management of Plodia interpunctella (Lepidoptera: Pyralidae) in stored products. J. Stored Prod. Res. 2007, 43, 302–311. [Google Scholar] [CrossRef]
- Phillips, T.W.; Strand, M.R. Larval secretions and food odors affect orientation in female Plodia interpunctella. Entomol. Exp. Appl. 1994, 71, 185–192. [Google Scholar] [CrossRef]
- Olsson, P.-O.C.; Anderbrant, O.; Löfstedt, C. Flight and oviposition behavior of Ephestia cautella and Plodia interpunctella in response to odors of different chocolate products. J. Insect Behav. 2005, 18, 363–380. [Google Scholar] [CrossRef]
- Olsson, P.-O.C.; Anderbrant, O.; Löfstedt, C. Experience influences oviposition behaviour in two pyralid moths, Ephestia cautella and Plodia interpunctella. Anim. Behav. 2006, 72, 545–551. [Google Scholar] [CrossRef]
- Olsson, P.-O.C.; Anderbrant, O.; Löfstedt, C.; Borg-Karlson, A.-K.; Liblikas, I. Electrophysiological and behavioral responses to chocolate volatiles in both sexes of the pyralid moths Ephestia cautella and Plodia interpunctella. J. Chem. Ecol. 2005, 31, 2947–2961. [Google Scholar] [CrossRef] [PubMed]
- Uechi, K.; Matsuyama, S.; Suzuki, T. Oviposition attractants for Plodia interpunctella (Hübner) (Lepidoptera: Pyralidae) in the volatiles of whole wheat flour. J. Stored Prod. Res. 2007, 43, 193–201. [Google Scholar] [CrossRef]
- Sambaraju, K.R.; Phillips, T.W. Ovipositional preferences and larval performances of two populations of Indianmeal moth, Plodia interpunctella. Entomol. Exp. Appl. 2008, 128, 283–293. [Google Scholar] [CrossRef]
- Nansen, C.; Phillips, T.W. Ovipositional responses of the Indianmeal moth, Plodia interpunctella (Hübner) (Lepidoptera : Pyralidae) to oils. Ann. Entomol. Soc. Am. 2003, 96, 524–531. [Google Scholar]
- Sambaraju, K.R.; Phillips, T.W. Effects of physical and chemical factors on oviposition by Plodia interpunctella (Lepidoptera: Pyralidae). Ann. Entomol. Soc. Am. 2008, 101, 955–963. [Google Scholar] [CrossRef]
- Gautam, S.G.; Opit, G.P.; Margosan, D.; Hoffmann, D.; Tebbets, J.S.; Walse, S. Comparative egg morphology and chorionic ultrastructure of key stored-product insect pests. Ann. Entomol. Soc. Am. 2015, 108, 43–56. [Google Scholar] [CrossRef]
- Huang, F.; Subramanyam, B. Effects of delayed mating on reproductive performance of Plodia interpunctella (Hübner) (Lepidoptera: Pyralidae). J. Stored Prod. Res. 2003, 39, 53–63. [Google Scholar] [CrossRef]
- SAS Institute Inc. The SAS System for Windows, Release 9.3; SAS Institute Inc.: Cary, NC, USA, 2011. [Google Scholar]
- Zar, J.H. Biostatistical Analysis, 4th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 1999. [Google Scholar]
- Justus, K.A.; Mitchell, B.K. Oviposition site selection by the diamondback moth, Plutella xylostella (L.) (Lepidoptera: Plutellidae). J. Insect Behav. 1996, 9, 887–898. [Google Scholar] [CrossRef]
- Hora, K.H.; Roessingh, P. Oviposition in Yponomeuta cagnagellus: The importance of contact cues for host plant acceptance. Physiol. Entomol. 1999, 24, 109–120. [Google Scholar] [CrossRef]
- ODell, T.M.; Shields, K.S.; Mastro, V.C.; Kring, T.J. The epiphysis of the gypsy moth, Lymantria dispar (Lepidoptera: Lymantriidae): Structure and function. Can. Entomol. 1982, 114, 751–761. [Google Scholar] [CrossRef]
- Robbins, R.K. Systematic implications of butterfly leg structures that clean the antennae. Psyche 1989, 96, 209–222. [Google Scholar] [CrossRef]
- Munroe, E.; Solis, A. The Pyraloidea. In Lepidoptera, Moths and Butterflies, Volume 1: Evolution, Systematics, and Biogeography; Handbook of Zoology 35; Kristensen, N.P., Ed.; Walter de Gruyter & Co.: Berlin, Germany, 1999; pp. 233–256. [Google Scholar]
- Anderson, P.; Hallberg, E. Structure and distribution of tactile and bimodal taste/tactile sensilla on the ovipositor, tarsi and antennae of the flour moth, Ephestia kuehniella (Zeller) (Lepidoptera: Pyralidae). Int. J. Insect Morphol. Embryol. 1990, 19, 13–23. [Google Scholar] [CrossRef]
- Faucheux, M.J. Morphology and distribution of sensilla on the cephalic appendages, tarsi and ovipositor of the European sunflower moth, Homoeosoma nebulella Den. & Schiff. (Lepidoptera: Pyralidae). Int. J. Insect Morphol. Embryol. 1991, 20, 291–307. [Google Scholar]
- Ndomo-Moualeu, A.F.; Ulrichs, C.; Radek, R.; Adler, C. Structure and distribution of antennal sensilla in the Indianmeal moth, Plodia interpunctella (Hübner, 1813) (Lepidoptera: Pyralidae). J. Stored Prod. Res. 2014, 59, 66–75. [Google Scholar] [CrossRef]
- Deseo, K.V. The oviposition of the Indian meal moth (Plodia interpunctella Hübner, Lep., Phycitidae) influenced by olfactory stimuli and antennectomy. Symp. Biol. Hung. 1976, 16, 61–65. [Google Scholar]
