Male Armaments and Reproductive Behavior in “Nutcracker” Camel Crickets (Rhaphidophoridae, Pristoceuthophilus)
Abstract
:1. Introduction
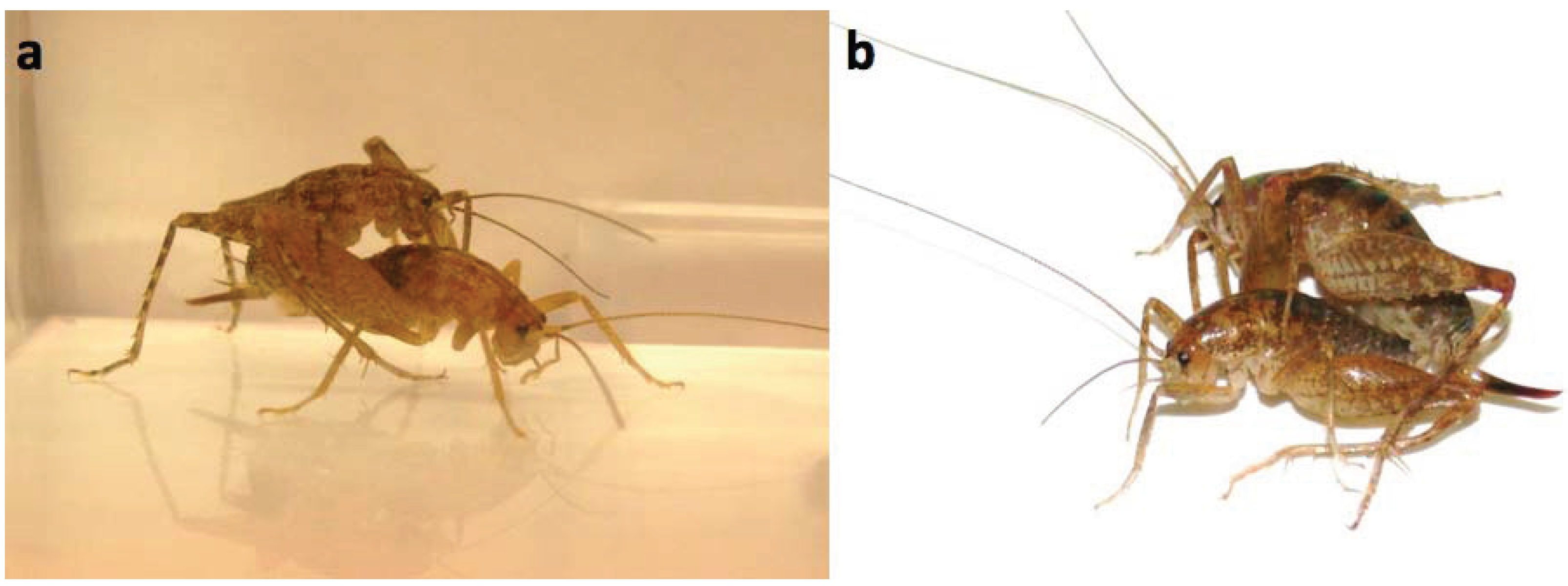
2. Experimental Section
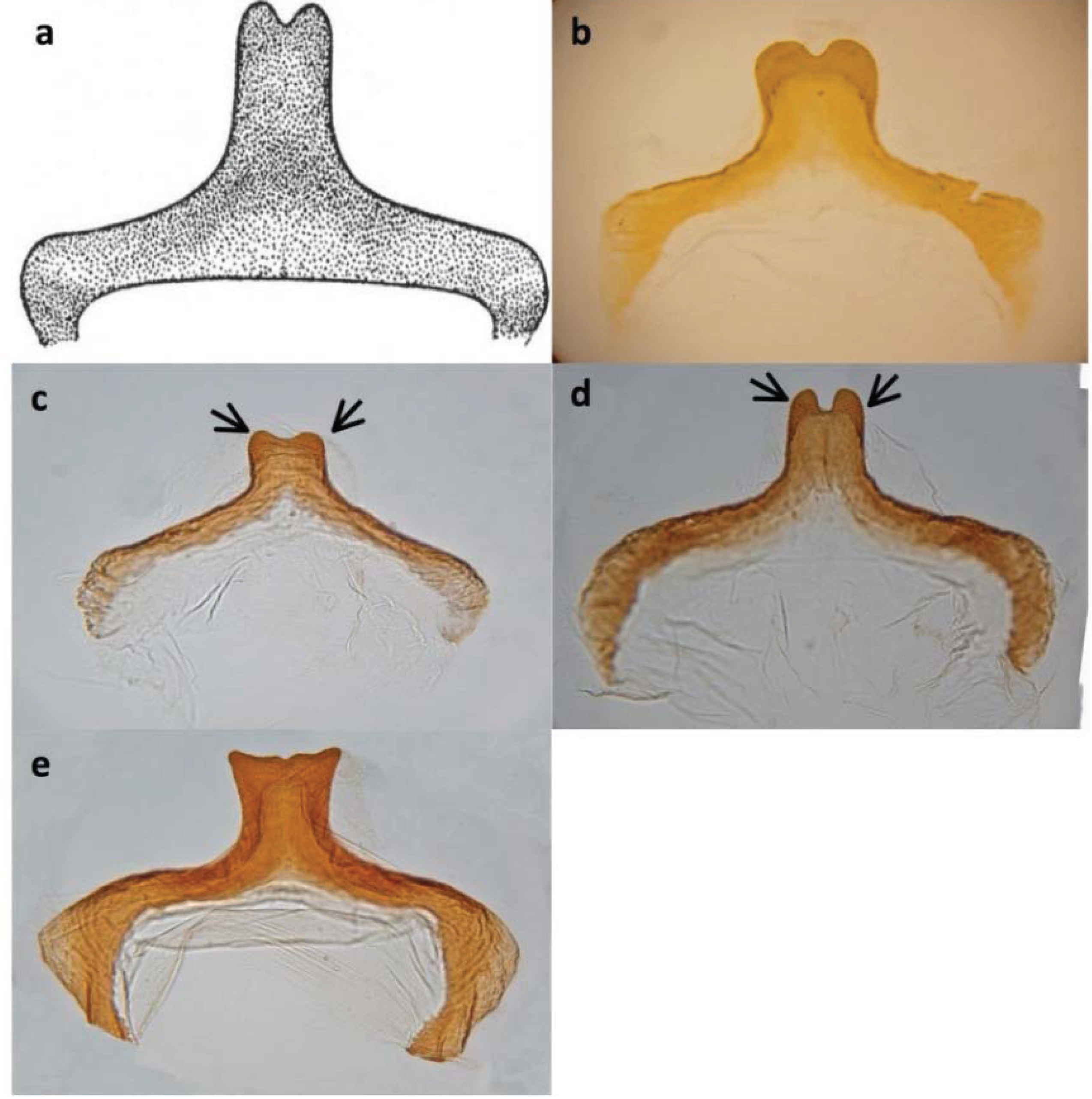
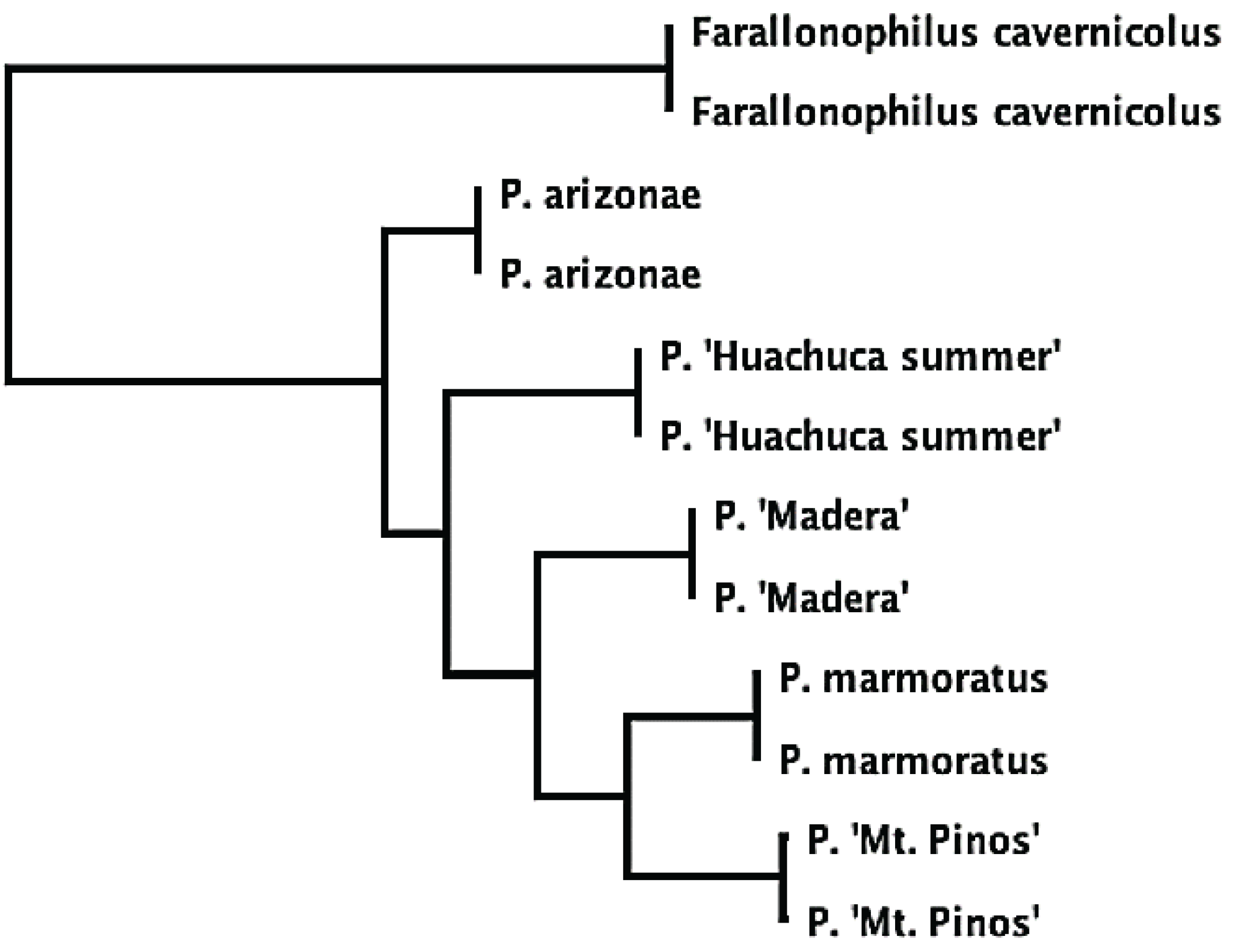
2.1. Taxonomic Issues and Species Status
2.2. Collection and Housing
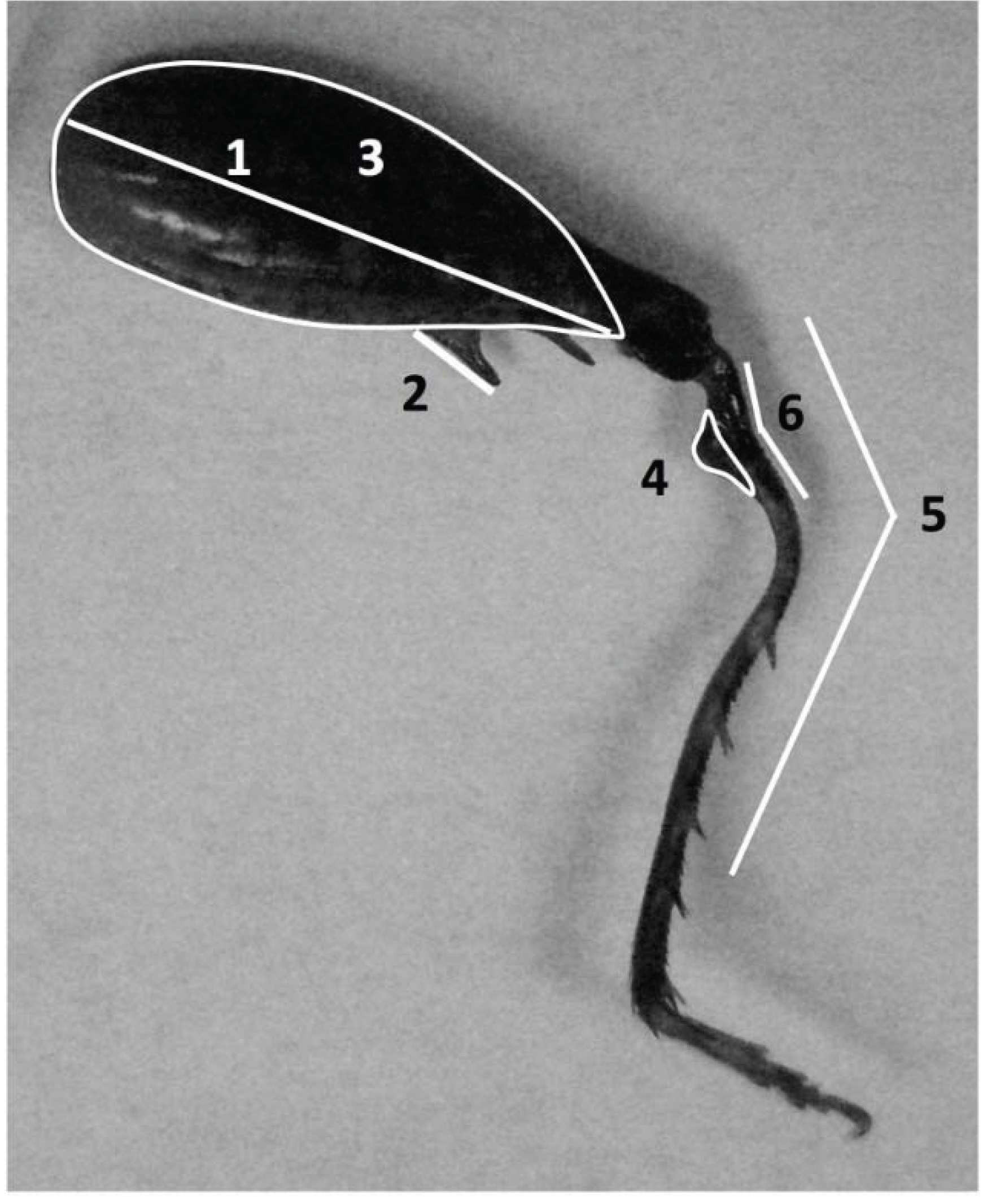
2.3. Measurement of Body Size, Armaments and Allometry
2.4. Behavioral Trials: Male-Male Fights and Male-Female Mating
2.5. Statistical Analyses
3. Results and Discussion
3.1. Armament Allometry
| Species | Spine Length | Flange Area | Primary Tibial Deflection | Secondary Tibial Deflection | Femur Area | Femur Length |
|---|---|---|---|---|---|---|
| P. ‘Huachuca summer’ | 6.24 ± 0.48 (0.71) | 4.05 ± 0.33 (0.67) | 6.23 ± 0.56 (0.61) | 6.44 ± 0.68 (0.45) | 1.13 ± 0.08 (0.74) | 0.94 ± 0.07 (0.72) |
| P. arizonae | 3.66 ± 0.68 (0.56) | 2.77 ± 0.68 (0.40) | 2.09 ± 0.53 (0.36) | 2.48 ± 0.54 (0.54) | 1.07 ± 0.14 (0.82) | 0.89 ± 0.12 (0.82) |
| P. ‘Madera’ | 5.28 ± 0.70 (0.81) | 3.85 ± 0.57 (0.76) | 3.04 ± 0.47 (0.74) | 2.41 ± 0.60 (0.32) | 1.2 ± 0.1 (0.92) | 1.17 ± 0.17 (0.77) |
