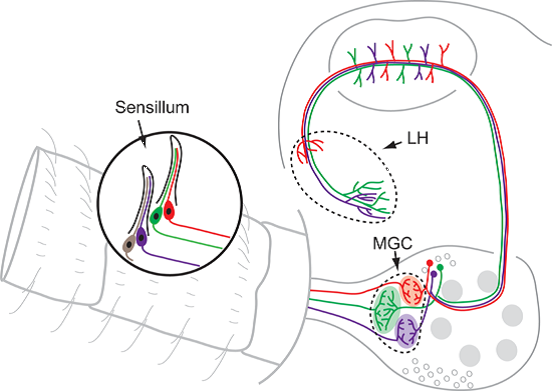
Processing of Pheromone Information in Related Species of Heliothine Moths
Abstract
:1. Introduction
2. Female-Produced Compounds Evoke Behavioral Responses in Conspecific and Heterospecific Males
| Species | H. virescens 1 | H. subflexa 2 | H. zea 3 | H. gelotopoeon 4 | H. assulta 5 | H. armigera 6 | |
|---|---|---|---|---|---|---|---|
| Pheromones | Primary component | Z11-16:AL | Z11-16:AL | Z11-16:AL | 16:AL | Z9-16:AL | Z11-16:AL |
| Secondary component(s) | Z9-14:AL | Z9-16:AL Z11-16:OH | Z9-16:AL | Z9-16:AL | Z11-16:AL | Z9-16:AL | |
| Ratio emitted from the female gland | 100:3.9 | 100:66:40.7 | 100:1.8 | 100:84 | 100:6.7 | 100:4.5 | |
| Antagonists | Z11-16:AC Z11-16:OH | - | Z9-14:AL Z11-16:AC | Z11-16:AL | Z9-14:AL Z9-16:OH | Z9-14:AL Z11-16:OH |
3. Peripheral Arrangement of the Male-Specific Olfactory Pathway
3.1. The Pheromone Components Are Generally Recognized by Two Neuron Types Being Separated in Distinct Sensilla
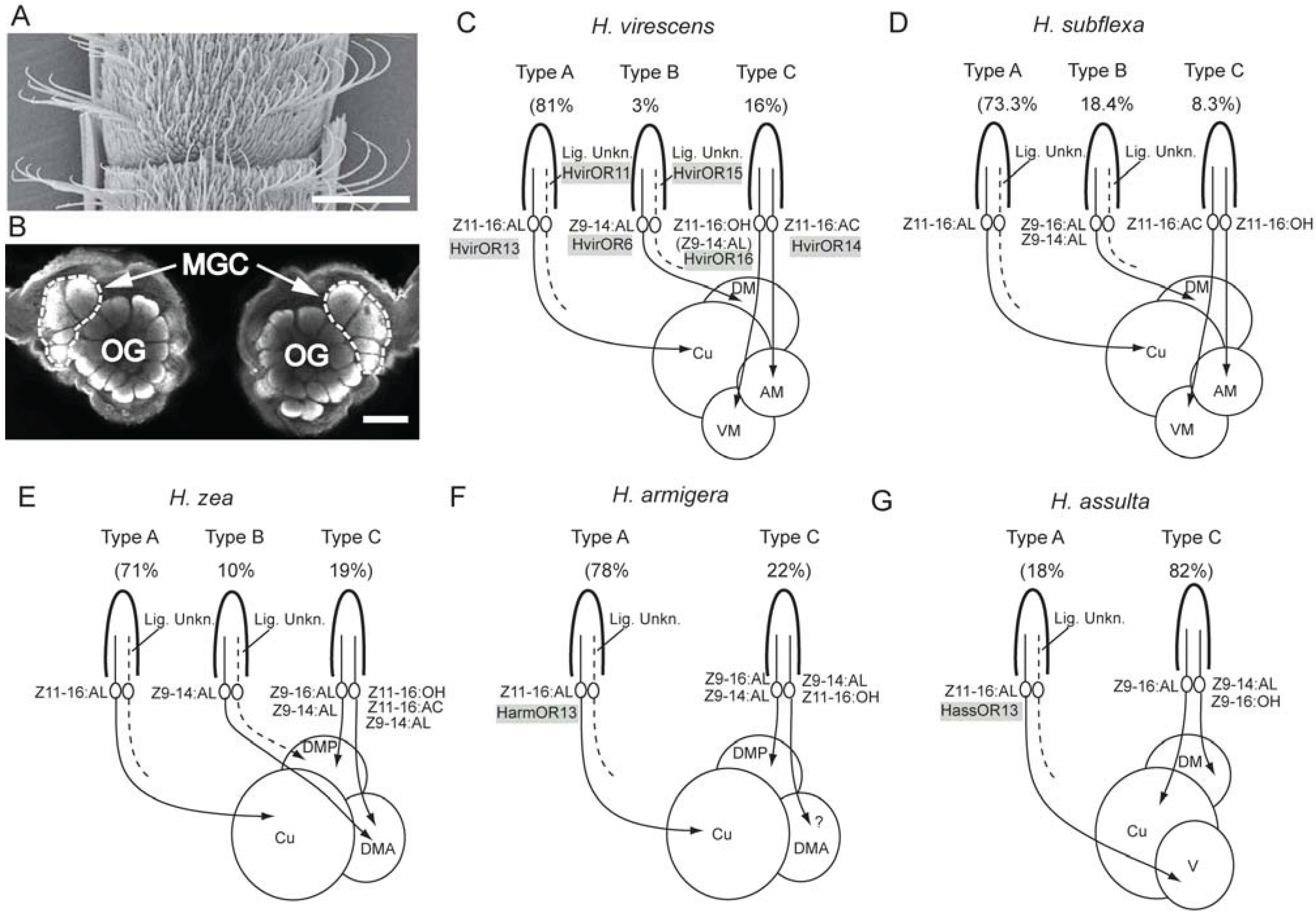
3.2. Interspecific Antagonism Is Ensured via Sensory Neurons Often Being Paired with Pheromone Neurons
3.3. Comparison of Physiological Sensillum Types across the Various Species
3.4. Hybrids as Models for Studying Basic Determinants Underlying Speciation
3.5. Molecular Basis for Detection of the Female-Produced Compounds
4. Central Processing of Information about Pheromones and Interspecific Signals
4.1. Anatomy and Physiology of the Male-Specific MGC in a Comparative Perspective
4.2. The Anatomical Separation of Pheromone and Plant Odor Signals Is Maintained in the Antennal-Lobe Projection Neurons
4.3. Most Male-Specific Projection Neurons Are Confined to the mALT Display Relatively Narrowly-Tuned Response Profiles
4.4. Odor Signals Associated with Distinct Behaviors Are Differently Represented in the Lateral Horn
4.5. Modulatory Input to the Antennal Lobe
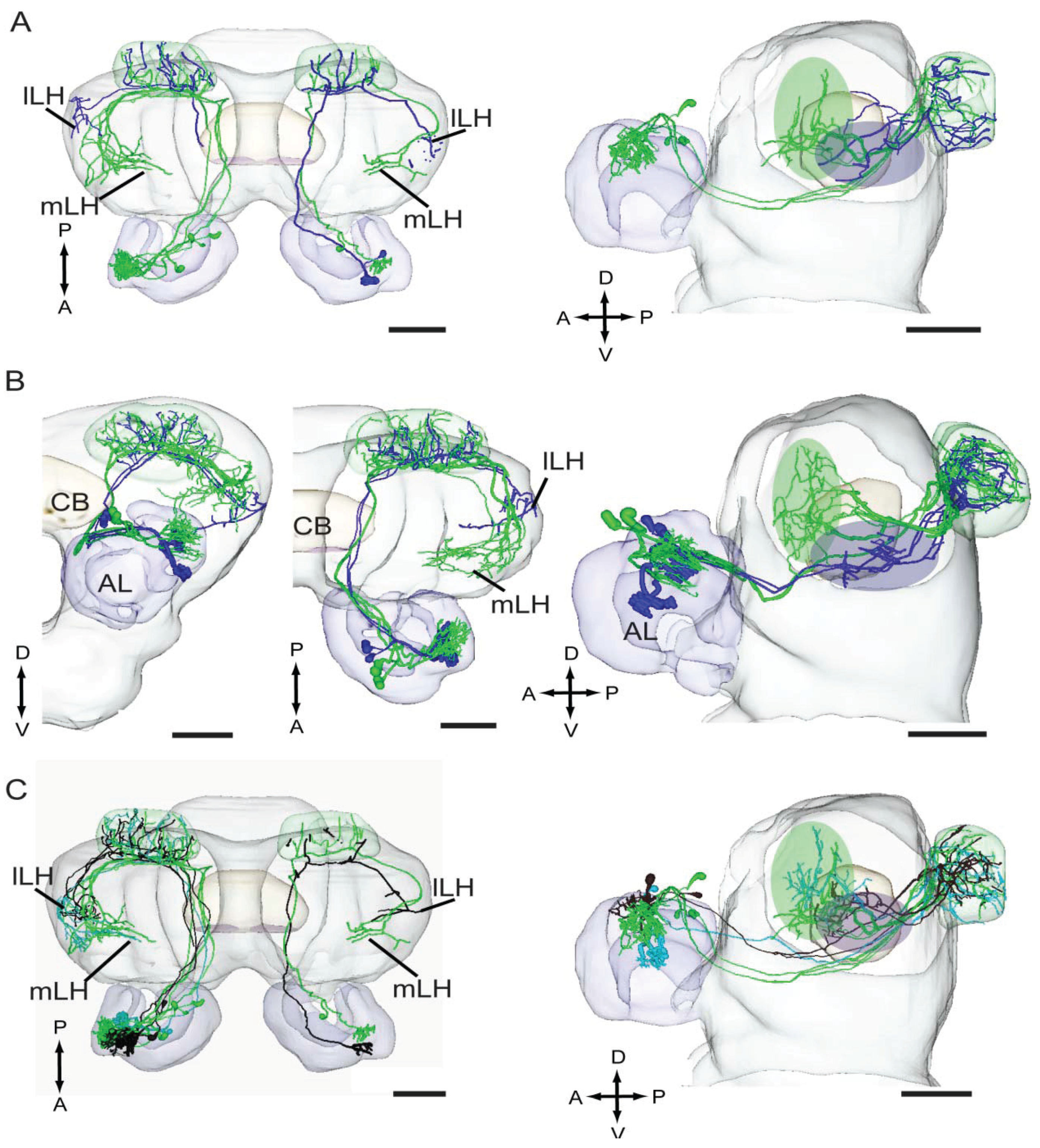
5. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Priesner, E.; Witzgall, P.; Voerman, S. Field attaction response of raspberry clearwing moths, Pennisethia hylaeiformis Lasp. (Lepidoptera: Sesiidae), to candidate pheromone chemicals. J. Appl. Entomol. 1986, 102, 195–210. [Google Scholar] [CrossRef]
- Cho, S.; Mitchell, A.; Mitter, C.; Regier, J.; Matthews, M.; Robertson, R. Molecular phylogenetics of heliothine moths (Lepidoptera: Noctuidae: Heliothinae), with comments on the evolution of host range and pest status. Syst. Entomol. 2008, 33, 581–594. [Google Scholar] [CrossRef]
- Mustaparta, H. Central mechanisms of pheromone information processing. Chem. Senses 1996, 21, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Fitt, G.P. The ecology of Heliothis species in relation to agroecosystems. Ann. Rev. Entomol. 1989, 34, 17–52. [Google Scholar] [CrossRef]
- Roelofs, W.L.; Hill, A.S.; Cardé, R.T.; Baker, T.C. Two sex-pheromone components of the tobacco budworm moth, Heliothis virescens. Life Sci. 1974, 14, 1555–1562. [Google Scholar] [CrossRef] [PubMed]
