Within-Colony Variation in the Immunocompetency of Managed and Feral Honey Bees (Apis mellifera L.) in Different Urban Landscapes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Colony Location and Landscape Analysis
2.2. Sampling
2.3. Encapsulation Response

2.4. Phenoloxidase Activity
2.5. Statistical Analyses
3. Results
3.1. Study Sites
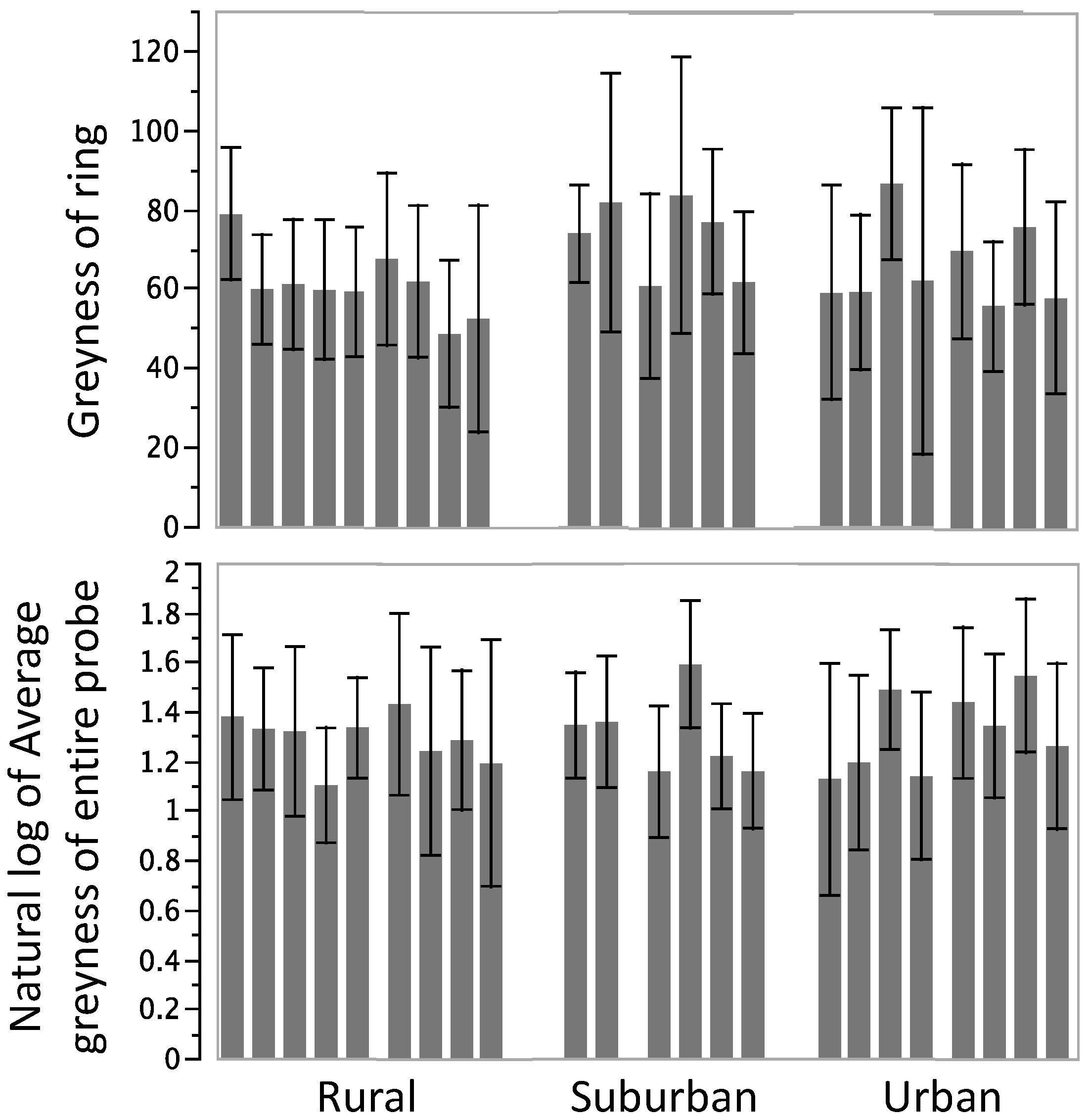
3.2. Encapsulation Response
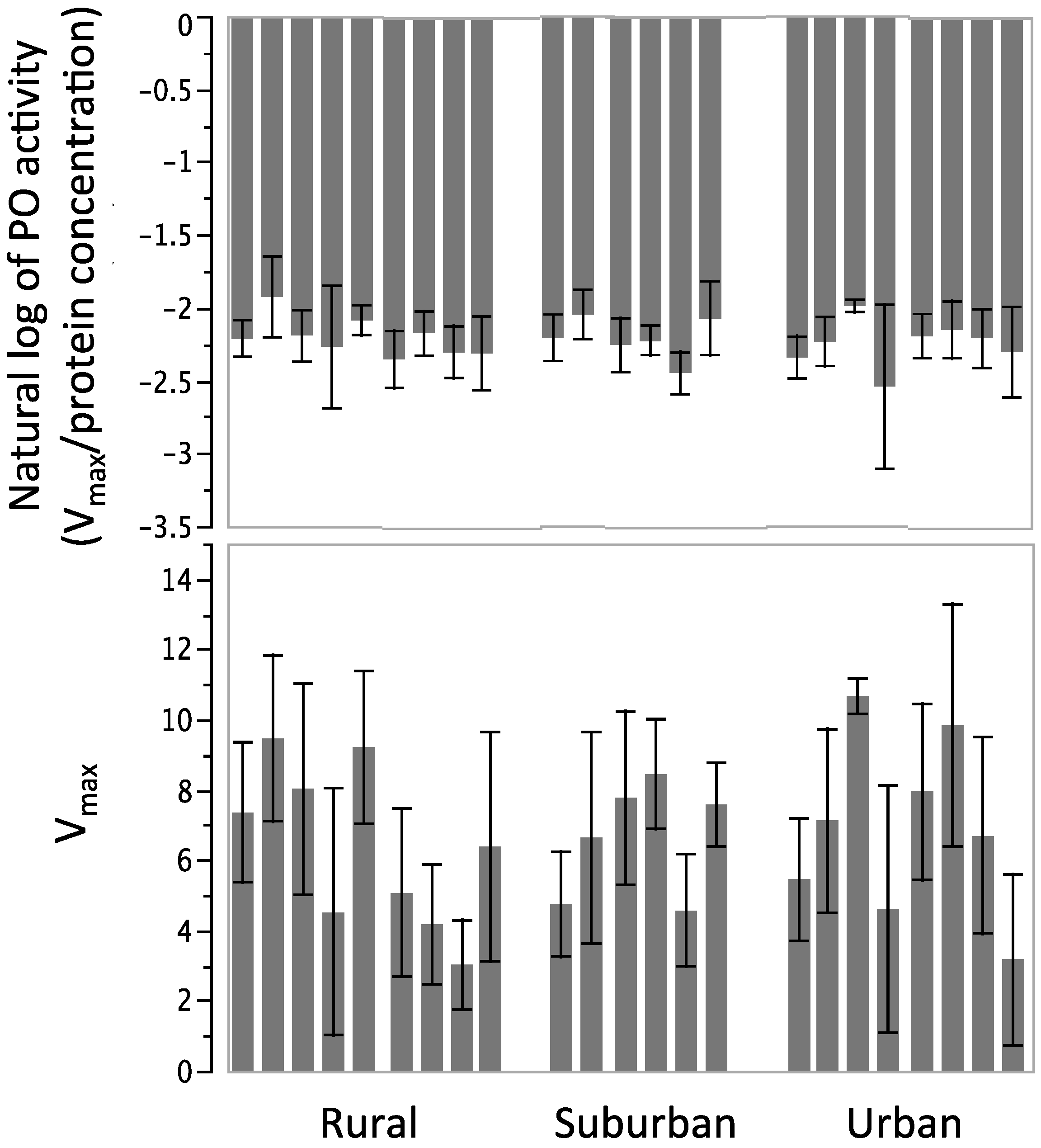
3.3. Phenoloxidase Activity
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wilson, E.O. The Insect Societies; Harvard University Press: Cambridge, MA, USA, 1971. [Google Scholar]
- Seeley, T.D. The Wisdom of the Hive: The Social Physiology of Honey Bee Colonies; Harvard University Press: Cambridge, MA, USA, 1995; p. 289. [Google Scholar]
- Oldroyd, B.P.; Fewell, J.H. Genetic diversity promotes homeostasis in insect colonies. Trends Ecol. Evol. 2007, 22, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Schmid-Hempel, P. Parasites in Social Insects; Princeton University Press: Princeton, NJ, USA, 1998; p. 409. [Google Scholar]
- Cremer, S.; Sixt, M. Analogies in the evolution of individual and social immunity. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Wilson-Rich, N.; Spivak, M.; Fefferman, N.H.; Starks, P.T. Genetic, individual, and group facilitation of disease resistance in insect docieties. Annu. Rev. Entomol. 2009, 54, 405–423. [Google Scholar] [CrossRef] [PubMed]
- Simone-Finstrom, M.; Spivak, M. Propolis and bee health: The natural history and significance of resin use by honey bees. Apidologie 2010, 41, 295–311. [Google Scholar] [CrossRef]
- Wilson-Rich, N.; Dres, S.T.; Starks, P.T. The ontogeny of immunity: Development of innate immune strength in the honey bee (Apis mellifera). J. Insect Physiol. 2008, 54, 1392–1399. [Google Scholar] [CrossRef] [PubMed]
- Lowe, E.C.; Simmons, L.W.; Baer, B. Worker heterozygosity and immune response in feral and managed honeybees (Apis mellifera). Aust. J. Zool. 2011, 59, 73–78. [Google Scholar] [CrossRef]
- Laughton, A.M.; Boots, M.; Siva-Jothy, M.T. The ontogeny of immunity in the honey bee, Apis mellifera L. Following an immune challenge. J. Insect Physiol. 2011, 57, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Bailey, N.W.; Zuk, M. Changes in immune effort of male field crickets infested with mobile parasitoid larvae. J. Insect Physiol. 2008, 54, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Iwama, R.; Ashida, M. Biosynthesis of prophenoloxidase in hemocytes of larval hemolymph of the silkworm, Bombyx mori. Insect Biochem. 1986, 16, 547–555. [Google Scholar] [CrossRef]
