Efficacy of Commercially Available Invertebrate Predators against Drosophila suzukii
Abstract
:1. Introduction
2. Experimental Section
2.1. Source of Insects
2.2. Experimental Arenas
2.3. The Feeding Rate of Orius majusculus and Orius laevigatus
2.4. The Feeding Rate of Atheta coriaria
2.5. The Feeding Rate of Hypoaspis miles
2.6. The Feeding Rate of Anthocoris nemoralis
2.7. Data Analysis
3. Results and Discussion
3.1. Orius majusculus and Orius laevigatus
3.2. Atheta coriaria



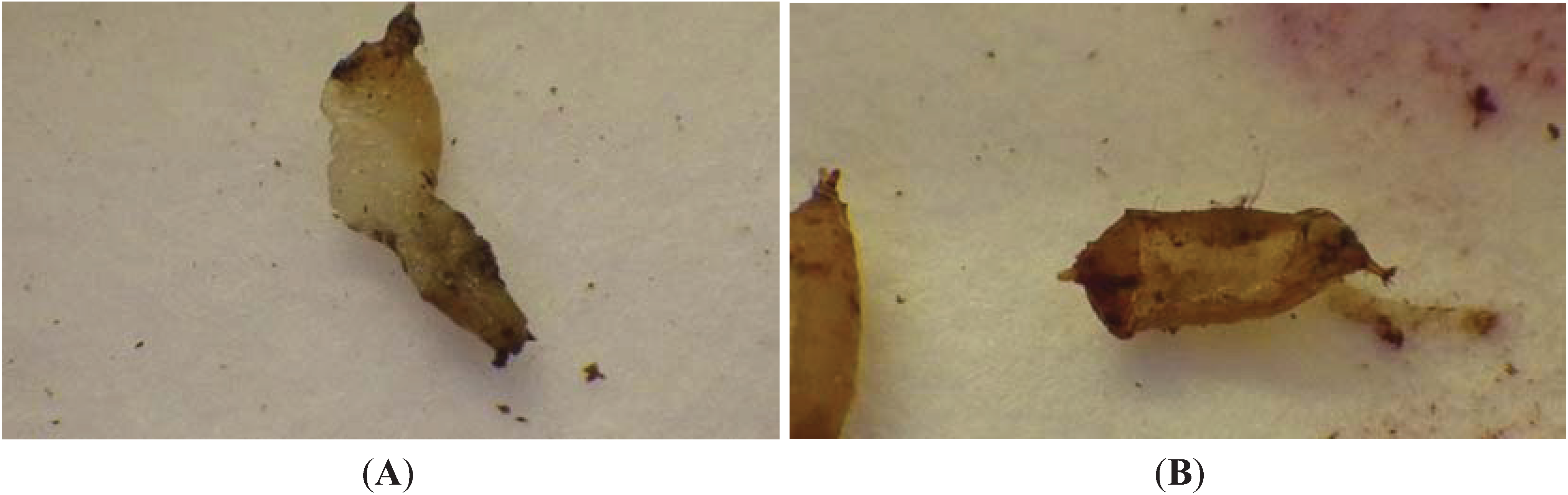
3.3. Hypoaspis miles
3.4. Anthocoris nemoralis


4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lee, J.C.; Bruck, D.J.; Dreves, A.J.; Ioriatti, C.; Vogt, H.; Baufeld, P. In focus: Spotted wing drosophila, Drosophila suzukii, across perspectives. Pest. Manag. Sci. 2011, 67, 1349–1351. [Google Scholar] [PubMed]
- Walsh, D.B.; Bolda, M.P.; Goodhue, R.E.; Dreves, A.J.; Lee, J.; Bruck, D.J.; Walton, V.M.; O’Neal, S.D.; Zalom, F.G. Drosophila suzukii (Diptera: Drosophilidae): Invasive pest of ripening soft fruit expanding its geographic range and damage potential. J. Integr. Pest. Manag. 2011, 2, 1–7. [Google Scholar] [CrossRef]
- Goodhue, R.E.; Bolda, M.; Farnsworth, D.; Williams, J.C.; Zalom, F.G. Spotted wing drosophila infestation of California strawberries and raspberries: Economic analysis of potential revenue losses and control costs. Pest. Manag. Sci. 2011, 67, 1396–1402. [Google Scholar] [CrossRef] [PubMed]
- Cini, A.; Ioriatti, C.; Anfora, G. A review of the invasion of Drosophila suzukii in Europe and a draft research agenda for integrated pest management. Bull. Insectol. 2012, 65, 149–160. [Google Scholar]
- Cuthbertson, A.G.S.; Collins, D.A.; Blackburn, L.F.; Audsley, N.; Bell, H.A. Preliminary screening of potential control products against Drosophila suzukii. Insects 2014, 5, 488–498. [Google Scholar] [CrossRef]
- Cuthbertson, A.G.S.; The Food and Environment Research Agency, Sand Hutton, York, UK. Personal Communication, 2014.