- Klun, J.A.; Bierl-Leonhardt, B.A.; Plimmer, J.R.; Sparks, A.N.; Primiani, M.; Chapman, O.L.; Lepone, G.; Lee, G.H. Sex pheromone chemistry of the female tobacoo budworm moth, Heliothis virescens. J. Chem. Ecol. 1980, 6, 177–183. [Google Scholar] [CrossRef]
- Klun, J.A.; Plimmer, J.R.; Bierl-Leonhardt, B.A.; Sparks, A.N.; Primiani, M.; Chapman, O.L.; Lee, G.H.; Lepone, G. Sex pheromone chemistry of female corn earworm moth, Heliothis zea. J. Chem. Ecol. 1980, 6, 165–175. [Google Scholar] [CrossRef]
- Teal, P.E.A.; Heath, R.R.; Tumlinson, J.H.; McLaughlin, J.R. Identification of a sex pheromone of Heliothis subflexa (GN.) (Lepidoptera: Noctuidae) and field trapping studies using different blends of components. J. Chem. Ecol. 1981, 7, 1011–1022. [Google Scholar] [CrossRef] [PubMed]
- Kehat, M.; Dunkelblum, E. Behavioral responses of male Heliothis armigera (Lepidoptera: Noctuidae) moths in a flight tunnel to combinations of components identified from female sex pheromone glands. J. Insect Behav. 1990, 3, 75–83. [Google Scholar] [CrossRef]
- Heath, R.R.; Mitchell, E.R.; Tovar, J.C. Effect of release rate and ratio of (Z)-11-hexadecen-1-ol from synthetic pheromone blends on trap capture of Heliothis subflexa (Lepidoptera: Noctuidae). J. Chem. Ecol. 1990, 16, 1259–1268. [Google Scholar] [CrossRef] [PubMed]
- Cork, A.; Boo, K.S.; Dunkelblum, E.; Hall, D.R.; Jee-Rajunga, K.; Mehat, M.; Kong, E.J.; Park, K.C.; Tepgidagarn, P.; Liu, X. Female sex pheromone of oriental tobacco budworm, Helicoverpa assulta (Guenée) (Lepidoptera: Noctuidae): Identification and field testing. J. Chem. Ecol. 1992, 18, 403–418. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.Y.; Cai, J.P. Sex pheromone components of the oriental tobacco budworm, Helicoverpa assulta Guenée: Identification and field trials. Entomol. Sinica. 1994, 1, 77–85. [Google Scholar]
- Cork, A.; Lobos, E.A. Female sex pheromone components of Helicoverpa gelotopoeon: First heliothine pheromone without (Z)-11-hexadecenal. Ent. Exp. Applic. 2003, 107, 201–206. [Google Scholar] [CrossRef]
- Vetter, R.S.; Baker, T.C. Behavioral responses of male Heliothis virescens in a sustained-flight tunnel to combinations of seven compounds identified from female sex pheromone glands. J. Chem. Ecol. 1983, 9, 747–759. [Google Scholar] [CrossRef] [PubMed]
- Vetter, R.S.; Baker, T.C. Behavioral responses of male Heliothis zea moths in sustained-flight tunnel to combinations of 4 compounds identified from female sex pheromone gland. J. Chem. Ecol. 1984, 19, 193–202. [Google Scholar] [CrossRef]
- Teal, P.E.A.; Tumlinson, J.H.; Heath, R.R. Chemical and behavioral analyses of volatile sex pheromone components re-leased by calling Heliothis virescens (F.) females (Lepidoptera: Noctuidae). J. Chem. Ecol. 1986, 12, 107–126. [Google Scholar] [CrossRef] [PubMed]
