Improvement of the Insecticidal Capacity of Two Purpureocillium Lilacinum Strains against Tribolium Confusum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Isolates
2.2. Estimation of Enzymatic Activity of P. Lilacinum
Plate Assay Method
2.3. Growth Studies: Effect of Different Substrates on the Rate of P. Lilacinum Growth
2.4. Quantitative Studies for Estimating the Production of Extracellular Enzymes by P. Lilacinum
2.4.1. Culture Media
2.4.2. Experimental Conditions and Fungal Inoculation
2.4.2.1. Spectrophotometric Analysis of Proteases and Amylases
2.4.2.2. Tritimetric Method
2.5. Stimulation of Pathogenicity against T. Confusum
2.5.1. Fungal Growth on Synthetic Hydrocarbon-Enriched Media
2.5.2. Insect Rearing
2.5.3. Pathogenicity Bioassay
2.6. Statistical Analysis
3. Results
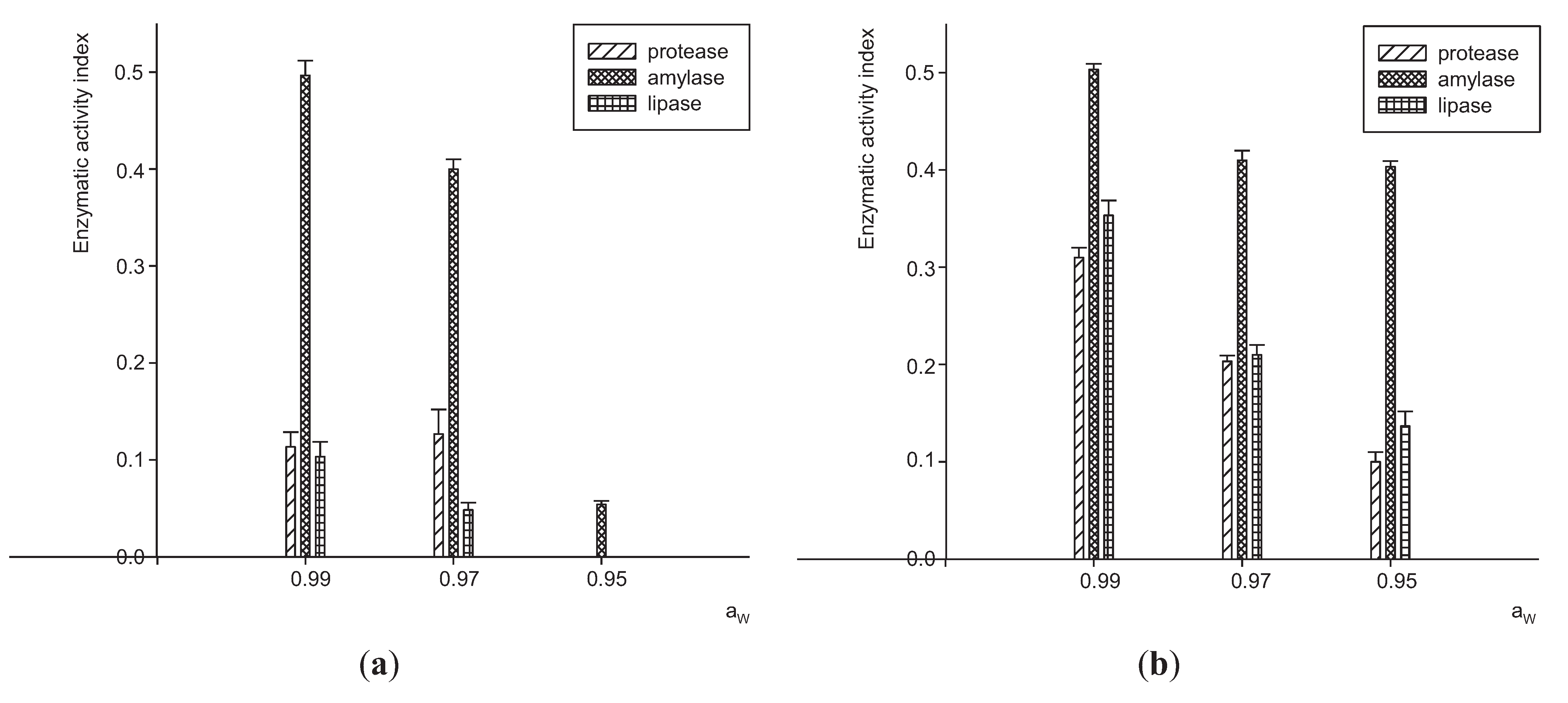
3.1. Extracellular Enzymes Activity by P. Lilacinum
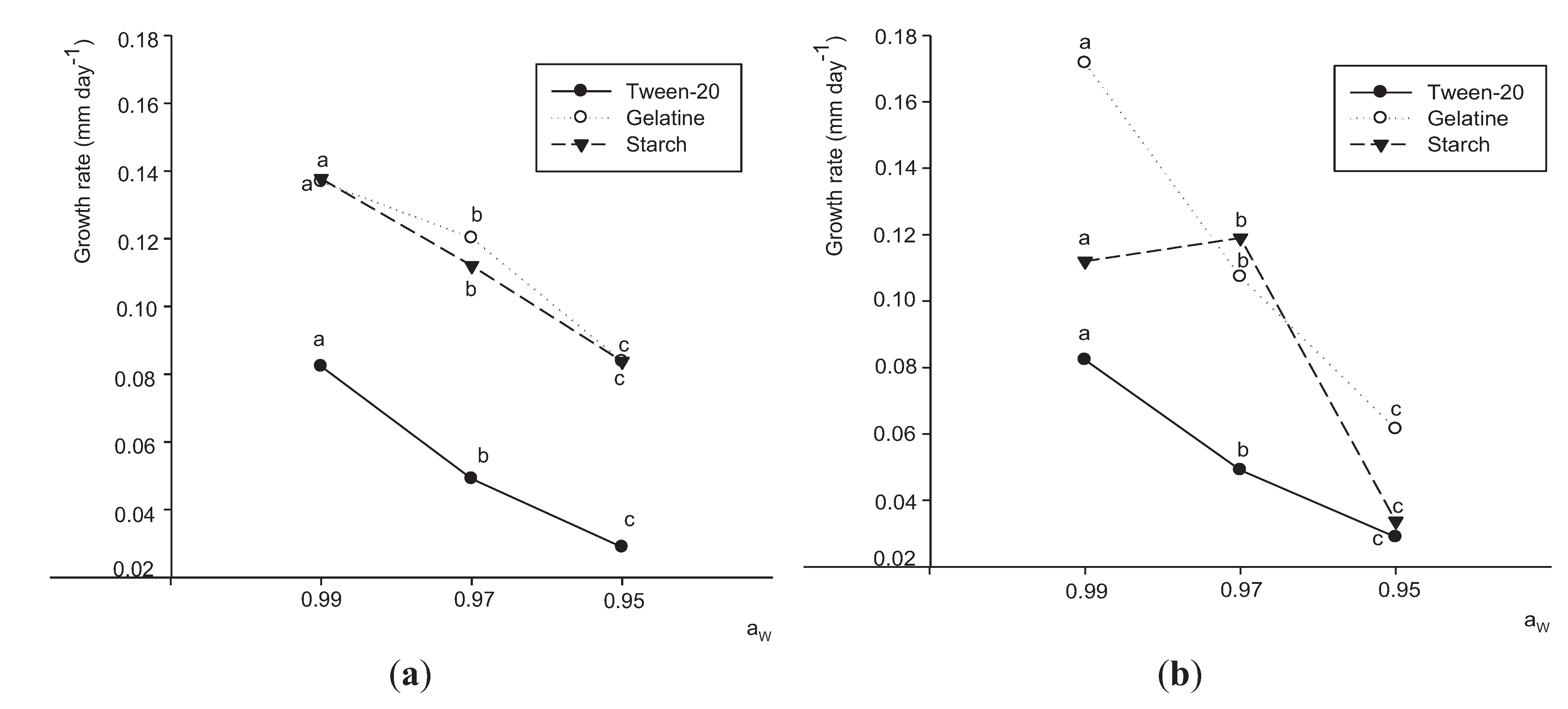
3.2. Effects of Aw and Substrates on Growth
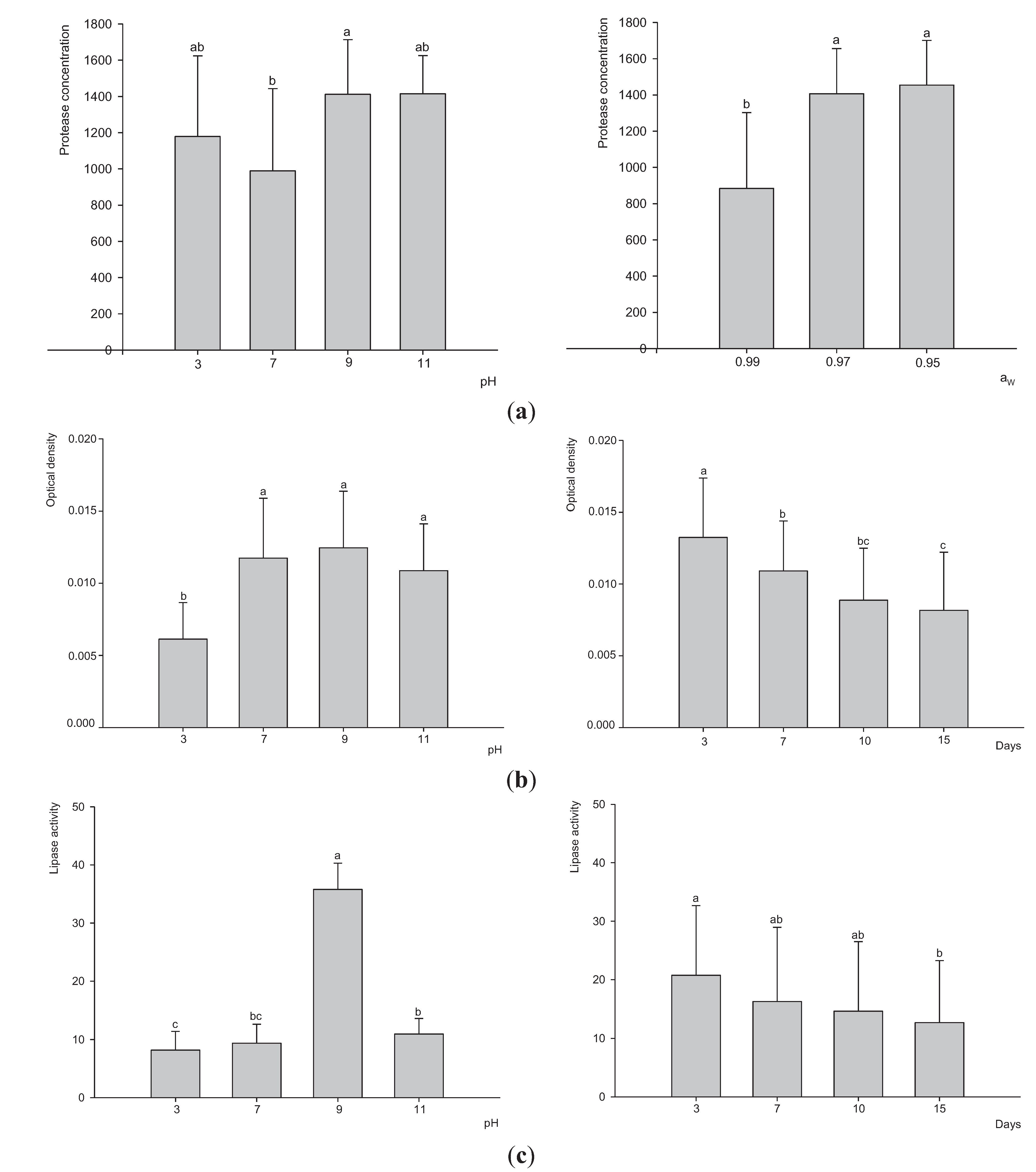
3.3. Extracellular Enzyme Production by P. Lilacinum
3.4. Pathogenicity Analysis

4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Descamps, L.R.; Reviriego, M.E.; Suárez, A.A.; Ferrero, A.A. Reproducción de Sitophilus oryzae L. (Coleoptera: Curculionidae) y de Tribolium castaneum Herbst. (Coleoptera: Tenebrionidae) en cultivares de trigo argentinos. Bol. San. Veg. Plagas 2004, 30, 171–176. (in Spanish). [Google Scholar]
- Cassini, C.; Rodriguez, J.; Bartosik, R.; Peiretti, J.; Cabral, G. TRIGO Eficiencia de Cosecha y Postcosecha de granos. Manual Técnico N. 1. Sección Postcosecha; Ediciones INTA: Ciudad de Buenos Aires, Argentina, 2005; p. 120. (in Spanish) [Google Scholar]
- Fusé, C.B.; Villaverde, M.L.; Padín, S.B.; de Giusto, M.; Juarez, M.P. Evaluación de la actividad insecticida de tierras de diatomeas de yacimientos argentinos. Rev. Investig. Agropecuarias 2013, 2, 207–213. (in Spanish). [Google Scholar]
- Mejía, D. Food and Agriculture Organization of the United Nations (FAO). Available online: http://www.fao.org/inpho/content/compend/text/ch23 04.htm (accessed on 20 February 2015).
