Evaluation of Wood and Cellulosic Materials as Fillers in Artificial Diets for Lyctus africanus Lesne (Coleoptera: Bostrichidae)
Abstract
:1. Introduction
2. Experimental Section
2.1. Insect Sources
2.2. Diet Sources
2.3. Diet Preparation
| Composition | Diet 1 (%, w/w) | Diet 2 (%, w/w) | Diet 3 (%, w/w) |
|---|---|---|---|
| Wood powder | 26 | − | − |
| Cellulose powder | − | 26 | − |
| Alpha-cellulose | − | − | 26 |
| Dry yeast | 24 | 24 | 24 |
| Starch | 50 | 50 | 50 |
2.4. Total Larvae
2.5. Population, Sex Ratio and Body Weight of Newly Emerged Adults
3. Results
3.1. Total Larvae (Immature-Stage)
| Artificial diet | Total larvae/5 female (mean ± S.E) |
|---|---|
| Diet 1 | 141.00 ± 10.41 a* |
| Diet 2 | 134.17 ± 24.48 a |
| Diet 3 | 113.83 ± 25.00 a |
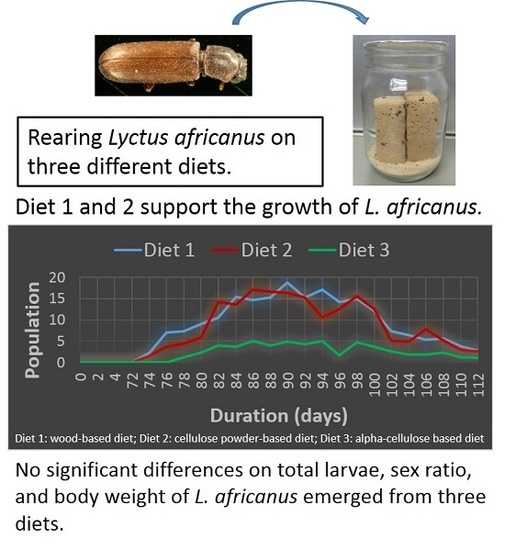
3.2. Total Adults
3.3. Sex Ratio
3.4. Body Weight

| Artificial diet | Total adults | Sex ratio (M/F) | Body weight (mg) |
|---|---|---|---|
| Diet 1 | 206.00 ± 49.37 a* | 0.94 ± 0.06 a | 1.98 ± 0.04 a |
| Diet 2 | 188.87 ± 30.60 a | 0.80 ± 0.05 a | 1.87 ± 0.06 a |
| Diet 3 | 55.87 ± 16.18 b | 0.76 ± 0.08 a | 2.01 ± 0.21 a |
4. Discussions ad Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gerberg, E.J. A Revision of the New World Species of Powder-post Beetles Belonging to the Family Lyctidae; US Department of Agriculture: Washington, DC, USA, 1957.
- Halperin, J.; Geis, K.U. Lyctidae (coleoptera) of Israel, their damage and its prevention. Phytoparasitica 1999, 27, 257–262. [Google Scholar] [CrossRef]
- Borowski, J.; Mazur, S. Contribution to the knowledge of the bostrichidae and associated histeridae of morocco (insecta, coleóptera). Il Nat. Valtellin. 2001, 12, 69–75. [Google Scholar]
- Liu, L.Y.; Schönitzer, K. Phylogenetic analysis of the family bostrichidae auct. At suprageneric levels. Mitt. Münch. Entomol. Ges. 2011, 101, 99–132. [Google Scholar]
- Australia, P.H. National Plantation Timber Industry: Biosecurity Plan; Plant Health Australia: Canberra, Australia, 2007. [Google Scholar]
- Peters, B.C.; Creffield, J.W.; Eldridge, R.H. Lyctine (coleoptera: Bostrichidae) pests of timber in Australia: A literature review and susceptibility testing protocol. Aust. For. 2002, 65, 107–119. [Google Scholar] [CrossRef]
- Stanaway, M.; Zalucki, M.; Gillespie, P.; Rodriguez, C.; Maynard, G. Pest risk assessment of insects in sea cargo containers. Aust. J. Entomol. 2001, 40, 180–192. [Google Scholar] [CrossRef]
- Brockerhoff, E.G.; Bain, J. Biosecurity implications of exotic beetles attacking trees and shrubs in new zealand. In Proceedings of the New Zealand Plant Protection Conference, Christchurch, New Zealand, 7–10 August 2000.