| P. marmoratus | 4.67 ± 0.29 (0.71) | – | 2.35 ± 0.13 (0.76) | – | 1.06 ± 0.03 (0.94) | 1.02 ± 0.04 (0.91) |
| P. ‘Mt. Pinos’ | 2.53 ± 0.18 (0.69) | 2.40 ± 1.15 (0.76) | 3.62 ± 0.35 (0.43) | 3.62 ± 0.44 (0.14) | 1.03 ± 0.04 (0.91) | 1.02 ± 0.05 (0.84) |

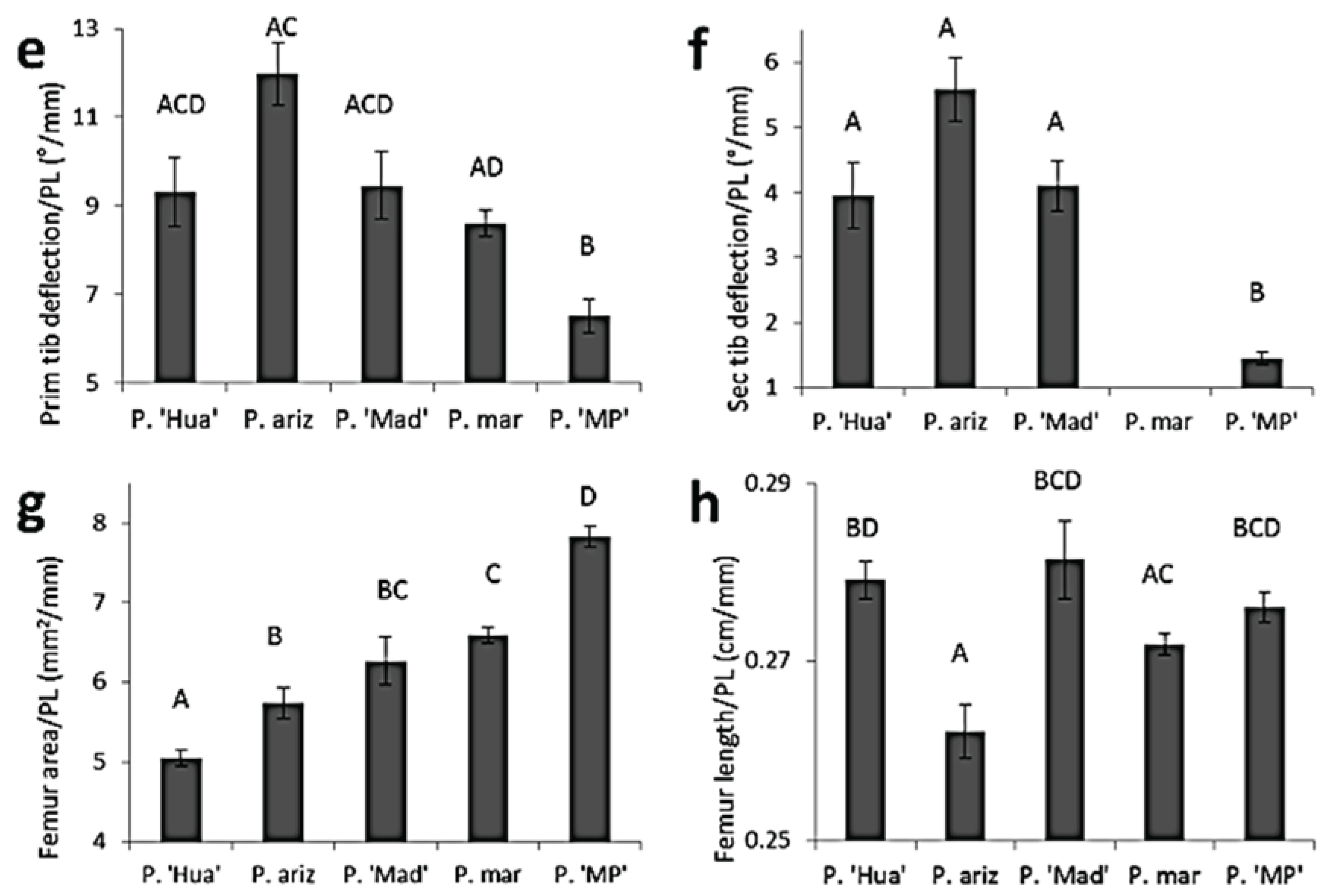
3.2. Comparisons of Body Size and Relative Armament Size among Species
3.3. Behavioral Trials
| Species | No. of Trials | No. of Trials with: | |||
|---|---|---|---|---|---|
| Male-Male Fights | Voluntary Copulation | Attempted Forced Copulation (Unsuccessful) | Successful Forced Copulation | ||
| P. ‘Huachuca summer’ | 21 | 11 | 15 | 4 | 0 |
| P. ‘Madera’ | 16 | 2 | 4 | 0 | 0 |
| P. marmoratus | 20 | 12 | 2 | 2 | 2 |
| P. ‘Mt. Pinos’ | 47 | 1 | 2 | 2 | 0 |
| Species | Total No. of trials | Total No. of Trials with | ||||
|---|---|---|---|---|---|---|
| Copulation Attempted (Successful/Unsuccessful) | Unsuccessful Attempted Copulation | Successful Copulation | ||||
| Forceful | Voluntary | Forceful | Voluntary | |||
| P. ‘Huachuca summer’ | 16 | 14 | 3 | 0 | 0 | 11 |
| P. ‘Madera’ | 11 | 0 | 0 | 0 | 0 | 0 |
| P. marmoratus | 101 | 33 | 15 | 0 | 4 | 14 |