- Vickers, N.J. Defining a synthetic pheromone blend attractive to male Heliothis subflexa under wind tunnel conditions. J. Chem. Ecol. 2002, 28, 1255–1267. [Google Scholar] [CrossRef]
- Vickers, N.J.; Baker, T.C. Chemical communication in heliothine moths. VII. Correlation between diminished responses to point-source plumes and single filaments similarly tainted with a behavioral antagonist. J. Comp. Physiol. A 1997, 180, 523–536. [Google Scholar] [CrossRef]
- Fadamiro, H.Y.; Baker, T.C. Helicoverpa zea males (Lepidoptera: Noctuidae) respond to the intermittent fine structure of their sex pheromone plume and an antagonist in a flight tunnel. Phys. Entomol. 1997, 22, 316–324. [Google Scholar] [CrossRef]
- Groot, A.T.; Horovitz, J.L.; Hamilton, J.; Santangelo, R.G.; Schal, C.; Gould, F. Experimental evidence for interspecific directional selection on moth pheromone communication. Proc. Natl. Acad. Sci. USA 2006, 103, 5858–5863. [Google Scholar] [CrossRef] [PubMed]
- Quero, C.; Baker, T.C. Antagonistic effect of (Z)-11-hexadecen-1-ol on the pheromone-mediated flight of Helicoverpa zea (Boddie) (Lepidoptera: Noctuidae). J. Insect Behav. 1999, 12, 701–709. [Google Scholar] [CrossRef]
- Quero, C.; Fadamiro, H.Y.; Baker, T.C. Responses of male Helicoverpa zea to single pulses of sex pheromone and behavioral antagonist. Physiol. Entomol. 2001, 26, 106–115. [Google Scholar] [CrossRef]
- Vickers, N.J.; Christensen, T.A.; Mustaparta, H.; Baker, T.C. Chemical communication in heliothine moths. III. Flight behavior of male Helicoverpa zea and Heliothis virescens in response to varying ratios of intra- and interspecific sex pheromone components. J. Comp. Physiol. A 1991, 169, 275–280. [Google Scholar] [CrossRef]
- Boo, K.S.; Park, K.C.; Hall, D.R.; Cork, A.; Berg, B.G.; Mustaparta, H. (Z)-9-tetradecenal: A potent inhibitor of pheromone-mediated communication in the oriental tobacco budworm moth, Helicoverpa assulta. J. Comp. Physiol. A 1995, 177, 695–699. [Google Scholar] [CrossRef]
- Dunkelblum, E.; Kehat, M. Female sex pheromone components of Heliothis peltigera (Lepidoptera: Noctuidae) chemical identification from gland extracts and male response. J. Chem. Ecol. 1989, 15, 2233–2245. [Google Scholar] [CrossRef] [PubMed]
- Steinbrecht, R.A.; Ozaki, M.; Ziegelberger, G. Immunocytochemical localization of pheromone-binding protein in moth antennae. Cell Tiss. Res. 1992, 270, 287–302. [Google Scholar] [CrossRef]
- Almaas, T.J.; Mustaparta, H. Pheromone reception in the tobacco budworm moth, Heliothis virescens. J. Chem. Ecol. 1990, 16, 1331–1347. [Google Scholar] [CrossRef]
- Almaas, T.J.; Mustaparta, H. Heliothis virescens: Response characteristics of receptor neurons in sensilla trichodea type 1 and type 2. J. Chem. Ecol. 1991, 17, 953–972. [Google Scholar] [CrossRef] [PubMed]
- Baker, T.C.; Ochieng, S.A.; Cossé, A.A.; Lee, S.G.; Todd, J.L.; Quero, C.; Vickers, N.J. A comparison of responses from olfactory receptor neurons of Heliothis subflexa and Heliothis virescens to components of their sex pheromone. J. Comp. Physiol. A 2004, 190, 155–165. [Google Scholar] [CrossRef]