- Kawabata, T.; Yasuhara, Y.; Ochiai, M.; Matsuura, S.; Ashida, M. Molecular-cloning of insect pro-phenol oxidase––A copper––Containing protein homologous to arthropod hemocyanin. Proc. Natl. Acad. Sci. USA 1995, 92, 7774–7778. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.D.; Pettis, J.S. Colony-level impacts of immune responsiveness in honey bees, Apis mellifera. Evolution 2005, 59, 2270–2274. [Google Scholar] [CrossRef] [PubMed]
- Mallon, E.B.; Brockmann, A.; Schmid-Hempel, P. Immune response inhibits associative learning in insects. Proc. R. Soc. B-Biol. Sci. 2003, 270, 2471–2473. [Google Scholar] [CrossRef] [PubMed]
- Sadd, B.M.; Siva-Jothy, M.T. Self-harm caused by an insect’s innate immunity. Proc. Biol. Sci. 2006, 273, 2571–2574. [Google Scholar] [CrossRef] [PubMed]
- Wilson-Rich, N.; Tarpy, D.R.; Starks, P.T. Within- and across-colony effects of hyperpolyandry on immune function and body condition in honey bees (Apis mellifera). J. Insect Physiol. 2012, 58, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Aizen, M.A.; Harder, L.D. The global stock of domesticated honey bees is growing slower than agricultural demand for pollination. Curr. Biol. 2009, 19, 915–918. [Google Scholar] [CrossRef] [PubMed]
- Rader, R.; Howlett, B.G.; Cunningham, S.A.; Westcott, D.A.; Newstrom-Lloyd, L.E.; Walker, M.K.; Teulon, D.A.J.; Edwards, W. Alternative pollinator taxa are equally efficient but not as effective as the honeybee in a mass flowering crop. J. Appl. Ecol. 2009, 46, 1080–1087. [Google Scholar] [CrossRef]
- Gambino, P.; Hoelmer, K.; Daly, H.V. Nest sites of feral honey-bees in California, USA. Apidologie 1990, 21, 35–45. [Google Scholar] [CrossRef]
- Oleksa, A.; Gawronski, R.; Tofilski, A. Rural avenues as a refuge for feral honey bee population. J. Insect Conserv. 2013, 17, 465–472. [Google Scholar] [CrossRef]
- Seeley, T.D.; Morse, R.A. Nest site selection by the honey bee, Apis mellifera. Insectes Sociaux 1978, 25, 323–337. [Google Scholar] [CrossRef]
- Steinhauer, N.; Rennich, K.; Wilson, M.E.; Caron, D.M.; Lengerich, E.J.; Pettis, J.; Rose, R.; Skinner, J.A.; Tarpy, D.R.; Wilkes, J.T.; et al. A national survey of managed honey bee 2012–2013 annual colony losses in the USA: Results from the bee informed partnership. J. Apic. Res. 2014, 53, 1–18. [Google Scholar] [CrossRef]