| P. ‘Mt. Pinos’ | 69 | 1 | 0 | 0 | 0 | 1 |

3.4. General Observations of Voluntary Copulatory Behavior
3.5. Status of P. ‘Huachuca Summer’
3.6. Comparisons of Allometries and Armaments
3.7. Dual-Purpose Armaments in Other Pristoceuthophilus?
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Emlen, D.J. The evolution of animal weapons. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 387–413. [Google Scholar] [CrossRef]
- Andersson, M. Sexual Selection; Princeton University Press: Princeton, NJ, USA, 1994.
- Berglund, A.; Bisazza, A.; Pilastro, A. Armaments and ornaments: An evolutionary explanation of traits of dual utility. Biol. J. Linnaean Soc. 1996, 58, 385–399. [Google Scholar] [CrossRef]
- McKinney, F.; Derrickson, S.R.; Mineau, P. Forced copulation in waterfowl. Behaviour 1983, 86, 250–294. [Google Scholar] [CrossRef]
- Wilgers, D.J.; Nicholas, A.C.; Reed, D.H.; Stratton, G.E.; Hebets, E.A. Condition-dependent alternative mating tactics in a sexually cannibalistic wolf spider. Behav. Ecol. 2009, 20, 891–900. [Google Scholar] [CrossRef]
