Mating Success, Longevity, and Fertility of Diabrotica virgifera virgifera LeConte (Chrysomelidae: Coleoptera) in Relation to Body Size and Cry3Bb1-Resistant and Cry3Bb1-Susceptible Genotypes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Resistant and Susceptible Populations
2.2. Handling of Experimental Insects
2.3. Mating and Female Fertility Experiments
| Sex | Genotype | n | Weight ± SD | Range | Age ± SD | Range |
|---|---|---|---|---|---|---|
| Male | Resistant | 138 | 11.4 ± 1.8 | 6.6–17.1 | 6.9 ± 2.6 | 2–14 |
| Susceptible | 151 | 11.8 ± 1.8 | 7.4–16.6 | 7.5 ± 3.2 | 3–18 | |
| Female | Resistant | 140 | 12.6 ± 2.2 | 7.7–18.9 | 5.2 ± 2.7 | 1–11 |
| Susceptible | 149 | 12.8 ± 2.0 | 8.3–17.3 | 6.7 ± 3.3 | 1–16 |
2.4. Statistical Analysis
3. Results
| Cross | Proportion Mating (%) | Duration (min) | |||
|---|---|---|---|---|---|
| Courtship | Copulation | ||||
| Mean ± SD | Range | Mean ± SD | Range | ||
| SMSF | 33/89 (37) | 14.7 ± 22.6 | 1–103 | 174.6 ± 30.0 | 103–231 |
| SMRF | 30/62 (48) | 11.6 ± 16.9 | 0–83 | 179.7 ± 31.4 | 127–292 |
| RMSF | 28/60 (47) | 16.8 ± 30.9 | 1–118 | 160.4 ± 31.3 | 110–223 |
| RMRF | 29/78 (37) | 18.7 ± 23.4 | 1–79 | 176.4 ± 36.4 | 120–270 |
| Cross | n | Longevity * | Total Eggs † | Potentially Viable Eggs ‡ | |||
|---|---|---|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range | Mean ± SD | Range | ||
| SMSF | 32 | 133 ± 45 | 22–191 | 1860 ± 789 | 68–3092 | 1780 ± 766 | 68–3024 |
| SMRF | 29 | 139 ± 47 | 26–210 | 1728 ± 871 | 1–3187 | 1646 ± 850 | 0–3142 |
| RMSF | 27 | 130 ± 51 | 12–220 | 1885 ± 701 | 122–3014 | 1822 ± 707 | 105–2980 |
| RMRF | 29 | 106 ± 57 | 7–241 | 1686 ± 851 | 387–3398 | 1610 ± 835 | 382–3380 |
| Male | Longevity * | Total Eggs | Potentially Viable Eggs † | |||
|---|---|---|---|---|---|---|
| n | Mean ± SD | n ‡ | Mean ± SD | n | Mean ± SD | |
| Resistant | 56 | 117 ± 55 a | 51 | 1787 ± 777 | 51 | 1718 ± 772 |
| Susceptible | 61 | 136 ± 45 b | 62 | 1798 ± 825 | 62 | 1717 ± 802 |
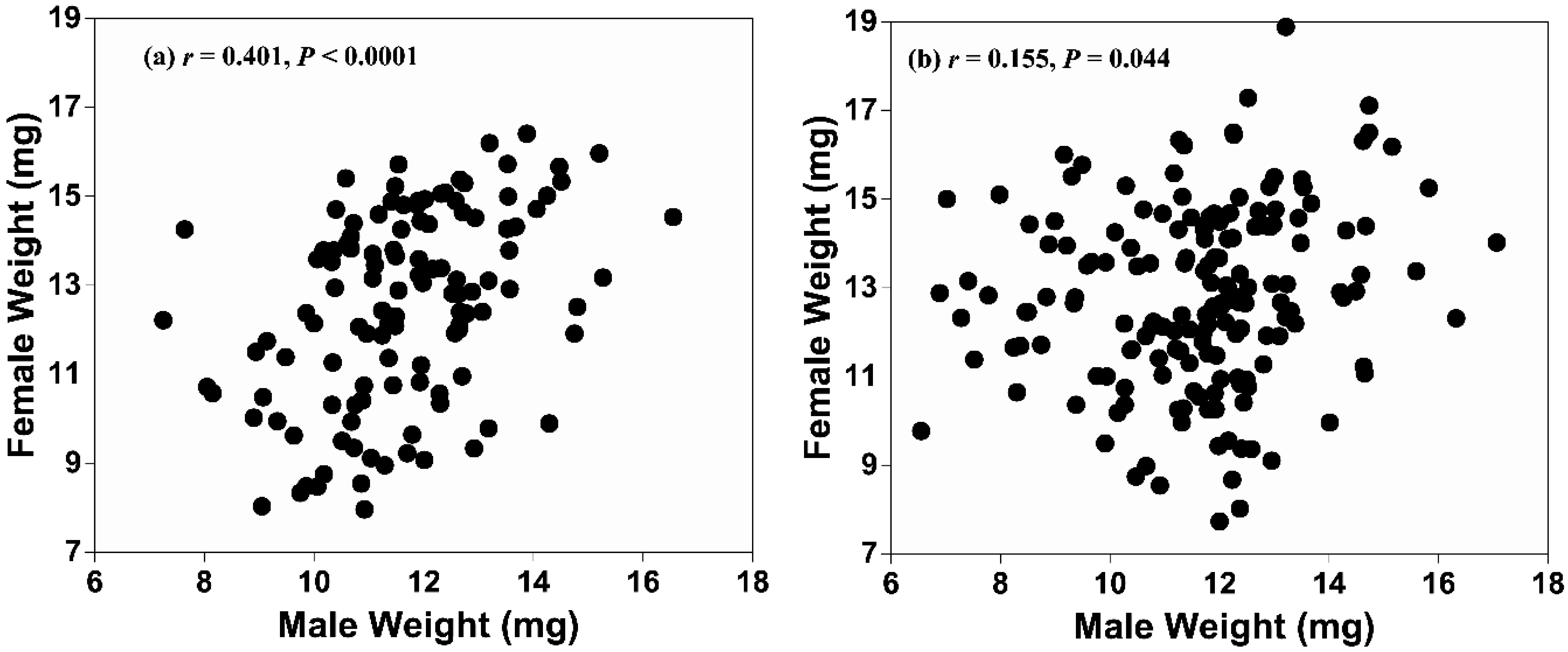
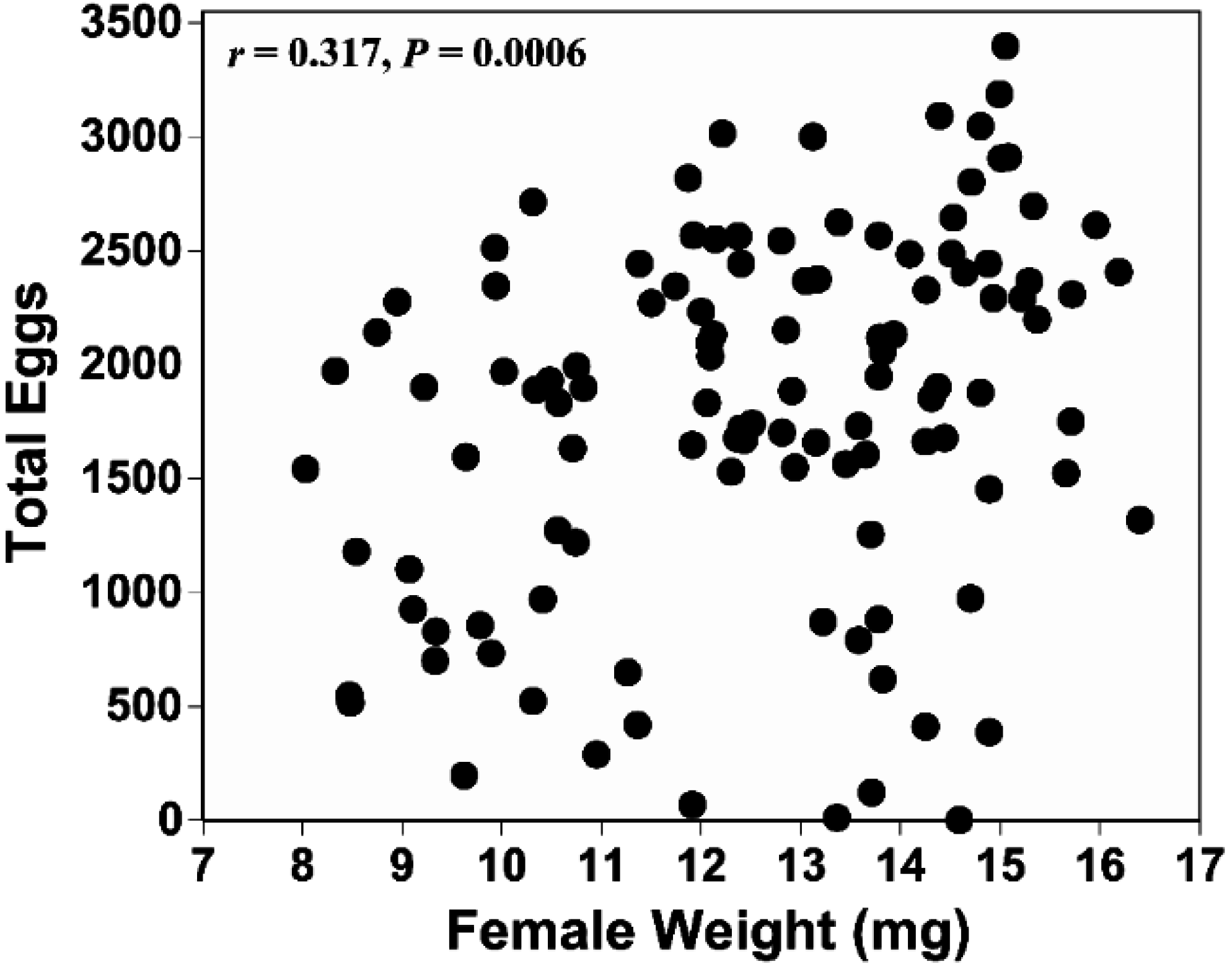
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix
| Colony a | Sex | n | Pupae | Adult | Difference | r b |
|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | ||||
| Lab (D) | Male | 261 | 11.4 ± 1.8 | 9.6 ± 1.9 | −1.8 ± 1.0 | 0.84 |
| Female | 298 | 12.9 ± 2.1 | 11.1 ± 2.0 | −1.7 ± 1.1 | 0.87 | |
| Lab (ND) | Male | 301 | 13.7 ± 2.7 | 12.2 ± 2.3 | −1.6 ± 1.1 | 0.91 |
| Female | 322 | 16.2 ± 2.8 | 14.4 ± 2.5 | −1.8 ± 1.4 | 0.85 | |
| Field | Male | 164 | 10.2 ± 2.2 | 8.7 ± 2.0 | −1.4 ± 0.8 | 0.92 |
| Female | 325 | 10.5 ± 2.1 | 9.1 ± 1.9 | −1.5 ± 0.8 | 0.92 |
References
- Metcalf, R.L. Foreword. In Methods for the Study of Pest Diabrotica; Krysan, J.L., Miller, T.A., Eds.; Springer: New York, NY, USA, 1986; pp. vii–xv. [Google Scholar]
- Sappington, T.W.; Siegfried, B.D.; Guillemaud, T. Coordinated Diabrotica genetics research: Accelerating progress on an urgent insect pest problem. Am. Entomol. 2006, 52, 90–97. [Google Scholar] [CrossRef]
