Development of a Real-Time qPCR Assay for Quantification of Covert Baculovirus Infections in a Major African Crop Pest
Abstract
:1. Introduction
2. Experimental Section
2.1. Culture of S. exempta
2.2. Overt Baculovirus Provenance
2.3. Total Genomic DNA Extraction from Insects
2.4. Real-Time Quantitative PCR (qPCR)
2.5. Covert Virus Dynamics during S. exempta Development
2.6. Localisation of SpexNPV Infection within Body Regions of Adult Moths
2.7. Baculovirus Dynamics Following SpexNPV Ingestion
3. Results
3.1. Analytical Sensitivity of the qPCR Assay
3.2. Covert SpexNPV Dynamics during Host Developmental Cycle

3.3. Localisation of Baculovirus in Adult Moths

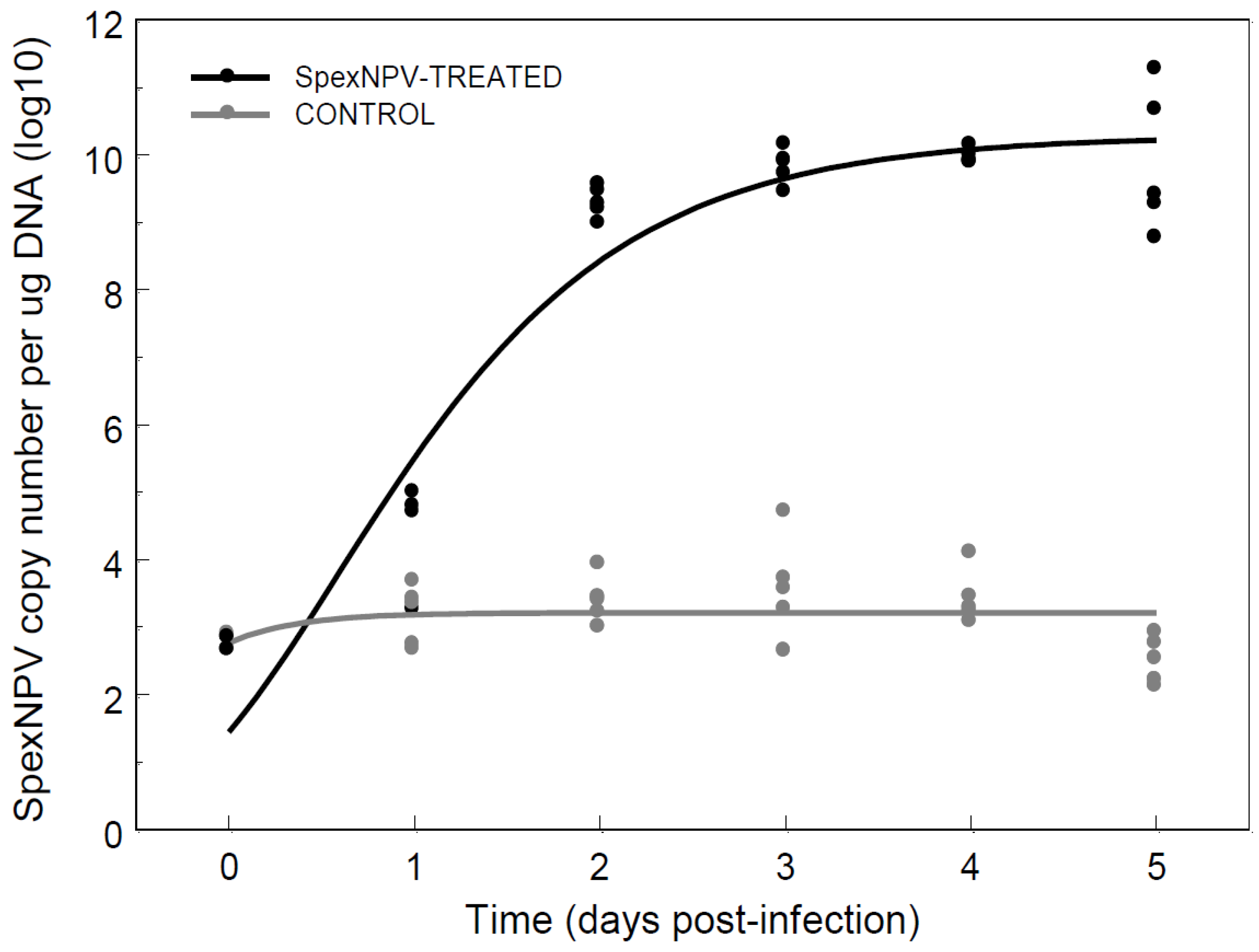
3.4. Baculovirus Dynamics Following Oral Ingestion
4. Discussion
5. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jehle, J.A.; Blissard, G.W.; Bonning, B.C.; Cory, J.S.; Herniou, E.A.; Rohrmann, G.F.; Theilmann, D.A.; Thiem, S.M.; Vlak, J.M. On the classification and nomenclature of baculoviruses: A proposal for revision. Arch. Virol. 2006, 151, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, G.; Dushoff, J.; Elkinton, J.S.; Levin, S.A. Pathogen-driven outbreaks in forest defoliators revisited: Building models from experimental data. Am. Nat. 2000, 156, 105–120. [Google Scholar] [CrossRef] [PubMed]
- Kukan, B. Vertical transmission of nucleopolyhedrovirus in insects. J. Invertebr. Pathol. 1999, 74, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.S.; Possee, R.D.; King, L.A. Activation and detection of a latent baculovirus resembling Mamestra brassicae nuclear polyhedrosis virus in M. brassica insects. Virology 1993, 194, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.S.; Possee, R.D.; King, L.A. Evidence for the presence of a low-level, persistent baculovirus infection of Mamestra brassicae insects. J. Gen. Virol. 1997, 78, 1801–1805. [Google Scholar] [PubMed]
- Burden, J.P.; Griffiths, C.M.; Cory, J.S.; Smith, P.; Sait, S.M. Vertical transmission of sublethal granulovirus infection in the Indian meal moth, Plodia interpunctella. Mol. Ecol. 2002, 11, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Burden, J.P.; Nixon, C.P.; Hodgkinson, A.E.; Possee, R.D.; Sait, S.M.; King, L.A.; Hails, R.S. Covert infections as a mechanism for long-term persistence of baculoviruses. Ecol. Lett. 2003, 6, 524–531. [Google Scholar] [CrossRef]
- Vilaplana, L.; Wilson, K.; Redman, E.M.; Cory, J.S. Pathogen persistence in migratory insects: High levels of vertically-transmitted virus infection in field populations of the African armyworm. Evol. Ecol. 2010, 24, 147–160. [Google Scholar] [CrossRef]