- Lee, K.V.; Steinhauer, N.; Rennich, K.; Wilson, M.E.; Tarpy, D.R.; Caron, D.M.; Rose, R.; Delaplane, K.S.; Baylis, K.; Lengerich, E.J.; et al. A national survey of managed honey bee 2013–2014 annual colony losses in the USA: Results from the bee informed partnership. Apidologie 2015, 46, 292–305. [Google Scholar] [CrossRef]
- Kraus, B.; Page, R.E., Jr. Population growth of Varroa jacobsoni Oud in Mediterranean climates of California. Apidologie 1995, 26, 149–157. [Google Scholar] [CrossRef]
- Tarpy, D.R.; Delaney, D.A.; Seeley, T.D. Mating frequencies of honey bee queens (Apis mellifera L.) in a population of feral colonies in the northeastern united states. PLoS ONE 2015, 10, e0118734. [Google Scholar] [CrossRef] [PubMed]
- Seeley, T.D.; Delaney, D.A.; Tarpy, D.R. A survivor population of wild colonies of european honeybees in the Northeastern United States: Investigating its genetic structure. Apidologie 2015, 46, 654–666. [Google Scholar] [CrossRef]
- McKinney, M.L. Urbanization, biodiversity, and conservation. Bioscience 2002, 52, 883–890. [Google Scholar] [CrossRef]
- Shochat, E.; Warren, P.S.; Faeth, S.H.; McIntyre, N.E.; Hope, D. From patterns to emerging processes in mechanistic urban ecology. Trends Ecol. Evol. 2006, 21, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Valiela, I.; Martinetto, P. Changes in bird abundance in Eastern North America: Urban sprawl and global footprint? Bioscience 2007, 57, 360–370. [Google Scholar] [CrossRef]
- Ball, A.; Truskewycz, A. Polyaromatic hydrocarbon exposure: An ecological impact ambiguity. Environ. Sci. Pollut. Res. 2013, 20, 4311–4326. [Google Scholar] [CrossRef] [PubMed]
- Grimm, N.B.; Faeth, S.H.; Golubiewski, N.E.; Redman, C.L.; Wu, J.; Bai, X.; Briggs, J.M. Global change and the ecology of cities. Science 2008, 319, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Molina, M.J.; Molina, L.T. Megacities and atmospheric pollution. J. Air Waste Manag. Assoc. 2004, 54, 644–680. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.B.; Roberts, S.P.; Elekonich, M.M. Age and natural metabolically-intensive behavior affect oxidative stress and antioxidant mechanisms. Exp. Gerontol. 2008, 43, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Chocholouskova, Z.; Pysek, P. Changes in composition and structure of urban flora over 120 years: A case study of the city of Plzen. Flora 2003, 198, 366–376. [Google Scholar] [CrossRef]
- Hope, D.; Gries, C.; Zhu, W.X.; Fagan, W.F.; Redman, C.L.; Grimm, N.B.; Nelson, A.L.; Martin, C.; Kinzig, A. Socioeconomics drive urban plant diversity. Proc. Natl. Acad. Sci. USA 2003, 100, 8788–8792. [Google Scholar] [CrossRef] [PubMed]