- Almaas, T.J.; Christensen, T.A.; Mustaparta, H. Chemical communication in heliothine moths. I. Antennal receptor neurons encode several features of intra- and interspecific odorants in the male corn earworm moth Helicoverpa zea. J. Comp. Physiol. A 1991, 169, 249–258. [Google Scholar] [CrossRef]
- Berg, B.G.; Mustaparta, H. The significance of major pheromone components and interspecific signals as expressed by receptor neurons in the oriental budworm moth, Helicoverpa assulta. J. Comp. Physiol. A 1995, 177, 683–694. [Google Scholar]
- Berg, B.G.; Tumlinson, J.H.; Mustaparta, H. Chemical communication in heliothine moths. IV. Receptor neuron responses to pheromone compounds and formate analogues in the male tobacco budworm moth Heliothis virescens. J. Comp. Physiol. A 1995, 177, 527–534. [Google Scholar]
- Hansson, B.S.; Almaas, T.J.; Anton, S. Chemical communication in heliothine moths. V. Antennal lobe projection patterns of pheromone-detecting olfactory receptor neurons in the male Heliothis virescens (Lepidoptera: Noctuidae). J. Comp. Physiol. A 1995, 177, 535–543. [Google Scholar] [CrossRef]
- Cossé, A.A.; Todd, J.L.; Baker, T.C. Neurons discovered in male Helicoverpa zea antennae that correlate with pheromone-mediated attraction and interspecific antagonism. J. Comp. Physiol. A 1998, 182, 585–594. [Google Scholar] [CrossRef]
- Lee, S.G.; Vickers, N.J.; Baker, T.C. Glomerular targets of Heliothis subflexa male olfactory receptor neurons housed within long trichoid sensilla. Chem. Senses 2006, 31, 821–834. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Hou, C.; Huan, L.Q.; Yan, F.S.; Wang, C.Z. Peripheral coding of sex pheromone blends with reverse ratios in two Helicoverpa species. PLoS One. 2013, 8, e70078. [Google Scholar] [CrossRef] [PubMed]
- Berg, B.G.; Almaas, T.J.; Bjaalie, J.G.; Mustaparta, H. The macroglomerular complex of the antennal lobe in the tobacco budworm moth Heliothis virescens: Specified subdivision in four compartments according to information about biologically significant compounds. J. Comp. Physiol. A 1998, 183, 669–682. [Google Scholar] [CrossRef]
- Berg, B.G.; Almaas, T.J.; Bjaalie, J.G.; Mustaparta, H. Projections of male-specific receptor neurons in the antennal lobe of the oriental tobacco budworm moth, Helicoverpa assulta: A unique glomerular organization among related species. J. Comp. Neurol. 2005, 486, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Ljungberg, H.; Anderson, P.; Hansson, B.S. Physiology and morphology of pheromone specific sensilla on the antennae of male and female Spodoptera littoralis (Lepidoptera: Noctuidae). J. Insect Physiol. 1993, 39, 253–260. [Google Scholar] [CrossRef]
- Su, C.Y.; Menuz, K.; Reisert, J.; Carlson, J.R. Non-synaptic inhibition between grouped neurons in an olfactory circuit. Nature 2012, 492, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Hansson, B.S.; Löfstedt, C. Inheritance of olfactory response to sex pheromone components in Ostrinia nubilalis. Naturwissenschaften 1987, 74, 497–499. [Google Scholar] [CrossRef]