- Beers, E.H.; van Steenwyk, R.A.; Shearer, P.W.; Coates, W.W.; Grant, J.A. Developing Drosophila suzukii management programs for sweet cherry in the western United States. Pest. Manag. Sci. 2011, 67, 1386–1395. [Google Scholar] [CrossRef] [PubMed]
- Roubos, C.R.; Rodriguez-Saona, C.; Holdcraft, R.; Mason, K.S.; Isaacs, R. Relative toxicity and residual activity of insecticides used in berry pest management: Mortality of natural enemies. J. Econ. Entomol. 2014, 107, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Naranjo-Lazaro, J.M.; Mellin-Rosas, M.A.; Gonzalez-Padilla, V.D.; Sanchez-Gonzalez, J.A.; Moreno-Carrillo, G.; Arredondo-Bernal, H.C. Susceptibility of Drosophila suzukii Matsumura (Diptera: Drosophilidae) to entomopathogenic fungi. Southwest Entomol. 2014, 39, 201–203. [Google Scholar] [CrossRef]
- Arnó, J.; Riudavets, J.; Gabarra, R. Survey of host plants and natural enemies of Drosophila suzukii in an area of strawberry production in Catalonia (northeast Spain). IOBC Bull. 2012, 80, 29–34. [Google Scholar]
- Malagnini, V.; Zanotelli, L.; Tolotti, G.; Profaizer, D.; Ahgeli, G. Evaluation of predatory activity of Orius laevigatus (Fieber) and O. maiusculus Reuter towards Drosophila suzukii (Matsumura) under laboratory conditions. In Proceedings of IOBC VIII Workshop on Integrated Soft Fruit Production, Vigalzano di Pergine, Trento, Italy, 26–28 May 2014; p. 123.
- Chabert, S.; Allemand, R.; Poyet, M.; Eslin, P.; Gibert, P. Ability of European parasitoids (Hymenoptera) to control a new invasive Asiatic pest, Drosophila suzukii. Biol. Control 2012, 63, 40–47. [Google Scholar] [CrossRef]
- Rossi Stacconi, M.V.; Grassi, A.; Dalton, D.T.; Miller, B.; Ouantar, M.; Loni, A.; Ioriatti, C.; Walton, V.M.; Anfora, G. First field records of Pachycrepoideus vindemiae as a parasitoid of Drosophila suzukii in European and Oregon small fruit production areas. Entomolgia 2013, 1, 11–16. [Google Scholar]
- Marris, G.; Cuthbertson, A.G.S.; Mathers, J.J.; Blackburn, L.F. Containing the small hive beetle for research purposes. Bee Craft 2010, 92, 17–21. [Google Scholar]
- Tashiro, H. Self-watering acrylic cages for confining insects and mites on detached leaves. J. Econ. Entomol. 1967, 60, 354–356. [Google Scholar]
- Cuthbertson, A.G.; Mathers, J.J.; Croft, P.; Nattriss, N.; Blackburn, L.F.; Luo, W.; Northing, P.; Murai, T.; Jacobson, R.J.; Walters, K.F. Prey consumption rates and compatibility with pesticides of four predatory mites from the family Phytoseiidae attacking Thrips palmi Karny (Thysanoptera: Thripidae). Pest Manag. Sci. 2012, 68, 1289–1295. [Google Scholar] [CrossRef] [PubMed]
- Cuthbertson, A.G.S.; The Food and Environment Research Agency, Sand Hutton, York, UK. Personal Observation, 2014.
- Cuthbertson, A.G.S.; The Food and Environment Research Agency, Sand Hutton, York, UK. Unpublished Data. 2014.
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuthbertson, A.G.S.; Blackburn, L.F.; Audsley, N. Efficacy of Commercially Available Invertebrate Predators against Drosophila suzukii. Insects 2014, 5, 952-960. https://doi.org/10.3390/insects5040952
Cuthbertson AGS, Blackburn LF, Audsley N. Efficacy of Commercially Available Invertebrate Predators against Drosophila suzukii. Insects. 2014; 5(4):952-960. https://doi.org/10.3390/insects5040952
Chicago/Turabian StyleCuthbertson, Andrew G. S., Lisa F. Blackburn, and Neil Audsley. 2014. "Efficacy of Commercially Available Invertebrate Predators against Drosophila suzukii" Insects 5, no. 4: 952-960. https://doi.org/10.3390/insects5040952