- White, N.D.G. Insects, mites and insecticides in stored-grain ecosystems. In Stored Grain Ecosystems; Jayas, P., White, N.D.G., Muir, W.E., Eds.; Marcel Dekker: New York, NY, USA, 1995; pp. 123–167. [Google Scholar]
- Wallace, H.A.H.; Sinha, R.N. Causal Factors Operative in Distributional Patterns and Abundance of Fungi: A Multivariate Study. In The Fungal Community. Its Organisation and Role in Ecosystems; Wicklow, D.T., Carroll, G.C., Eds.; Marcell Dekker Inc.: New York, NY, USA, 1981; pp. 233–247. [Google Scholar]
- Nesci, A.; Barra, P.; Etcheverry, M. Integrated management of insect vectors of Aspergillus flavus in stored maize using synthetic antioxidants and natural phytochemicals. J. Stored Prod. Res. 2011, 47, 231–237. [Google Scholar] [CrossRef]
- Nesci, A.; Montemarani, A.; Etcheverry, M. Assessment of mycoflora and infestation of insects, vector of Aspergillus section Flavi, in stored peanut from Argentina. Mycotoxin Res. 2011, 27, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Barra, P.; Nesci, A.; Etcheverry, M. In vitro compatibility of natural and food grade fungicide and insecticide substances with Purpureocillium lilacinum and their effect against Aspergillus flavus. J. Stored Prod. Res. 2013, 54, 67–73. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). Monograph on the Evaluation of Carcinogenic Risk to Humans; IARC: Lyon, France, 1993. [Google Scholar]
- Cassini, C.; Santajuliana, M. Proyecto de Eficiencia de Cosecha, Postcosecha de Granos y Agroindustria en Origen; Instituto Nacional de Tecnología agropecuaria (INTA): Ciudad de Buenos Aires, Argentina, 2012. (in Spanish) [Google Scholar]
- Sartori, M.R.; Pacheco, I.A.; Vilar, R.M. Resistance to Phosphine in Stored Grain Insects in Brazil. In Proceedings of the Fifth International Working Conference on Stored-Product Protection, Bordeaux, France, 9–14 September 1990; Fleurat-Lessard, F., Ducom, P., Eds.; Imprimerie Medocaine: Blanquefort Cedex, France, 1991; pp. 1041–1050. [Google Scholar]
- Leemon, D.M.; Jonsson, N.N. Laboratory studies on Australian isolates of Metarhizium anisopliae as a biopesticide for the cattle tick Boophilus microplus. J. Invertebr. Pathol. 2008, 97, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.A.; Pope, E.C.; Carpenter, S.; Scholte, E.J.; Butt, T.M. Entomopathogenic fungus as a biological control for an important vector of livestock disease: The Culicoides biting midge. PLOS ONE 2011, 6, e16108. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, T.; Takken, W.; Koenraadt, C. Development of Metarhizium anisopliae and Beauveria bassiana formulations for control of malaria mosquito larvae. Parasit. Vectors 2011, 4, 23. [Google Scholar] [CrossRef] [PubMed]
- Morgan-Jones, G.; White, G.F.; Rodriguez-Kabana, R. Phytonematode pathology: Ultrastructural studies. II. Parasitism of Meloidogyne arenaria eggs and larvae by Paecilomyces lilacinus. Nematropica 1984, 14, 57–71. [Google Scholar]
- Jatala, P. Biological control of plant parasitic nematodes. Ann. Rev. Phytopathol. 1986, 24, 453–489. [Google Scholar] [CrossRef]
- Dube, B.; Smart, G.C. Biological control of Meloidogyne by Paecilomyces lilacinus and Pasteuria penetrans. J. Nematol. 1987, 19, 222–227. [Google Scholar] [PubMed]
- Khan, A.; Williams, K.L.; Nevalainen, H.K.M. Control of plant parasitic nematodes by Paecilomyces lilacinus and Monacrosporium lysipagum in pot trials. BioControl 2006, 51, 643–658. [Google Scholar] [CrossRef]
- Barra, P.; Rosso, L.; Nesci, A.; Etcheverry, M. Isolation and identification of entomopathogenic fungi and their evaluation against Tribolium confusum, Sitophilus zeamais, and Rhyzopertha dominica in stored maize. J. Pest Sci. 2013, 86, 217–226. [Google Scholar] [CrossRef]