- Akers, R.P.; O’Connel, R.J. The contribution of olfactory receptor neurons to the preservation of pheromone component ratios in male redbanded leafroller moths. J. Comp. Physiol. A. 1988, 163, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Baker, T.C.; Fadamiro, H.Y.; Cossé, A.A. Moth uses fine tuning for odour resolution. Nature 1998, 393, 530. [Google Scholar]
- Binyameen, M.; Jankuvová, J.; Blaženec, M.; Jakuš, R.; Song, L.; Schlyter, F.; Andersson, M.N. Co-localization of insect olfactory sensory cells improves the discrimination of closely separated odour sources. Funct. Ecol. 2014, 28, 1216–1223. [Google Scholar] [CrossRef]
- Lucas, P.R.; Renou, M. Responses to pheromone compounds in Mamestra suasa (Lepidoptera: Noctuidae) olfactory neurons. J. Insect Physiol. 1989, 11, 837–845. [Google Scholar] [CrossRef]
- Lee, S.G.; Carlsson, M.A.; Hansson, B.S.; Todd, J.L.; Baker, T.C. Antennal lobe projection destinations of Helicoverpa zea male olfactory receptor neurons responsive to heliothine sex pheromone components. J. Comp. Physiol. A 2006, 192, 351–363. [Google Scholar]
- Baker, T.C. Nearest neural neighbors: Moth sex pheromone receptors HR11 and HR13. Chem. Senses 2009, 34, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Krieger, J.; Gondesen, I.; Forstner, M.; Gohl, T.; Dewer, Y.; Breer, H. HR11 and HR13 receptor-expressing neurons are housed together in pheromone-responsive sensilla trichodea of male Heliothis virescens. Chem. Senses 2009, 34, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Todd, J.; Baker, T.C. Function of peripheral olfactory organs. In Insect Olfaction; Hansson, B.S., Ed.; Springer-Verlag: Berlin, Germany, 1999; pp. 97–124. [Google Scholar]
- Wang, C.Z.; Dong, J.F. Interspecific hybridization of Helicoverpa armigera and Helicoverpa assulta (Lepidoptera: Noctuidae). Chin. Sci. Bull. 2001, 46, 489–491. [Google Scholar] [CrossRef]
- Laster, M.L. Interspecific hybridization of Heliothis virescens and H. subflexa. Environ. Entomol. 1972, 6, 682–687. [Google Scholar]
- Zhao, X.C.; Yan, Y.H.; Wang, C.Z. Behavioral and electrophysiological responses of Helicoverpa assulta, H. armigera (Lepidoptera: Noctuidae), their F1 hybrids and backcross progenies to sex pheromone component blends. J. Comp. Physiol. A Neuroethol. Sens. Neural. Behav. Physiol. 2006, 192, 1037–1047. [Google Scholar] [CrossRef] [PubMed]
- Baker, T.C.; Quero, C.; Ochieng, S.A.; Vickers, N.J. Inheritance of olfactory preferences II. Olfactory receptor neuron responses from Heliothis subflexa × Heliothis virescens hybrid male moths. Brain Behav. Evol. 2006, 68, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Vickers, N.J. Inheritance of olfactory preferences I. Pheromone-mediated behavioral responses of Heliothis subflexa × Heliothis virescens hybrid male moths. Brain Behav. Evol. 2006, 68, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Vickers, N.J. Inheritance of olfactory preferences III. Processing of pheromonal signals in the antennal lobe of Heliothis subflexa × Heliothis virescens hybrid male moths. Brain Behav. Evol. 2006, 68, 90–108. [Google Scholar] [CrossRef] [PubMed]