- Kareiva, P.; Watts, S.; McDonald, R.; Boucher, T. Domesticated nature: Shaping landscapes and ecosystems for human welfare. Science 2007, 316, 1866–1869. [Google Scholar] [CrossRef] [PubMed]
- Mimet, A.; Pellissier, V.; Quenol, H.; Aguejdad, R.; Dubreuil, V.; Roze, F. Urbanisation induces early flowering: Evidence from platanus acerifolia and prunus cerasus. Int. J. Biometeorol. 2009, 53, 287–298. [Google Scholar] [PubMed]
- Di Pasquale, G.; Salignon, M.; le Conte, Y.; Belzunces, L.P.; Decourtye, A.; Kretzschmar, A.; Suchail, S.; Brunet, J.-L.; Alaux, C. Influence of pollen nutrition on honey bee health: Do pollen quality and diversity matter? PLoS ONE 2013, 8, e72016. [Google Scholar] [PubMed]
- Oke, T.R. The energetic basis of the urban heat-island. Q. J. R. Meteorol. Soc. 1982, 108, 1–24. [Google Scholar] [CrossRef]
- Kuhnholz, S.; Seeley, T.D. The control of water collection in honey bee colonies. Behav. Ecol. Sociobiol. 1997, 41, 407–422. [Google Scholar]
- Starks, P.T.; Gilley, D.C. Heat shielding: A novel method of colonial thermoregulation in honey bees. Naturwissenschaften 1999, 86, 438–440. [Google Scholar] [CrossRef] [PubMed]
- Ribbands, C.R. The flight range of the honey-bee. J. Anim. Ecol. 1951, 20, 220–226. [Google Scholar] [CrossRef]
- Dale, A.G.; Frank, S.D. The effects of urban warming on herbivore abundance and street tree condition. PLoS ONE 2014, 9, e102996. [Google Scholar] [CrossRef] [PubMed]
- Meineke, E.K.; Dunn, R.R.; Sexton, J.O.; Frank, S.D. Urban warming drives insect pest abundance on street trees. PLoS ONE 2013, 8, e59687. [Google Scholar] [CrossRef] [PubMed]
- Xian, G.; Homer, C.; Demitz, J.; Fry, J.; Hossain, N.; Wickham, J. Change of impervious surface area between 2001 and 2006 in the conterminous United States. Photogramm. Eng. Remote Sens. 2011, 77, 758–762. [Google Scholar]
- Richard, F.-J.; Tarpy, D.R.; Grozinger, C.M. Effects of insemination quantity on honey bee queen physiology. PLoS ONE 2007, 2, e980. [Google Scholar] [CrossRef] [PubMed]
- Moret, Y.; Schmid-Hempel, P. Survival for immunity: The price of immune system activation for bumblebee workers. Science 2000, 290, 1166–1168. [Google Scholar] [CrossRef] [PubMed]
- Randolt, K.; Gimple, O.; Geissendoerfer, J.; Reinders, J.; Prusko, C.; Mueller, M.J.; Albert, S.; Tautz, J.; Beier, H. Immune-related proteins induced in the hemolymph after aseptic and septic injury differ in honey bee worker larvae and adults. Arch. Insect Biochem. Physiol. 2008, 69, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Laughton, A.M.; Siva-Jothy, M.T. A standardised protocol for measuring phenoloxidase and prophenoloxidase in the honey bee, Apis mellifera. Apidologie 2011, 42, 140–149. [Google Scholar] [CrossRef]