- Leschen, R.A.; Lawrence, J.F.; Kuschel, G.; Thorpe, S.; Wang, Q. Coleoptera genera of New Zealand. N. Z. Entomol. 2003, 26, 15–28. [Google Scholar] [CrossRef]
- Bytinski-Salz, H. An annotated list of insects and mites introduced into israel. Isr. J. Entomol. 1966, 1, 15–48. [Google Scholar]
- Mito, T.; Uesugi, T. Invasive alien species in Japan: The status quo and the new regulation for prevention of their adverse effects. Glob. Environ. Res. 2004, 8, 171–193. [Google Scholar]
- Furukawa, N.; Yoshimura, T.; Imamura, Y. Survey of lyctine damages on houses in Japan: Identification of species and infested area. Wood Preserv. 2009, 35, 260–264. (In Japanese) [Google Scholar] [CrossRef]
- Beaver, R.A.; Sittichaya, W.; Liu, L.-Y. A review of the powder-post beetles of thailand (coleoptera: Bostrichidae). Trop. Nat. Hist. 2011, 11, 135–158. [Google Scholar]
- Khalsa, H.; Nigam, B.; Agarwal, P. Breeding of powder post beetle, lyctus africanus lense (coleoptera) in an artificial medium. Indian J. Entomol. 1962, 24, 139–142. [Google Scholar]
- Parkin, E.A. A study of the food relations of the lyctus powder-post beetles. In Annals of Applied Biology; Association of Applied Biologists: Warwickshire, UK, 1936; Volume 23, pp. 369–401. [Google Scholar]
- Iwata, R.; Nishimoto, K. Studies on the autecology of Lyctus brunneus (stephens): Artificial diets in relationship to beetle supply. Mokuzai Gakkaishi 1983, 29, 336–343. (In Japanese) [Google Scholar]
- Iwata, R.; Nishimoto, K. Studies on autoecology of Lyctus brunneus (stephens): Mass culture of Lyctus brunneus. Wood Res. Tech. Notes 1980, 15, 34–44. (In Japanese) [Google Scholar]
- Antrim, R.L.; Chan, Y.-C.; Crary, J.R., Jr.; Harris, D.W. Method for Obtaining a Purified Cellulose Product from Corn Hulls. U.S. Patent 4,239,906, 16 December 1980. [Google Scholar]
- Ito, T. Rearing of Lyctus brunneus stephens (coleoptera: Lyctidae) on an artificial diet. Appl. Entomol. Zool. 1980, 15, 496–497. [Google Scholar]
- Parkin, E. The digestive enzymes of some wood-boring beetle larvae. J. Exp. Biol. 1940, 17, 364–377. [Google Scholar]
- Gay, F.J. Observations on the biology of Lyctus brunneus (steph). Aust. J. Zool. 1953, 1, 102–110. [Google Scholar] [CrossRef]
- Rosel, A. Oviposition, egg development and other features of five species of lyctidae (coleoptera). Aust. J. Entomol. 1969, 8, 145–152. [Google Scholar] [CrossRef]
- Altson, A.M. On the method of oviposition and the egg of Lyctus brunneus, steph. J. Linn. Soc. Lond. Zool. 1923, 35, 217–227. [Google Scholar] [CrossRef]
- Helal, H. Some biological informations about the small powder post beetle Lyctus africanus lesne, in Egypt. Agric. Res. Rev. 1981, 59, 167–175. [Google Scholar]
- Timmins, W.; Bellward, K.; Stamp, A.; Reynolds, S. Food intake, conversion efficiency, and feeding behaviour of tobacco hornworm caterpillars given artificial diet of varying nutrient and water content. Physiol. Entomol. 1988, 13, 303–314. [Google Scholar] [CrossRef]
- Iwata, R.; Nishimoto, K. Studies on the autecology of Lyctus brunneus (stephens): Investigations on the composition of artificial diets for Lyctus brunneus (stephens). Mater. Org. 1982, 17, 51–63. [Google Scholar]
- Mauldin, J.K.; Lambremont, E.N.; Graves, J.B. Principal lipid classes and fatty acids synthesized during growth and development of beetle lyctus-planicollis. Insect Biochem. 1971, 1, 316–326. [Google Scholar] [CrossRef]
- Iwata, R. Mass Culture method and biology of the wood-boring beetle, Lyctus Brunneus (Stephens). Ph.D. Thesis, Kyoto University, Kyoto, Japan.
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kartika, T.; Yoshimura, T. Evaluation of Wood and Cellulosic Materials as Fillers in Artificial Diets for Lyctus africanus Lesne (Coleoptera: Bostrichidae). Insects 2015, 6, 696-703. https://doi.org/10.3390/insects6030696
Kartika T, Yoshimura T. Evaluation of Wood and Cellulosic Materials as Fillers in Artificial Diets for Lyctus africanus Lesne (Coleoptera: Bostrichidae). Insects. 2015; 6(3):696-703. https://doi.org/10.3390/insects6030696
Chicago/Turabian StyleKartika, Titik, and Tsuyoshi Yoshimura. 2015. "Evaluation of Wood and Cellulosic Materials as Fillers in Artificial Diets for Lyctus africanus Lesne (Coleoptera: Bostrichidae)" Insects 6, no. 3: 696-703. https://doi.org/10.3390/insects6030696