- Krieger, J.; Grosse-Wilde, E.; Gohl, T.; Dewer, Y.M.E.; Raming, K.; Breer, H. Genes encoding candidate pheromone receptors in a moth (Heliothis virescens). Proc. Natl. Acad. Sci. USA 2004, 101, 11845–11850. [Google Scholar] [CrossRef] [PubMed]
- Grosse-Wilde, E.; Gohl, T.; Bouché, E.; Breer, H.; Krieger, J. Candidate pheromone receptors provide the basis for the response of distinct antennal neurons to pheromonal compounds. Eur. J. Neurosci. 2007, 25, 2364–2373. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Vásquez, G.M.; Schal, C.; Zwiebel, L.J.; Gould, F. Functional characterization of pheromone receptors in the tobacco budworm Heliothis virescens. Insect Mol. Biol. 2011, 20, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.D.; Zhu, K.Y.; Wang, C.Z. Sequencing and characterization of six cDNAs putatively encoding three pairs of pheromone receptors in two sibling species, Helicoverpa armigera and Helicoverpa assulta. J. Insect Physiol. 2010, 56, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gu, S.; Zhang, Y.; Guo, Y.; Wang, G. Candidate olfaction genes identified within the Helicoverpa armigera antennal transcriptome. PLoS One 2012, 7, e48260. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, C.; Lin, K.; Wang, G. Functional specificity of sex pheromone receptors in the cotton bollworm Helicoverpa armigera. PLoS One 2013, 8, e48260. [Google Scholar]
- Jiang, X.J.; Guo, H.; Di, C.; Yu, S.; Zhu, L.; Huang, L.Q.; Wang, C.Z. Sequence similarity and functional comparisons of pheromone receptor orthologs in two closely related Helicoverpa species. Insect Biochem. Mol. Biol. 2014, 48, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Vásquez, G.M.; Zainulabeuddin, S.; Estes, P.A.; Leal, W.S.; Gould, F. Specificity of the receptor for the major sex pheromone component in Heliothis virescens. J. Insect Sci. 2013, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mustaparta, H.; Department of Psychology, Norwegian University of Science and Technology, Trondheim, Norway. Personal communication. 2014.
- Krieger, J.; Gaenssle, H.; Raming, K.; Breer, H. Odorant binding proteins of Heliothis virescens. Insect Biochem. Mol. Biol. 1993, 23, 449–456. [Google Scholar] [PubMed]