- Sakaluk, S.K.; Bangert, P.J.; Eggert, A.-K.; Gack, C.; Swanson, L.V. The gin trap as a device facilitating coercive mating in sagebrush crickets. Proc. R. Soc. Lond. Ser. B 1995, 261, 65–71. [Google Scholar] [CrossRef]
- Vahed, K. Coercive copulation in the alpine bushcricket Anonconotus alpinus Yersin (Tettigoniidae: Tettigoniinae: Platycleidini). Ethology 2002, 108, 1065–1075. [Google Scholar] [CrossRef]
- Vahed, K.; Carron, G. Comparison of forced mating behaviour in four taxa of Anonconotus, the Alpine bushcricket. J. Zool. 2008, 276, 313–321. [Google Scholar] [CrossRef]
- Khila, A.; Abouheif, E.; Rowe, L. Function, developmental genetics, and fitness consequences of a sexually antagonistic trait. Science 2012, 336, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Haley, E.L.; Gray, D.A. Mating behavior and dual-purpose armaments in a camel cricket. Ethology 2012, 118, 49–56. [Google Scholar] [CrossRef]
- Mitchell, P.L. Combat and territorial defense of Acanthophora femorata (Hemiptera: Coreidae). Ann. Entomol. Soc. Am. 1980, 73, 404–408. [Google Scholar]
- Miyatake, T. Functional morphology of the hind legs as weapons for male contests in Leptoglossus australis (Heteroptera: Coreidae). J. Insect Behav. 1997, 10, 727–735. [Google Scholar]