- Magan, N. Physiological Approaches to Improving the Ecological Fitness of Fungal Biocontrol Agents. In Fungi as Biocontrol Agents; Butt, T.M., Jackson, C., Magan, N., Eds.; CAB International: Wallingford, UK, 2001. [Google Scholar]
- Hallsworth, J.E.; Magan, N. Effects of KCl concentration on accumulation of acyclic sugar alcohols and trehalose in conidia of three entomopathogenic fungi. Lett. Appl. Microbiol. 1994, 18, 8–11. [Google Scholar] [CrossRef]
- Hallsworth, J.E.; Magan, N. Effect of carbohydrate type and concentration on polyhydroxy alcohol and trehalose content of conidia of three entomopathogenic fungi. Microbiology 1994, 140, 2705–2713. [Google Scholar] [CrossRef]
- Frey, S.; Magan, N. Production of the fungal biocontrol agent Ulocladium atrum by submerged fermentation: Accumulation of endogenous reserves and shelf-life studies. Appl. Microbiol. Biotechnol. 2001, 56, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, J.F.; Crowe, J.H. The mechanism of cryoprotection of proteins by solutes. Cryobiology 1988, 25, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Deacon, J. Significance of ecology in the development of biocontrol agents against soil-borne plant pathogens. Biocontrol Sci. Technol. 1991, 1, 5–20. [Google Scholar] [CrossRef]
- Samson, R.A.; Evans, H.C.; Latgé, J.P. Atlas of Entomopathogenic Fungi; Springer Verlag: New York, NY, USA, 1988; p. 187. [Google Scholar]
- Hajek, A.E.; St. Leger, R.J. Interactions between fungal pathogens and insect hosts. Annu. Rev. Entomol. 1994, 39, 293–322. [Google Scholar] [CrossRef]
- Charnley, A.K.; Collins, S.A. Entomopathogenic Fungi and Their Role in Pest Control. In The Mycota IV: Environmental and Microbial Relationships, 2nd ed.; Kubicek, C.P., Druzhinina, I.S., Eds.; Springer-Verlag: Berlin, Germany, 2007; pp. 159–187. [Google Scholar]
- Bidochka, M.J.; St. Leger, R.S.; Roberts, D.W. Mechanisms of Deuteromycete fungal infections in grasshoppers and locusts: An overview. Mem. Entomol. Soc. Can. 1997, 171, 213–224. [Google Scholar] [CrossRef]
- Boucias, D.; Pendland, J. Attachment of Mycopathogens to Cuticle. In The Fungal Spore and Disease Initiation in Plants and Animals; Cole, G.T., Hoch, H.C., Eds.; Plenum Press: New York, NY, USA, 1991; pp. 101–127. [Google Scholar]
- Fang, W.G.; Feng, J.; Fan, Y.H.; Zhang, Y.J.; Bidochka, M.J.; Leger, R.J.S.; Pei, Y. Expressing a fusion protein with protease and chitinase activities increases the virulence of the insect pathogen Beauveria bassiana. J. Invertebr. Pathol. 2009, 102, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Blomquist, G.J.; Dillwith, J.W. Cuticular Lipids. In Comprehensive Insect Physiology, Biochemistry, and Pharmacology; Kerkutand, G.A., Gilbert, L.I., Eds.; Pergamon Press: Oxford, UK, 1985; pp. 117–154. [Google Scholar]
- Lockey, K.H. Lipids of the insect cuticle: Origin, composition and function. Comp. Biochem. Physiol. 1988, 89, 595–645. [Google Scholar] [CrossRef]
- Buckner, J.S. Cuticular Polar Lipids of Insects. In Insect Lipids: Chemistry, Biochemistry and Biology; Stanley-Samuelson, D.W., Nelson, D.R., Eds.; University of Nebraska Press: Lincoln, NE, USA, 1993; pp. 227–270. [Google Scholar]
- Nelson, D.R.; Blomquist, G.J. Insect Waxes. In Waxes Chemistry, Molecular Biology and Functions; Hamilton, R.J., Ed.; The Oily Press, Ltd.: Dundee, UK, 1995; pp. 1–90. [Google Scholar]
- Blomquist, G.J.; Nelson, D.R.; de Renobales, M. Chemistry, biochemistry and physiology of insect cuticular lipids. Arch. Insect Biochem. 1987, 6, 227–265. [Google Scholar] [CrossRef]
- Boucias, D.G.; Pendland, J.C. Nutritional requirements for conidial germination of several host range pathotypes of the entomopathogenic fungus Nomuraea rileyi. J. Invertebr. Pathol. 1984, 43, 288–292. [Google Scholar] [CrossRef]