- Miller, J.R.; Strickler, K.L. Finding and accepting host plants. In Chemical Ecology of Insects; Bell, W.J., Cardé, R.T., Eds.; Sinauer Associates, Inc.: Sunderland, MA, USA, 1984; pp. 127–157. [Google Scholar]
- Faucheux, M.J. Sensilla on the antennae, mouthparts, tarsi and ovipositor of the sunflower moth, Homoeosoma electellum (Hulster) (Lepidoptera, Pyralidae): A scanning electron microscopic study. Ann. Sci. Nat. Zool. Biol. Anim. 1995, 16, 121–136. [Google Scholar]
- Walters, B.D.; Albert, P.J.; Zacharuk, R.Y. Morphology and ultrastructure of sensilla on the proboscis of the adult spruce budworm, Choristoneura fumiferana (Clem.) (Lepidoptera: Tortricidae). Can. J. Zool. 1998, 76, 466–479. [Google Scholar] [CrossRef]
- Banga, N.; Albert, P.J.; Kapoor, N.N.; McNeil, J.N. Structure, distribution, and innervation of sensilla on the ovipositor of the spruce budworm, Choristoneura fumiferana, and evidence of a gustatory function for type II sensilla. Can. J. Zool. 2003, 81, 2032–2037. [Google Scholar] [CrossRef]
- Yamaoka, K.; Hirao, T. Releasing signals of oviposition behavior in Bombyx mori. J. Insect Physiol. 1973, 19, 2215–2223. [Google Scholar] [CrossRef]
- Maher, N.; Thiery, D. Distribution of chemo- and mechanoreceptors on the tarsi and ovipositor of female European grapevine moth, Lobesia botrana. Entomol. Exp. Appl. 2004, 110, 135–143. [Google Scholar] [CrossRef]
- Chadha, G.K.; Roome, R.E. Oviposition behaviour and the sensilla of the ovipositor of Chilo partellus and Spodoptera littoralis (Lepidoptera: Noctuidae). J. Zool. 1980, 192, 169–178. [Google Scholar] [CrossRef]
- Hattori, M. Host-plant factors responsible for oviposition behaviour in the limabean pod borer, Etiella zinckenella Treitschke. J. Insect Physiol. 1988, 34, 191–196. [Google Scholar] [CrossRef]
- Ramaswamy, S.B. Periodicity of oviposition, feeding, and calling by mated female Heliothis virescens in a field cage. J. Insect Behav. 1990, 3, 417–427. [Google Scholar] [CrossRef]
- Weston, P.A.; Rattlingourd, P.L. Ovipositional stimuli of Angoumois grain moth (Lepidoptera: Gelechiidae), a primary pest of stored grains. J. Entomol. Sci. 1999, 34, 445–451. [Google Scholar]
- Fadamiro, H.Y.; Wyatt, T.D. Flight initiation by Prostephanus truncatus in relation to time of day, temperature, relative humidity and starvation. Entomol. Exp. Appl. 1995, 75, 273–277. [Google Scholar] [CrossRef]
- Aslam, M.; Hagstrum, D.W.; Dover, B.A. The effect of photoperiod on the flight activity and biology of Rhyzopertha dominica (Coleoptera: Bostrichidae). J. Kans. Entomol. Soc. 1994, 67, 107–115. [Google Scholar]
- Wright, E.J.; Morton, R. Daily flight activity of Trogoderma variabile (Coleoptera: Dermestidae) and Rhyzopertha dominica (Coleoptera: Bostrichidae). J. Stored Prod. Res. 1995, 31, 177–184. [Google Scholar] [CrossRef]
- Lum, P.T.M.; Flaherty, B.R. Regulating oviposition by Plodia interpunctella in laboratory by light and dark conditions. J. Econ. Entomol. 1970, 63, 236–239. [Google Scholar] [CrossRef]
- Madrid, F.J.; Sinha, R.N. Movement and oviposition of Ephestia cautella (Walker) and Plodia interpunctella (Hübner) (Lepidoptera: Pyralidae) of different ages in response to seasonal light changes. Can. J. Zool. 1983, 61, 1726–1732. [Google Scholar] [CrossRef]
- Sambaraju, K.R.; Phillips, T.W. Responses of adult Plodia interpunctella (Hübner) (Lepidoptera: Pyralidae) to light and combinations of attractants and light. J. Insect Behav. 2008, 21, 422–439. [Google Scholar] [CrossRef]
- Quentin, M.E.; Spencer, J.L.; Miller, J.R. Bean tumbling as a control measure for the common bean weevil, Acanthoscelides obtectus. Entomol. Exp. Appl. 1991, 60, 105–109. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sambaraju, K.R.; Donelson, S.L.; Bozic, J.; Phillips, T.W. Oviposition by Female Plodia interpunctella (Lepidoptera: Pyralidae): Description and Time Budget Analysis of Behaviors in Laboratory Studies. Insects 2016, 7, 4. https://doi.org/10.3390/insects7010004
Sambaraju KR, Donelson SL, Bozic J, Phillips TW. Oviposition by Female Plodia interpunctella (Lepidoptera: Pyralidae): Description and Time Budget Analysis of Behaviors in Laboratory Studies. Insects. 2016; 7(1):4. https://doi.org/10.3390/insects7010004
Chicago/Turabian StyleSambaraju, Kishan R., Sarah L. Donelson, Janko Bozic, and Thomas W. Phillips. 2016. "Oviposition by Female Plodia interpunctella (Lepidoptera: Pyralidae): Description and Time Budget Analysis of Behaviors in Laboratory Studies" Insects 7, no. 1: 4. https://doi.org/10.3390/insects7010004