- Eberhard, W.G. The function of female resistance behavior: intromission by male coercin vs. female cooperation in sepsid flies (Diptera: Sepsidae). Rev. Biol. Trop. 2002, 50, 484–505. [Google Scholar]
- Conroy, L.P.; Gray, D.A. Forced copulation as a conditional alternative strategy in camel crickets. Behav. Ecol. Sociobiol. 2014, 68, 1431–1439. [Google Scholar] [CrossRef] [PubMed]
- Bonduriansky, R.; Day, T. The evolution of static allometry in sexually selected traits. Evolution 2003, 57, 2450–2458. [Google Scholar] [CrossRef] [PubMed]
- Kodric-Brown, A.; Sibly, R.M.; Brown, J.H. The allometry of ornaments and weapons. Proc. Natl. Acad. Sci. USA 2006, 103, 8733–8738. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, R.L.; Araya-Salas, M.; Gray, D.A.; Reichert, M.S.; Symes, L.B.; Wilkins, M.R.; Safran, R.J.; Höbel, G. How acoustic signals scale with individual body size: Common trends across diverse taxa. Behav. Ecol. 2014. [Google Scholar] [CrossRef]
- Cohn, T.J.; University of Michigan, Ann Arbor, MI, USA. Personal communication, 2011.
- Hebard, M. Studies in the Orthoptera of Arizona. Part I. New genera, species, and geographical races. Trans. Am. Entomol. Soc. 1935, 61, 111–153. [Google Scholar]
- Gray, D.A.; California State University, Northridge, CA, USA. Unpublished data. 2014.
- O’Hara, J.; Gray, D.A. Two new Orthopteran hosts of North American Polideini (Diptera: Tachinidae). Entomol. News 2004, 115, 177–178. [Google Scholar]
- Bohonak, A.J.; van der Linde, K. RMA: Software for Reduced Major Axis Regression, Java version. 2004. Available online: http://www.kimvdlinde.com/professional/rma.html (accessed on 20 November 2014).
- Mosimann, J.E. Size allometry: Size and shape variables with characteristics of the lognormal and generalized gamma distributions. J. Am. Stat. Assoc. 1970, 65, 930–945. [Google Scholar] [CrossRef]
- Haley, E.; Gray, D.A. Abdominal tubercles of adult male camel crickets, Pristoceuthophilus marmoratus Rehn (Orthoptera: Rhaphidophoridae), produce cues attractive to females. J. Insect Behav. 2013, 26, 804–811. [Google Scholar]
- Bonduriansky, R. Sexual selection and allometry: A critical reappraisal of the evidence and ideas. Evolution 2007, 61, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Polis, G.A.; Sisson, W.D. Life history. In The Biology of Scorpions; Polis, G.A., Ed.; Stanford University Press: Stanford, CA, USA, 1990; pp. 161–223. [Google Scholar]
- Gould, S.J.; Vrba, E.S. Exaptation—A missing term in the science of form. Paleobiology 1982, 8, 4–15. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conroy, L.P.; Gray, D.A. Male Armaments and Reproductive Behavior in “Nutcracker” Camel Crickets (Rhaphidophoridae, Pristoceuthophilus). Insects 2015, 6, 85-99. https://doi.org/10.3390/insects6010085
Conroy LP, Gray DA. Male Armaments and Reproductive Behavior in “Nutcracker” Camel Crickets (Rhaphidophoridae, Pristoceuthophilus). Insects. 2015; 6(1):85-99. https://doi.org/10.3390/insects6010085
Chicago/Turabian StyleConroy, Lauren P., and David A. Gray. 2015. "Male Armaments and Reproductive Behavior in “Nutcracker” Camel Crickets (Rhaphidophoridae, Pristoceuthophilus)" Insects 6, no. 1: 85-99. https://doi.org/10.3390/insects6010085
APA StyleConroy, L. P., & Gray, D. A. (2015). Male Armaments and Reproductive Behavior in “Nutcracker” Camel Crickets (Rhaphidophoridae, Pristoceuthophilus). Insects, 6(1), 85-99. https://doi.org/10.3390/insects6010085