- Boucias, D.G.; Pendland, J.C.; Latge, J.P. Nonspecific factors involved in attachment of entomopathogenic Deuteromycetes to host insect cuticle. Appl. Environ. Microbiol. 1988, 54, 1795–1805. [Google Scholar] [PubMed]
- Lord, J.C.; Howard, R.W. A proposed role for the cuticular fatty amides of Liposcelis bostrychophila (Psocoptera: Liposcelidae) in preventing adhesion of entomopathogenic fungi with dry-conidia. Mycopathologia 2004, 158, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Aro, N.; Pakula, T.; Penttila, M. Transcriptional regulation of plant cell wall degradation by filamentous fungi. FEMS Microbiol. Rev. 2005, 29, 719–739. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Urquiza, A.; Keyhani, N.O. Action on the surface: Entomopathogenic fungi versus the insect cuticle. Insects 2013, 4, 357–374. [Google Scholar]
- Hankin, L.; Anagnostakis, S.L. The use of solid media for detection of enzyme production by fungi. Mycologia 1975, 67, 597–607. [Google Scholar] [CrossRef]
- Dallyn, H.; Fox, A. Spoliage of Material of Reduced Water Activity by Xerophilic Fungi. In Microbial Growth and Survival in Extreme Environments; Gould, G.H., Corry, E.L., Eds.; Academic Press: London, UK, 1980; pp. 129–139. [Google Scholar]
- Pitt, J.I. The Genus Penicillium and its Teleomorphic States Eupenicillium and Talaromyces; Academic Press: London, UK, 1979. [Google Scholar]
- Price, M.F.; Wilkinson, I.D.; Gentry, L.O. Plate method for detection of phospholipase activity in Candida albicans. Sabouraudia 1982, 20, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Gornall, A.G.; Bardawill, C.J.; David, M.M. Determination of serum proteins by means of the biuret reaction. J. Biol. Chem. 1949, 177, 751–766. [Google Scholar]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Kamimura, E.S.; Medieta, O.; Rodrigues, M.L.; Maugeri, F. Studies on lipase-affinity adsorption using response-surface analysis. Biotech. Appl. Biochem. 2001, 33, 153–159. [Google Scholar] [CrossRef]
- Crespo, R.; Juárez, M.P.; dal Bello, G.M.; Padín, S.; Calderón Fernández, G.; Pedrini, N. Increased mortality of Acanthoscelides obtectus by alkane-grown Beauveria bassiana. BioControl 2002, 47, 685–696. [Google Scholar] [CrossRef]
- Goettel, M.S.; Inglis, G.D. Fungi: Hyphomycetes. In Manual of Techniques in Insect Pathology; Lacey, L.A., Ed.; Academic Press: San Diego, CA, USA, 1997; pp. 213–249. [Google Scholar]
- Padín, S.B.; dal Bello, G.M.; Vasicek, A.L. Pathogenicity of Beauveria bassiana for adults of Tribolium castaneum (Coleoptera: Tenebrionidae) in stored grains. Entomophaga 1997, 42, 569–574. [Google Scholar] [CrossRef]
- Wicklow, D.T.; Weaver, D.K.; Throne, J.E. Fungal colonists of maize grain conditioned at constant temperatures and humidities. J. Stored Prod. Res. 1998, 34, 355–361. [Google Scholar] [CrossRef]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzalez, L.; Tablada, M.; Robledo, C.W. InfoStat, versión 2008; Grupo InfoStat, FCA: Universidad Nacional de Córdoba, Córdoba, Argentina, 2008. [Google Scholar]
- Lacey, L.A.; Frutos, R.; Kaya, H.K.; Vail, P. Insect pathogens as biological control agents: Do they have a future? Biol. Control. 2001, 21, 230–248. [Google Scholar] [CrossRef]
- Juárez, M.P.; Crespo, R.; Calderón Fernández, G.; Lecouna, R.; Cafferata, L.F.R. Characterization and carbon metabolism in fungi pathogenic to Triatoma infestans, a Chagas disease vector. J. Invertebr. Pathol. 2000, 76, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Crespo, R.; Juárez, M.P.; Cafferatta, L.F.R. Biochemistry of the interaction between entomopathogenous fungi and their insect host-like hydrocarbons. Mycologia 2000, 92, 528–536. [Google Scholar] [CrossRef]