- Callahan, F.E.; Vogt, R.G.; Tucker, M.L.; Dickens, J.C.; Mattoo, A.K. High level expression of “male specific” pheromone binding proteins (PBPs) in the antennae of female noctuiid moths. Insect Biochem. Mol. Biol. 2000, 30, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.R.; Wu, K.M.; Guo, Y.Y. Cloning, expression and immunocytochemical localization of a general odorant-binding protein gene from Helicoverpa armigera (Hübner). Insect Biochem. Mol. Biol. 2003, 33, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.R.; Wu, K.M.; Guo, Y.Y. Molecular Cloning and Bacterial Expression of Pheromone Binding Protein in the Antennae of Helicoverpa armigera (Hübner). Arch. Insect Biochem. Physiol. 2004, 57, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.Y.; Wang, G.R.; Wu, K.M.; Guo, Y.Y.; Yuan, G.; Guo, X. Molecular cloning and sequence analysis of genes encoding.GOBP1 and PBP in the antenna of Helicoverpa assulta (Guenée). Sci. Agric. Sinica 2005, 38, 1817–1824. [Google Scholar]
- Liu, X.G.; An, S.H.; Luo, M.H.; Guo, X.R.; Yuan, G.H. Cloning and sequencing of cDNA encoding pheromone binding protein 3 from the Helicoverpa assulta (Guenée) and its expression in Escherichia coli. Acta Entomol. Sin. 2006, 49, 733–739. [Google Scholar]
- Li, L.; Yang, W.L.; Guo, X.R.; Luo, M.H.; Yuan, G.H.; Qiao, Q.; Fu, X.W. Cloning, sequence analysis and spatio-temporal expression of a pheromone binding protein 2 (PBP2) gene from Helicoverpa assulta (Guenée) (Lepidoptera: Noctuidae). Acta Entomol. Sin. 2009, 52, 1199–1205. [Google Scholar] [CrossRef]
- Zhang, T.T.; Mei, X.D.; Feng, J.N.; Berg, B.G.; Zhang, Y.J.; Guo, Y.Y. Characterization of three pheromone-binding proteins (PBPs) of Helicoverpa armigera (Hüber) and their binding properties. J. Insect Physiol. 2012, 58, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Anton, S.; Homberg, U. Antennal lobe structure. In Insect Olfaction,, 2nd ed.; Hansson, B.S., Ed.; Springer-Verlag: Berlin, Germany, 1999; pp. 97–128. [Google Scholar]
- Berg, B.G.; Galizia, C.G.; Brandt, R.; Mustaparta, H. Digital atlases of the antennal lobe in two species of tobacco budworm moths, the oriental Helicoverpa assulta (male) and the american Heliothis virescens (male and female). J. Comp. Neurol. 2002, 446, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Skiri, H.T.; Rø, H.; Berg, B.G.; Mustaparta, H. Consistent organization of glomeruli in the antennal lobes of related species of helitohine moths. J. Comp. Neurol. 2005, 491, 367–380. [Google Scholar] [CrossRef]
- Løfaldli, B.B.; Kvello, P.; Mustaparta, H. Integration of the antennal lobe glomeruli and three projection neurons in the standard brain atlas of the moth Heliothis virescens. Front. Syst. Neurosci. 2010, 4, 5. [Google Scholar] [PubMed]
- Stranden, M.; Røstelien, T.; Liblikas, I.; Almaas, T.J.; Borg-Karlson, A.K.; Mustaparta, H. Receptor neurons in three heliothine moths responding to floral and inducible plant volatiles. Chemoecology 2002, 13, 143–154. [Google Scholar] [CrossRef]
- Hillier, N.K.; Kleineidam, C.; Vickers, N.J. Physiology and glomerular projections of olfactory receptor neurons on the antenna of female Heliothis virescens (Lepidoptera: Noctuidae) responsive to behaviorally relevant odors. J. Comp. Physiol. A Neuroethol. Sens. Neural. Behav. Physiol. 2006, 192, 199–219. [Google Scholar] [CrossRef] [PubMed]