- Gray, M.E.; Sappington, T.W.; Miller, N.J.; Moeser, J.; Bohn, M.O. Adaptation and invasiveness of western corn rootworm: Intensifying research on a worsening pest. Annu. Rev. Entomol. 2009, 54, 303–321. [Google Scholar] [CrossRef] [PubMed]
- Ball, H.J.; Weekman, G.T. Differential resistance of corn rootworms to insecticides in Nebraska and adjoining states. J. Econ. Entomol. 1963, 56, 553–555. [Google Scholar] [CrossRef]
- Meinke, L.J.; Siegfried, B.D.; Wright, R.J.; Chandler, L.D. Adult susceptibility of Nebraska western corn rootworm (Coleoptera: Chrysomelidae) populations to selected insecticides. J. Econ. Entomol. 1998, 91, 594–600. [Google Scholar] [CrossRef]
- Wright, R.J.; Scharf, M.E.; Meinke, L.J.; Zhou, X.; Siegfried, B.D.; Chandler, L.D. Larval susceptibility of an insecticide-resistant western corn rootworm (Coleoptera: Chrysomelidae) population to soil insecticides: Laboratory bioassays, assays of detoxification enzymes, and field performance. J. Econ. Entomol. 2000, 93, 7–13. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhu, K.Y.; Wilde, G.E.; Higgins, R.A.; Sloderbeck, P.E.; Buschman, L.L.; Shufran, R.A.; Whitworth, R.J.; Starkey, S.R.; He, F. Evidence of evolving carbaryl resistance in western corn rootworm (Coleoptera: Chrysomelidae) in areawide-managed cornfields in north central Kansas. J. Econ. Entomol. 2001, 94, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Levine, E.; Spencer, J.L.; Isard, A.; Onstad, D.W.; Gray, M.E. Adaptation of the western corn rootworm to crop rotation: Evolution of a new strain in response to a management practice. Am. Entomol. 2002, 48, 94–107. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (USEPA). Bacillus thuringiensis Cry3Bb1 Protein and the Genetic Material Necessary for Its Production (Vector ZMIR13L) in Event MON863 Corn (006484) Biopesticide Registration Action Document (BRAD). 2007; USEPA: Washington, DC, USA. Available online: http://www.epa.gov/oppbppd1/biopesticides/ingredients_keep/tech_docs/brad_006484.htm (accessed on 16 June 2015). [Google Scholar]
- United States Environmental Protection Agency (USEPA). Biopesticides Registration Action Document (BRAD): MON 89034 × TC1507 × MON 88017 × DAS-59122–7 (SmartStax) Bt. Corn Seed Blend. 2011; USEPA: Washington, DC, USA. Available online: http://www.regulations.gov/#!docketDetail;D=EPA-HQ-OPP-2011–0362 (accessed on 22 June 2015). [Google Scholar]
- Vaughn, T.; Cavato, T.; Brar, G.; Coombe, T.; de Gooyer, T.; Ford, S.; Groth, M.; Howe, A.; Johnson, S.; Kolacz, K.; et al. A method of controlling corn rootworm feeding using a Bacillus thuringiensis protein expressed in transgenic maize. Crop Sci. 2005, 45, 931–938. [Google Scholar] [CrossRef]