- Murillo, R.; Hussey, M.S.; Possee, R.D. Evidence for covert baculovirus infections in a Spodoptera exigua laboratory culture. J. Gen. Virol. 2011, 92, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Kemp, E.M.; Woodward, D.T.; Cory, J.S. Detection of single and mixed covert baculovirus infections in eastern spruce budworm, Choristoneura fumiferana populations. J. Invertebr. Pathol. 2011, 107, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Virto, C.; Zarate, C.A.; Lopez-Ferber, M.; Murillo, R.; Caballero, P.; Williams, T. Gender-mediated differences in vertical transmission of a nucleopolyhedrovirus. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Virto, C.; Navarro, D.; Tellez, M.M.; Herrero, S.; Williams, T.; Murillo, R.; Caballero, P. Natural populations of Spodoptera exigua are infected by multiple viruses that are transmitted to their offspring. J. Invertebr. Pathol. 2014, 122, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Jakubowska, A.K.; D’Angiolo, M.; Gonzalez-Martinez, R.M.; Millan-Leiva, A.; Carballo, A.; Murillo, R.; Caballero, P.; Herrero, S. Simultaneous occurrence of covert infections with small RNA viruses in the lepidopteran Spodoptera exigua. J. Invertebr. Pathol. 2014, 121, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Liu, Y.; Graham, R.I.; Wilson, K.; Wu, K. Densovirus is a mutualistic symbiont of a global crop pest (Helicoverpa armigera) and protects against a baculovirus and Bt biopesticide. PLoS Pathog. 2014, 10. [Google Scholar] [CrossRef] [PubMed]
- Fuxa, J.R.; Richter, A.R.; Ameen, A.O.; Hammock, B.D. Vertical transmission of TnSNPV, TnCPV, AcMNPV, and possibly recombinant NPV in Trichoplusia ni. J. Invertebr. Pathol. 2002, 79, 44–50. [Google Scholar] [CrossRef]
- Rose, D.J.W.; Dewhurst, C.F.; Page, W.W. The African Armyworm Handbook, 2nd ed.; NRI: Chatham, UK, 2000. [Google Scholar]
- Brown, E.S.; Swaine, G. Virus disease of African armyworm Spodoptera exempta (Wlk). Bull. Entomol. Res. 1965, 56, 95–116. [Google Scholar] [CrossRef]
- Redman, E.M.; Wilson, K.; Grzywacz, D.; Cory, J.S. High levels of genetic diversity in Spodoptera exempta NPV from Tanzania. J. Invertebr. Pathol. 2010, 105, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Swaine, G. Generation to generation passage of nuclear polyhedral virus of Spodoptera exempta (Wlk). Nature 1966, 210, 1053–1054. [Google Scholar] [CrossRef] [PubMed]
- Vilaplana, L.; Redman, E.M.; Wilson, K.; Cory, J.S. Density-related variation in vertical transmission of a virus in the African armyworm. Oecologia 2008, 155, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Graham, R.I.; Grzywacz, D.; Mushobozi, W.L.; Wilson, K. Wolbachia in a major African crop pest increases susceptibility to viral disease rather than protects. Ecol. Lett. 2012, 15, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Reeson, A.F.; Wilson, K.; Gunn, A.; Hails, R.S.; Goulson, D. Baculovirus resistance in the noctuid Spodoptera exempta is phenotypically plastic and responds to population density. Proc. R Soc. Lond. B. 1998, 265, 1787–1791. [Google Scholar] [CrossRef]