- Pedrini, N.; Juárez, M.; Crespo, R.; de Alaniz, M. Clues on the role of Beauveria bassiana catalases in alkane degradation events. Mycologia 2006, 98, 528–534. [Google Scholar]
- Pedrini, N.; dal Bello, G.; Padín, S.; Juárez, M. Capacidad insecticida de Beauveria bassiana cultivada en hidrocarburos para control de coleópteros en granos almacenados. Agrociencia (Uruguay) 2011, 1, 64–69. (in Portuguese). [Google Scholar]
- Pedrini, N.; Crespo, R.; Juarez, M.P. Biochemistry of insect epicuticle degradation by entomopathogenic fungi. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 2007, 146, 124–137. [Google Scholar] [CrossRef]
- Pedrini, N.; Mijailovsky, S.J.; Girotti, J.R.; Stariolo, R.; Cardozo, R.M.; Gentile, A.; Juárez, M.P. Control of pyrethroid-resistant chagas disease vectors with entomopathogenic fungi. PLOS Negl. Trop. Dis 2009, 3, e434. [Google Scholar] [CrossRef] [PubMed]
- Scorsetti, A.C.; Elíades, L.A.; Stenglein, S.A.; Cabello, M.N.; Pelizza, S.A.; Saparrat, M.C.N. Pathogenic and enzyme activities of the entomopathogenic fungus Tolypocladium cylindrosporum (Ascomycota: Hypocreales) from Tierra del Fuego, Argentina. Rev. Biol. Trop. 2011, 60, 833–841. [Google Scholar]
- Charnley, A.K. Fungal pathogens of insects: Cuticle degrading enzymes and toxins. Adv. Bot. Res. 2003, 40, 241–321. [Google Scholar]
- Jackson, C.W.; Heale, J.B.; Hall, R.A. Traits associated with virulence to the aphid Macrosiphoniella sanborni in eighteen isolates of Verticillium lecanii. Ann. Biol. 1985, 106, 39–48. [Google Scholar] [CrossRef]
- Rosato, Y.B.; Messias, C.L.; Azevedo, J.L. Production of extracellular enzymes by isolates of Metarhizium anisopliae. J. Invertebr. Pathol. 1981, 38, 1–3. [Google Scholar] [CrossRef]
- Esteves, I.; Peteira, B.; Atkins, S.; Magan, N.; Kerry, B. Production of extracellular enzymes by different isolates of Pochonia chlamydosporia. Mycol. Res. 2009, 113, 867–876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segers, R. The nematophagus fungus Verticillium Chlamydosporium: Aspects of phatogenicity. Ph.D. Thesis, Nottingham University, Nottingham, UK, 1996. [Google Scholar]
- Carlsen, M.; Spohr, A.B.; Nielsen, J.; Villadsen, J. Morpgology and physiology of an α amylase producing strain of Aspergillus oryzae during batch cultivations. Biotechnol. Bioeng. 1996, 49, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Chandler, D.; Hay, D.; Reid, A.P. Sampling and occurrence of entomopathogenic fungi and nematodes in UK soils. Appl. Soil Ecol. 1997, 5, 133–141. [Google Scholar] [CrossRef]
- Bidochka, M.J.; Khachatourians, G.G. Identification of Beauveria bassiana extracellular protease as a virulence factor in pathogenicity toward the migratory grasshopper Melanoplus sanguinipes. J. Invertebr. Pathol. 1990, 59, 165–173. [Google Scholar] [CrossRef]
- Hepburn, H.R. Structure of the Integument. In Comprehensive Insect Physiology, Biochemistry and Pharmacology; Kerkut, G.A., Gilbert, L.I., Eds.; Pergamon: Oxford, UK, 1985; Volume 3, pp. 1–58. [Google Scholar]
- Bidochka, M.J. Interaction of the Entomopathogenic Fungus Beauveria Bassiana with the Migratory Grasshopper Melanoplus Sanguinipes a Systematic Study of Pathogenesis. Ph.D. Thesis, University of Saskatchewan, Saskatoon, SK, Canada, 1989. [Google Scholar]
- Andersen, S.O. Sclerotization and Tanning of the Cuticle. In Comprensive Insect Physiology, Biochemistry and Pharmacology; Kerkut, G.A., Gilbert, L.I., Eds.; Pergamon: Oxford, UK, 1985; pp. 59–74. [Google Scholar]
- Margaritis, L.H. Structure and Physiology of the Egg Shell. In Comprensive Insect Physiology, Biochemistry and Pharmacology; Kerkut, G.A., Gilbert, L.I., Eds.; Pergamon Press: Oxford, UK, 1985; Volume 1, pp. 153–230. [Google Scholar]