- Storer, N.P.; Babcock, J.M.; Edwards, J.M. Field measures of western corn rootworm (Coleoptera: Chrysomelidae) mortality caused by Cry34/35Ab1 proteins expressed in maize event 59122 and implications for trait durability. J. Econ. Entomol. 2006, 99, 1381–1387. [Google Scholar] [CrossRef] [PubMed]
- Lefko, S.A.; Nowatzki, T.M.; Thompson, S.D.; Binning, R.R.; Pascual, M.A.; Peters, M.L.; Simbro, E.J.; Stanley, B.H. Characterizing laboratory colonies of western corn rootworm (Coleoptera: Chrysomelidae) selected for survival on maize containing event DAS-59122-7. J. Appl. Entomol. 2008, 132, 189–204. [Google Scholar] [CrossRef]
- Meihls, L.N.; Higdon, M.L.; Siegfried, B.D.; Miller, N.J.; Sappington, T.W.; Ellersieck, M.R.; Spencer, T.A.; Hibbard, B.E. Increased survival of western corn rootworm on transgenic corn within three generations of on-plant greenhouse selection. Proc. Natl. Acad. Sci. USA 2008, 105, 19177–19182. [Google Scholar] [CrossRef] [PubMed]
- Meihls, L.N.; Higdon, M.L.; Ellersieck, M.; Hibbard, B.E. Selection for resistance to mCry3A-expressing transgenic corn in western corn rootworm. J. Econ. Entomol. 2011, 104, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Meihls, L.N.; Higdon, M.L.; Ellersieck, M.; Tabashnik, B.E.; Hibbard, B.E. Greenhouse-selected resistance to Cry3Bb1-producing corn in three western corn rootworm populations. PLoS ONE 2012, 7, e51055. [Google Scholar] [CrossRef] [PubMed]
- Tabashnik, B.E. Delaying insect resistance to transgenic crops. Proc. Natl. Acad. Sci. USA 2008, 105, 19029–19030. [Google Scholar] [CrossRef] [PubMed]
- Oswald, K.J.; French, B.W.; Nielson, C.; Bagley, M. Selection for Cry3Bb1 resistance in a genetically diverse population of nondiapausing western corn rootworm (Coleoptera: Chrysomelidae). J. Econ. Entomol. 2011, 104, 1038–1044. [Google Scholar] [CrossRef] [PubMed]
- Gassmann, A.J.; Petzold-Maxwell, J.L.; Keweshan, R.S.; Dunbar, M.W. Field-evolved resistance to Bt maize by western corn rootworm. PLoS ONE 2011, 6, e22629. [Google Scholar] [CrossRef] [PubMed]
- Gassmann, A.J.; Petzold-Maxwell, J.L.; Clifton, E.H.; Dunbar, M.W.; Hoffmann, A.M.; Ingber, D.A.; Keweshan, R.S. Field-evolved resistance by western corn rootworm to multiple Bacillus thuringiensis toxins in transgenic maize. Proc. Natl. Acad. Sci. USA 2014, 111, 5141–5146. [Google Scholar] [CrossRef] [PubMed]
- Wangila, D.S.; Gassmann, A.A.; Petzold-Maxwell, J.L.; French, B.W.; Meinke, L.J. Susceptibility of Nebraska western corn rootworm (Coleoptera: Chrysomelidae) populations to Bt corn events. J. Econ. Entomol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Onstad, D.W.; Guse, C.A.; Spencer, J.L.; Levine, E.; Gray, M.E. Modeling the dynamics of adaptation to transgenic corn by western corn rootworm (Coleoptera: Chrysomelidae). J. Econ. Entomol. 2001, 94, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Storer, N.P. A spatially explicit model simulating western corn rootworm (Coleoptera: Chrysomelidae) adaptation to insect-resistant maize. J. Econ. Entomol. 2003, 96, 1530–1547. [Google Scholar] [CrossRef] [PubMed]