- Hunter-Fujita, F.R.; Entwhistle, P.F.; Evans, H.F.; Crook, N.E. Insect Viruses and Pest Management; Wiley and Sons: Chichester, UK, 1998. [Google Scholar]
- Graham, R.I.; Tyne, W.I.; Possee, R.D.; Sait, S.M.; Hails, R.S. Genetically variable nucleopolyhedroviruses isolated from spatially separate populations of the winter moth Operophtera brumata (Lepidoptera: Geometridae) in Orkney. J. Invertebr. Pathol. 2004, 87, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotech. 1994, 3, 294–299. [Google Scholar]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.M.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative Real-Time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Escasa, S.; Cory, J.S.; Algoma University, Sault Set Marie, Ontario, Canada. Unpublished data. 2008.
- Nybo, K. qPCR efficiency calculations. BioTechniques 2011, 51, 401–402. [Google Scholar] [CrossRef] [PubMed]
- Fuxa, J.R.; Sun, J.Z.; Weidner, E.H.; LaMotte, L.R. Stressors and rearing diseases of Trichoplusia ni: Evidence of vertical transmission of NPV and CPV. J. Invertebr. Pathol. 1999, 74, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Fuxa, J.R.; Richter, A.R. Virulence and multigeneration passage of a nuclear polyhedrosis virus selected for an increased rate of vertical transmission. Biol. Control 1992, 2, 171–175. [Google Scholar] [CrossRef]
- Fuxa, J.R.; Weidner, E.H.; Richter, A.R. Polyhedra without virions in a vertically transmitted nuclear polyhedrosis-virus. J. Invertebr. Pathol. 1992, 60, 53–58. [Google Scholar] [CrossRef]
- Cooper, D.; Cory, J.S.; Theilmann, D.A.; Myers, J.H. Nucleopolyhedroviruses of forest and western tent caterpillars: Cross-infectivity and evidence for activation of latent virus in high-density field populations. Ecol. Entomol. 2003, 28, 41–50. [Google Scholar] [CrossRef]
- Longworth, J.F.; Cunningham, J.C. Activation of occult nuclear-polyhedrosis viruses by foreign nuclear polyhedra. J. Invertebr. Pathol. 1968, 10, 361–367. [Google Scholar] [CrossRef]
- Kouassi, L.N.G.; Tsuda, K.; Goto, C.; Mukawa, S.; Sakamaki, Y.; Kusigemati, K.; Nakamura, K. Prevalence of latent virus in Spodoptera. litura (Fabricius) (Lepidoptera: Noctuidae) and its activation by a heterologous virus. Appl. Entomol. Zool. 2009, 44, 95–102. [Google Scholar]
- Wilson, K.; Cotter, S.C.; Reeson, A.F.; Pell, J.K. Melanism and disease resistance in insects. Ecol. Lett. 2001, 4, 637–649. [Google Scholar] [CrossRef]
- Cotter, S.C.; Myatt, J.P.; Benskin, C.M.H.; Wilson, K. Selection for cuticular melanism reveals immune function and life-history trade-offs in Spodoptera littoralis. J. Evol. Biol. 2008, 21, 1744–1754. [Google Scholar] [CrossRef] [PubMed]
- Federici, B.A. Baculovirus pathogenesis. In The Baculoviruses; Miller, L.K., Ed.; Plenum Press: New York, NY, USA, 1997. [Google Scholar]