- Theopold, U.; Schmidt, O.; Soderhall, K.; Dushay, M.S. Coagulation in arthropods: Defence, wound closure and healing. Trends Immunol. 2004, 25, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Theopold, U.; Krautz, R.; Dushay, M.S. The drosophila clotting system and its messages for mammals. Dev. Comp. Immunol. 2014, 42, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Parker, R.; Guarna, M.M.; Melathopoulos, A.P.; Moon, K.-M.; White, R.; Huxter, E.; Pernal, S.F.; Foster, L.J. Correlation of proteome-wide changes with social immunity behaviors provides insight into resistance to the parasitic mite, varroa destructor, in the honey bee (Apis mellifera). Genome Biol. 2012, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Adamo, S.A.; Lovett, M.M.E. Some like it hot: The effects of climate change on reproduction, immune function and disease resistance in the cricket gryllus texensis. J. Exp. Biol. 2011, 214, 1997–2004. [Google Scholar] [CrossRef] [PubMed]
- Franke, K.; Fischer, K. Effects of inbreeding and temperature stress on life history and immune function in a butterfly. J. Evol. Biol. 2013, 26, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Karl, I.; Stoks, R.; de Block, M.; Janowitz, S.A.; Fischer, K. Temperature extremes and butterfly fitness: Conflicting evidence from life history and immune function. Glob. Chang. Biol. 2011, 17, 676–687. [Google Scholar] [CrossRef]
- Suwanchaichinda, C.; Paskewitz, S.M. Effects of larval nutrition, adult body size, and adult temperature on the ability of Anopheles gambiae (Diptera: Culicidae) to melanize sephadex beads. J. Med. Entomol. 1998, 35, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Nowak, D.J.; Greenfield, E.J. Tree and impervious cover change in US cities. Urban For. Urban Green. 2012, 11, 21–30. [Google Scholar] [CrossRef]
- Paul, M.J.; Meyer, J.L. Streams in the urban landscape. Annu. Rev. Ecol. Syst. 2001, 32, 207–231. [Google Scholar] [CrossRef]
- Dubovskiy, I.M.; Grizanova, E.V.; Ershova, N.S.; Rantala, M.J.; Glupov, V.V. The effects of dietary nickel on the detoxification enzymes, innate immunity and resistance to the fungus beauveria bassiana in the larvae of the greater wax moth Galleria mellonella. Chemosphere 2011, 85, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Christensen, B.M.; Li, J.Y.; Chen, C.C.; Nappi, A.J. Melanization immune responses in mosquito vectors. Trends Parasitol. 2005, 21, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Nappi, A.J.; Christensen, B.M. Melanogenesis and associated cytotoxic reactions: Applications to insect innate immunity. Insect Biochem. Mol. Biol. 2005, 35, 443–459. [Google Scholar] [CrossRef] [PubMed]