- Marquardt, P.T.; Krupke, C.H. Dispersal and mating behavior of Diabrotica virgifera virgifera (Coleoptera: Chrysomelidae) in Bt cornfields. Environ. Entomol. 2009, 38, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.L.; Hibbard, B.E.; Moeser, J.; Onstad, D.W. Behaviour and ecology of western corn rootworm (Diabrotica virgifera virgigera LeConte). Agric. For. Entomol. 2009, 11, 9–27. [Google Scholar] [CrossRef]
- Costa, S.D.; Barbercheck, M.E.; Kennedy, G.G. Sublethal acute and chronic exposure of Colorado potato beetle (Coleoptera: Chrysomelidae) to the *-endotoxin of Bacillus thuringiensis. J. Econ. Entomol. 2000, 93, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Bates, S.L.; Zhao, J.-T.; Roush, R.T.; Shelton, A.M. Insect resistance management in GM crops: Past, present and future. Nat. Biotech. 2005, 23, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Gassmann, A.J.; Carrière, Y.; Tabashnik, B.E. Fitness costs of insect resistance to Bacillus thuringiensis. Annu. Rev. Entomol. 2009, 54, 147–163. [Google Scholar] [CrossRef] [PubMed]
- Siegfried, B.D.; Vaughn, T.T.; Spencer, T. Baseline susceptibility of western corn rootworm (Coleoptera: Chrysomelidae) to Cry3Bb1 Bacillus thuringiensis toxin. J. Econ. Entomol. 2005, 98, 1320–1324. [Google Scholar] [CrossRef] [PubMed]
- Hibbard, B.E.; el Khishen, A.A.; Vaughn, T.T. Impact of MON863 transgenic roots is equivalent on western corn rootworm larvae for a wide range of maize phonologies. J. Econ. Entomol. 2009, 102, 1607–1613. [Google Scholar] [CrossRef] [PubMed]
- Oswald, K.J.; French, B.W.; Nielson, C.; Bagley, M. Assessment of fitness costs in Cry3Bb1-resistant and susceptible western corn rootworm (Coleoptera: Chrysomelidae) laboratory colonies. J. Appl. Entomol. 2012, 136, 730–740. [Google Scholar] [CrossRef]
- Petzold-Maxwell, J.L.; Cibils-Stewart, X.; French, B.W.; Gassmann, A.J. Adaptation by western corn rootworm (Coleoptera: Chrysomelidae) to Bt maize: Inheritance, fitness costs, and feeding preference. J. Econ. Entomol. 2012, 105, 1407–1418. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.M.; French, B.W.; Jaronski, S.; Gassmann, A.J. Effects of entomopathogens on mortality of western corn rootworm and fitness costs of resistance to Cry3Bb1 maize. J. Econ. Entomol. 2014, 107, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.M.; French, B.W.; Hellmich, R.L.; Lauter, N.; Gassmann, A.J. Fitness costs of resistance to Cry3Bb1 maize by western corn rootworm. J. Appl. Entomol. 2015, 139, 403–415. [Google Scholar] [CrossRef]
- Thornhill, R.; Alcock, J. The Evolution of Insect Mating Systems; Harvard University Press: Cambridge, MA, USA, 1983. [Google Scholar]
- Kokko, H.; Klug, H.; Jennions, M.D. Mating systems. In The Evolution of Insect Mating Systems; Shuker, D.M., Simmons, L.W., Eds.; Oxford University Press: Oxford, UK, 2014; pp. 42–58. [Google Scholar]