- Ghanem, K.M.; Al-Garni, S.M.; Al-Makishah, N.H. Statistical optimization of cultural conditions for chitinase production from fish scales waste by Aspergillus terreus. Afr. J. Biotechnol. 2010, 9, 5135–5146. [Google Scholar]
- Gómes Fernandes, E.; Maia Valério, H.; Feltrin, T.; van der Sand, S.T. Variability in the production of extracellular enzymes by entomopathogenic fungi grown on different substrates. Braz. J. Microbiol. 2012, 43, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Petlamul, W.; Prasertsan, P. Evaluation of strains of Metarhizium anisopliae and Beauveria bassiana against Spodoptera litura on the basis of their virulence, germination rate, conidia production, radial growth and enzyme activity. Mycobiology 2012, 40, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Dhar, P.; Gurvinder, K. Production of cuticle-degrading proteases by Beauveria bassiana and their induction in different media. Afr. J. Biochem. Res. 2010, 4, 65–72. [Google Scholar]
- Lopez-Llorca, L.V.; Olivares-Bernabeu, C.; Salinas, J.; Jansson, H.B.; Kolattukudy, P.E. Prepenetration events in fungal parasitism of nematode eggs. Mycol. Res. 2002, 106, 499–506. [Google Scholar] [CrossRef]
- Mendoza de Gives, P.; Behnke, J.M.; Davies, K.G. Extracellular enzyme production by nematophagous fungi in the presence and absence of nematodes. Int. J. Nemathol. 2003, 13, 27–36. [Google Scholar]
- Mortan, O.; Hirsch, P.R.; Peberdy, J.F.; Kerry, B.R. Cloning and genetic variation in protease VCP1 from the nematophagous fungus Pochonia chlamydosporia. Mycol. Res. 2003, 107, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Segers, R.; Butt, T.M.; Kerry, BR.; Peberdy, J.F. The nematophagous fungus Verticillium chlamydosporium produces a chymoelastase-like protease which hydrolyses host nematode proteins in situ. Microbiology 1994, 140, 2715–2723. [Google Scholar] [CrossRef] [PubMed]
- Maccheroni Jr., W.; Welington, L.A.; Azevedo, J.L. Ambient pH-regulated enzime secretion in endophytic and pathogenic isolates of the fungal genus Colletotrichum. Sci. Agric. 2004, 61, 298–302. [Google Scholar] [CrossRef]
- Silva, W.O.B.; Mitidieri, S.; Schrank, A.; Vainstein, M.H. Productionand extraction of an extracellular lipase from the entomopathogenic fungus Metarhizium anisopliae. Process Biochem. 2005, 40, 321–326. [Google Scholar] [CrossRef]
- Hasan, S.; Ahmad, A.; Purwar, A.; Khan, N.; Kundan, R.; Gupta, G. Production of extracellular enzymes in the entomopathogenic fungus Verticillium lecanii. Bioinformation 2013, 9, 238–242. [Google Scholar] [CrossRef] [PubMed]
- St. Leger, R.J.; Charnley, A.K.; Cooper, R.M. Cuticle-degrading enzymes of entomopathogenic fungi: Synthesis in culture on cuticle. J. Invertebr. Pathol. 1986, 48, 85–95. [Google Scholar] [CrossRef]
- Lopez-Llorca, L.V.; Carbonell, T. Characterization of spanish strains of Verticillium lecanii. Rev. Iberoam. Micol. 1999, 16, 136–142. [Google Scholar] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barra, P.; Etcheverry, M.; Nesci, A. Improvement of the Insecticidal Capacity of Two Purpureocillium Lilacinum Strains against Tribolium Confusum. Insects 2015, 6, 206-223. https://doi.org/10.3390/insects6010206
Barra P, Etcheverry M, Nesci A. Improvement of the Insecticidal Capacity of Two Purpureocillium Lilacinum Strains against Tribolium Confusum. Insects. 2015; 6(1):206-223. https://doi.org/10.3390/insects6010206
Chicago/Turabian StyleBarra, Paula, Miriam Etcheverry, and Andrea Nesci. 2015. "Improvement of the Insecticidal Capacity of Two Purpureocillium Lilacinum Strains against Tribolium Confusum" Insects 6, no. 1: 206-223. https://doi.org/10.3390/insects6010206