- Poelkki, M.; Kangassalo, K.; Rantala, M.J. Transgenerational effects of heavy metal pollution on immune defense of the blow fly protophormia terraenovae. PLoS ONE 2012, 7, e38832. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.D.; Aronstein, K.; Chen, Y.P.; Hetru, C.; Imler, J.L.; Jiang, H.; Kanost, M.; Thompson, G.J.; Zou, Z.; Hultmark, D. Immune pathways and defence mechanisms in honey bees Apis mellifera. Insect Mol. Biol. 2006, 15, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Barribeau, S.M.; Sadd, B.M.; du Plessis, L.; Brown, M.J.F.; Buechel, S.D.; Cappelle, K.; Carolan, J.C.; Christiaens, O.; Colgan, T.J.; Erler, S.; et al. A depauperate immune repertoire precedes evolution of sociality in bees. Genome Biol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Youngsteadt, E.; Dale, A.G.; Terando, A.J.; Dunn, R.R.; Frank, S.D. Do cities simulate climate change? A comparison of herbivore response to urban and global warming. Glob. Chang. Biol. 2015, 21, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Youngsteadt, E.; Appler, R.H.; López-Uribe, M.M.; Tarpy, D.R.; Frank, S.D. Urbanization increases pathogen pressure on feral and managed honey bees. PLoS ONE. in press.
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Appler, R.H.; Frank, S.D.; Tarpy, D.R. Within-Colony Variation in the Immunocompetency of Managed and Feral Honey Bees (Apis mellifera L.) in Different Urban Landscapes. Insects 2015, 6, 912-925. https://doi.org/10.3390/insects6040912
Appler RH, Frank SD, Tarpy DR. Within-Colony Variation in the Immunocompetency of Managed and Feral Honey Bees (Apis mellifera L.) in Different Urban Landscapes. Insects. 2015; 6(4):912-925. https://doi.org/10.3390/insects6040912
Chicago/Turabian StyleAppler, R. Holden, Steven D. Frank, and David R. Tarpy. 2015. "Within-Colony Variation in the Immunocompetency of Managed and Feral Honey Bees (Apis mellifera L.) in Different Urban Landscapes" Insects 6, no. 4: 912-925. https://doi.org/10.3390/insects6040912
APA StyleAppler, R. H., Frank, S. D., & Tarpy, D. R. (2015). Within-Colony Variation in the Immunocompetency of Managed and Feral Honey Bees (Apis mellifera L.) in Different Urban Landscapes. Insects, 6(4), 912-925. https://doi.org/10.3390/insects6040912