- Gwynne, D.T. Sexual conflict over nuptial gifts in insects. Annu. Rev. Entomol. 2008, 53, 83–101. [Google Scholar] [CrossRef] [PubMed]
- Kuijper, B.; Pen, I.; Weissing, F.J. A guide to sexual selection theory. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 287–311. [Google Scholar] [CrossRef]
- Hunt, J.; Sakaluk, S.K. Mate choice. In The Evolution of Insect Mating Systems; Shuker, D.M., Simmons, L.W., Eds.; Oxford University Press: Oxford, UK, 2014; pp. 129–158. [Google Scholar]
- Shuker, D.M. Sexual selection theory. In The Evolution of Insect Mating Systems; Shuker, D.M., Simmons, L.W., Eds.; Oxford University Press: Oxford, UK, 2014; pp. 20–41. [Google Scholar]
- Blanckenhorn, W.U. Behavioral causes and consequences of sexual size dimorphism. Ethology 2005, 111, 977–1016. [Google Scholar] [CrossRef]
- Branson, T.F.; Sutter, G.R. Influence of population density of immatures on size, longevity, and fecundity of adult Diabrotica virgifera virgifera (Coleoptera: Chrysomelidae). Environ. Entomol. 1985, 14, 687–690. [Google Scholar] [CrossRef]
- Jackson, J.J. Rearing and handling of Diabrotica virgifera and Diabrotica undecimpunctata howardi. In Methods for the Study of Pest Diabrotica; Krysan, J.L., Miller, T.A., Eds.; Springer: New York, NY, USA, 1986; pp. 25–47. [Google Scholar]
- Parimi, S.; Meinke, L.J.; French, B.W.; Chandler, L.D.; Siegfried, B.D. Stability and persistence of aldrin and methyl-parathion resistance in western corn rootworm populations (Coleoptera: Chrysomelidae). Crop Protect. 2006, 25, 269–274. [Google Scholar] [CrossRef]
- Krysan, J.L. Introduction: Biology, distribution, and identification of pest Diabrotica. In Methods for the Study of Pest Diabrotica; Krysan, J.L., Miller, T.A., Eds.; Springer: New York, NY, USA, 1986; pp. 1–23. [Google Scholar]
- Branson, T.F.; Jackson, J.J. An improved diet for adult Diabrotica virgifera virgifera (Coleoptera: Chrysomelidae). J. Kansas Entomol. Soc. 1988, 61, 353–355. [Google Scholar]
- Tallamy, D.W.; Powell, B.E.; McClafferty, J.A. Male traits under cryptic female choice in the spotted cucumber beetle (Coleoptera: Chrysomelidae). Behav. Ecol. 2002, 13, 511–518. [Google Scholar] [CrossRef]
- Tallamy, D.W.; Darlington, M.B.; Pesek, J.D.; Powell, B.E. Copulatory courtship signals male genetic quality in cucumber beetles. Proc. R. Soc. Lond. B 2003, 270, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Hammack, L. Calling behavior in female western corn rootworm beetles (Coleoptera: Chrysomelidae). Ann. Entomol. Soc. Am. 1995, 88, 562–569. [Google Scholar] [CrossRef]
- Hammack, L.; USDA-ARS, NCARL, 13786 Thomas Place, Keystone, SD 57751, USA. Unpublished data. 1995.
- French, B.W.; Hammack, L. Reproductive traits of northern corn rootworm (Coleoptera: Chrysomelidae) in relation to female and male body size. Ann. Entomol. Soc. Am. 2010, 103, 688–694. [Google Scholar] [CrossRef]
- Ruesink, W.G. Egg sampling techniques. In Methods for the Study of Pest Diabrotica; Krysan, J.L., Miller, T.A., Eds.; Springer: New York, NY, USA, 1986; pp. 83–99. [Google Scholar]