- Krokene, P.; Heldal, I.; Fossdal, C.G. Quantifying Neodiprion sertifer nucleopolyhedrovirus DNA from insects, foliage and forest litter using the quantitative real-time polymerase chain reaction. Agric. For. Entomol. 2013, 15, 120–125. [Google Scholar] [CrossRef]
- Dewhurst, C.F.; Page, W.W.; Rose, D.J.W. The relationship between outbreaks, rainfall and low density populations of the African armyworm, Spodoptera exempta, in Kenya. Entomol. Exp. Appl. 2001, 98, 285–294. [Google Scholar] [CrossRef]
- Boots, M.; Greenman, J.; Ross, D.; Norman, R.; Hails, R.; Sait, S. The population dynamical implications of covert infections in host-microparasite interactions. J. Anim. Ecol. 2003, 72, 1064–1072. [Google Scholar] [CrossRef]
- Jones, E.O.; White, A.; Boots, M. Interference and the persistence of vertically transmitted parasites. J. Theor. Biol. 2007, 246, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Sorrell, I.; White, A.; Pedersen, A.B.; Hails, R.S.; Boots, M. The evolution of covert, silent infection as a parasite strategy. Proc. R Soc. Lond. B 2009, 276, 2217–2226. [Google Scholar] [CrossRef] [PubMed]
- Bonsall, M.B.; Sait, S.M.; Hails, R.S. Invasion and dynamics of covert infection strategies in structured insect-pathogen populations. J. Anim. Ecol. 2005, 74, 464–474. [Google Scholar] [CrossRef]
- Cabodevilla, O.; Villar, E.; Virto, C.; Murillo, R.; Williams, T.; Caballero, P. Intra- and intergenerational persistence of an insect Nucleopolyhedrovirus: Adverse effects of sublethal disease on host development, reproduction, and susceptibility to superinfection. Appl. Environ. Microbiol. 2011, 77, 2954–2960. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Graham, R.I.; Tummala, Y.; Rhodes, G.; Cory, J.S.; Shirras, A.; Grzywacz, D.; Wilson, K. Development of a Real-Time qPCR Assay for Quantification of Covert Baculovirus Infections in a Major African Crop Pest. Insects 2015, 6, 746-759. https://doi.org/10.3390/insects6030746
Graham RI, Tummala Y, Rhodes G, Cory JS, Shirras A, Grzywacz D, Wilson K. Development of a Real-Time qPCR Assay for Quantification of Covert Baculovirus Infections in a Major African Crop Pest. Insects. 2015; 6(3):746-759. https://doi.org/10.3390/insects6030746
Chicago/Turabian StyleGraham, Robert I., Yamini Tummala, Glenn Rhodes, Jenny S. Cory, Alan Shirras, David Grzywacz, and Kenneth Wilson. 2015. "Development of a Real-Time qPCR Assay for Quantification of Covert Baculovirus Infections in a Major African Crop Pest" Insects 6, no. 3: 746-759. https://doi.org/10.3390/insects6030746
APA StyleGraham, R. I., Tummala, Y., Rhodes, G., Cory, J. S., Shirras, A., Grzywacz, D., & Wilson, K. (2015). Development of a Real-Time qPCR Assay for Quantification of Covert Baculovirus Infections in a Major African Crop Pest. Insects, 6(3), 746-759. https://doi.org/10.3390/insects6030746