- Boetel, M.A.; Fuller, B.W. Seasonal emergence-time effects on adult longevity, fecundity, and egg viability of northern and western corn rootworms (Coleoptera: Chrysomelidae). Environ. Entomol. 1997, 26, 1208–1212. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis, 5th ed.; Prentice-Hall, Inc.: Englewood Cliffs, NJ, USA, 2010. [Google Scholar]
- SAS Institute Inc. SAS/STAT® 9.2. User’s Guide; SAS Institute Inc.: Cary, NC, USA, 2008. [Google Scholar]
- Civetta, A.; Clark, A.G. Correlated effects of sperm competition and postmating female mortality. Proc. Natl. Acad. Sci. USA 2000, 97, 13162–13165. [Google Scholar] [CrossRef] [PubMed]
- Rice, W.R. Dangerous liaisons. Proc. Natl. Acad. Sci. USA 2000, 97, 12953–12955. [Google Scholar] [CrossRef] [PubMed]
- Branson, T.F.; Guss, P.L.; Jackson, J.J. Mating frequency of the western corn rootworm. Ann. Entomol. Soc. Am. 1977, 70, 506–508. [Google Scholar] [CrossRef]
- French, B.W.; Hammack, L.; Tallamy, D.W. Effect of maternity on offspring survival in western corn rootworm (Coleoptera: Chrysomelidae) in relation to Cry3Bb1-resistant and Cry3Bb1-susceptible genotypes. USDA-ARS, NCARL, Brookings, SD, USA, Unpublished data. 2015; in preparation. [Google Scholar]
- Gay, L.; Eady, P.E.; Vasudev, R.; Hosken, D.J.; Tregenza, T. Costly sexual harassment in a beetle. Physiol. Entomol. 2009, 34, 86–92. [Google Scholar] [CrossRef]
- Branson, T.F.; Johnson, R.D. Adult western corn rootworms: Oviposition, fecundity, and longevity in the laboratory. J. Econ. Entomol. 1973, 66, 417–418. [Google Scholar] [CrossRef]
- Hill, R.E. Mating, oviposition patterns, fecundity and longevity of the western corn rootworm. J. Econ. Entomol. 1975, 68, 311–315. [Google Scholar] [CrossRef]
- Elliott, N.C.; Gustin, R.D.; Hanson, S.L. Influence of adult diet on the reproductive biology and survival of the western corn rootworm Diabrotica virgifera virgifera. Entomol. Exp. Appl. 1990, 56, 15–21. [Google Scholar] [CrossRef]
- Lance, D.R.; Fisher, J.R. Food quality of various plant tissues for adults of the northern corn rootworm (Coleoptera: Chrysomelidae). J. Kansas Entomol. Soc. 1987, 60, 462–466. [Google Scholar]
- Naranjo, S.E.; Sawyer, A.J. Reproductive biology and survival of Diabrotica barberi (Coleoptera: Chrysomelidae): Effect of temperature, food, and seasonal time of emergence. Ann. Entomol. Soc. Am. 1987, 80, 841–848. [Google Scholar] [CrossRef]
- Cothran, R.D. The mechanistic basis of a large male mating advantage in two freshwater amphipod species. Ethology 2008, 114, 1145–1153. [Google Scholar] [CrossRef]
- Hunt, J.; Breuker, C.J.; Sadowski, J.A.; Moore, A.J. Male-male competition, female mate choice and their interaction: Determining total sexual selection. J. Evol. Biol. 2009, 22, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Labeyrie, E.; Blanckenhorn, W.U.; Rahier, M. Mate choice and toxicity in two species of leaf beetles with different types of chemical defense. J. Chem. Ecol. 2003, 29, 1665–1680. [Google Scholar] [CrossRef] [PubMed]
- Nahrung, H.F.; Allen, G.R. Sexual selection under scramble competition: Mate location and mate choice in the Eucalypt leaf beetle Chrysophtharta agricola (Chapuis) in the field. J. Insect Behav. 2004, 17, 353–366. [Google Scholar] [CrossRef]
- French, B.W.; Hammack, L. Male reproductive competition and components of female fitness in relation to body size in northern corn rootworm (Coleoptera: Chrysomelidae). Ann. Entomol. Soc. Am. 2014, 107, 279–287. [Google Scholar] [CrossRef]
- French, B.W.; Hammack, L. Spermatophore size in relation to body size and pairing duration in the northern corn rootworm (Coleoptera: Chrysomelidae). Ann. Entomol. Soc. Am. 2012, 105, 506–511. [Google Scholar] [CrossRef]
- Kang, J.; Krupke, C.H. Likelihood of multiple mating in Diabrotica virgifera virgifera (Coleoptera: Chrysomelidae). J. Econ. Entomol. 2009, 102, 2096–2100. [Google Scholar] [CrossRef] [PubMed]
- Carroll, S.P.; Hendry, A.P.; Reznick, D.N.; Fox, C.W. Evolution on ecological time-scales. Functional Ecol. 2007, 21, 387–393. [Google Scholar] [CrossRef]
- Kasumovic, M.M.; Andrade, M.C.B. A change in competitive context reverses sexual selection on male size. J. Evol. Biol. 2009, 22, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, D.R.; Levine, E. Copulation and its duration affects female weight, oviposition, hatching patterns, and ovarian development in the western corn rootworm (Coleoptera: Chrysomelidae). J. Econ. Entomol. 1993, 86, 1664–1671. [Google Scholar] [CrossRef]
- Brodt, J.F.; Tallamy, D.W.; Ali, J. Female choice by scent recognition in the spotted cucumber beetle. Ethology 2007, 112, 300–306. [Google Scholar] [CrossRef]
- Ali, J.; Tallamy, D.W. Female spotted cucumber beetles use own cuticular hydrocarbon signature to choose immunocompatible mates. Anim. Behav. 2010, 80, 9–12. [Google Scholar] [CrossRef]
- Thomas, M.L.; Simmons, L.W. Short-term phenotypic plasticity in long-chain cuticular hydrocarbons. Proc. R. Soc. B 2011. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.F. Changing Landscapes: Effects of Varying Refuge Structure on the Behavioral Ecology and Resistance Management of Western Corn Rootworm, Diabrotica virgifera virgifera LeConte. Ph.D. Thesis, Purdue University, Lafayette, IN, USA, 2011. [Google Scholar]
- Bieńkowski, A.O. Mating behaviour in Donaciinae (Coleoptera: Chrysomelidae). In Advances in Chrysomelidae Biology 1; Cox, M.L., Ed.; Backhuys Publishers: Leiden, The Netherlands, 1999; pp. 411–420. [Google Scholar]
- Lew, A.C.; Ball, H.J. The mating behavior of the western corn rootworm Diabrotica virgifera (Coleoptera: Chrysomelidae). Ann. Entomol. Soc. Am. 1979, 72, 391–393. [Google Scholar] [CrossRef]
- Tallamy, D.W. Mate choice after intromission in spotted cucumber beetles. In New Developments in the Biology of Chrysomelidae; Jolivet, P., Santiago-Blay, J.A., Schmitt, M., Eds.; SPB Academic Publishing: The Hague, The Netherlands, 2004; pp. 709–720. [Google Scholar]
- Lew, A.C.; Ball, H.J. Effect of copulation time on spermatozoan transfer of Diabrotica virgifera (Coleoptera: Chrysomelidae). Ann. Entomol. Soc. Am. 1980, 73, 360–361. [Google Scholar] [CrossRef]
- Kang, J.; Krupke, C.H. Influence of weight of male and female western corn rootworm (Coleoptera: Chrysomelidae) on mating behaviors. Ann. Entomol. Soc. Am. 2009, 102, 326–332. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (USEPA). Biopesticides Registration Action Document (BRAD): Event MON863 Bacillus thuringiensis Cry3bb1 Corn. 2003; USEPA: Washington, DC, USA. Available online: http://www.epa.gov/oppbppd1/biopesticides/ingredients_keep/tech_docs/cry3bb1/1_%20cry3bb1_exec_summ.pdf (accessed on 22 June 2015). [Google Scholar]
- Al-Deed, M.A.; Wilde, G.E. Effect of Bt corn expressing the Cry3Bb1 toxin on western corn rootworm (Coleoptera: Chrysomelidae) biology. J. Kansas Entomol. Soc. 2005, 78, 142–152. [Google Scholar] [CrossRef]
- Nowatzki, T.M.; Zhou, X.; Meinke, L.J.; Vaughn, T.; Siegfried, B.D. Effect of Bacillus thuringiensis Cry3Bb1 protein on the feeding behavior and longevity of adult western corn rootworm (Coleoptera: Chrysomelidae). J. Econ. Entomol. 2006, 99, 927–930. [Google Scholar] [CrossRef] [PubMed]
- Meissle, M.; Hellmich, R.L.; Romeis, J. Impact of Cry3Bb1-expresssing Bt maize on adults of the western corn rootworm, Diabrotica virgifera virgifera (Coleoptera: Chrysomelidae). Pest Manag. Sci. 2011, 67, 807–814. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
French, B.W.; Hammack, L.; Tallamy, D.W. Mating Success, Longevity, and Fertility of Diabrotica virgifera virgifera LeConte (Chrysomelidae: Coleoptera) in Relation to Body Size and Cry3Bb1-Resistant and Cry3Bb1-Susceptible Genotypes. Insects 2015, 6, 943-960. https://doi.org/10.3390/insects6040943
French BW, Hammack L, Tallamy DW. Mating Success, Longevity, and Fertility of Diabrotica virgifera virgifera LeConte (Chrysomelidae: Coleoptera) in Relation to Body Size and Cry3Bb1-Resistant and Cry3Bb1-Susceptible Genotypes. Insects. 2015; 6(4):943-960. https://doi.org/10.3390/insects6040943
Chicago/Turabian StyleFrench, Bryan Wade, Leslie Hammack, and Douglas W. Tallamy. 2015. "Mating Success, Longevity, and Fertility of Diabrotica virgifera virgifera LeConte (Chrysomelidae: Coleoptera) in Relation to Body Size and Cry3Bb1-Resistant and Cry3Bb1-Susceptible Genotypes" Insects 6, no. 4: 943-960. https://doi.org/10.3390/insects6040943
APA StyleFrench, B. W., Hammack, L., & Tallamy, D. W. (2015). Mating Success, Longevity, and Fertility of Diabrotica virgifera virgifera LeConte (Chrysomelidae: Coleoptera) in Relation to Body Size and Cry3Bb1-Resistant and Cry3Bb1-Susceptible Genotypes. Insects, 6(4), 943-960. https://doi.org/10.3390/insects6040943