- Christensen, T.A.; Mustaparta, H.; Hildebrand, J.G. Chemical communication in heliothine moths. II. Central processing of intra- and interspecific olfactory messages in the male corn earworm moth Helicoverpa zea. J. Comp. Physiol. A 1991, 169, 259–274. [Google Scholar] [CrossRef]
- Vickers, N.J.; Christensen, T.A.; Hildebrand, J.G. Combinatorial odor discrimination in the brain: Attractive and antagonist odor blends are represented in distinct combinations of uniquely identifiable glomeruli. J. Comp. Neurol. 1998, 400, 35–56. [Google Scholar] [CrossRef] [PubMed]
- Vickers, N.J.; Christensen, T.A. Functional divergence of spatially conserved olfactory glomeruli in two related moth species. Chem. Senses 2003, 28, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Christensen, T.A.; Mustaparta, H.; Hildebrand, J.G. Chemical communication in heliothine moths. VI. Parallel pathways for information processing in the macroglomerular complex of the male tobacco budworm moth Heliothis virescens. J. Comp. Physiol. A. 1995, 177, 545–557. [Google Scholar] [CrossRef]
- Zhao, X.C.; Berg, B.G. Arrangement of output information from the 3 macroglomerular units in the heliothine moth Helicoverpa assulta: Morphological and physiological features of male-specific projection neurons. Chem. Senses 2010, 35, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Homberg, U.; Christensen, T.A.; Hildebrand, J.G. Structure and function of the deutocerebrum in insect. Ann. Rev. Entomol. 1989, 34, 477–501. [Google Scholar] [CrossRef]
- Rø, H.; Müller, D.; Mustaparta, H. Anatomical organization of antennal lobe projection neurons in the moth Heliothis virescens. J. Comp. Neurol. 2007, 500, 658–675. [Google Scholar] [CrossRef]
- Ito, K.; Shinomiya, K.; Ito, M.; Armstrong, J.D.; Boyan, G.; Hartenstein, V.; Harzsch, S.; Heisenberg, M.; Homberg, U.; Jenett, A.; et al. A systematic nomenclature for the insect brain. Neuron 2014, 81, 755–765. [Google Scholar]
- Galizia, C.G.; Sachse, S.; Mustaparta, H. Calcium responses to pheromones and plant odours in the antennal lobe of the male and female moth Heliothis virescens. J. Comp. Physiol. A 2000, 186, 1049–1063. [Google Scholar] [CrossRef] [PubMed]
- Homberg, U.; Montague, R.A.; Hildebrand, J.G. Anatomy of antenno-cerebral pathways in the brain of the sphinx moth Manduca sexta. Cell Tiss. Res. 1988, 254, 255–281. [Google Scholar] [CrossRef]
- Zhao, X.C.; Kvello, P.; Løfaldli, B.B.; Lillevoll, S.C.; Mustaparta, H.; Berg, B.G. Representation of pheromones, interspecific signals, and plant odors in higher olfactory centers; mapping physiologically identified antennal-lobe projection neurons in the male heliothine moth. Front. Syst. Neurosci. 2014. [Google Scholar] [CrossRef]
- Trona, F.; Anfora, G.; Balkenius, A.; Bengtsson, M.; Tasin, M.; Knight, A.; Janz, N.; Witzgall, P.; Ignell, R. Neural coding merges sex and habitat chemosensory signals in an insect herbivore. Proc. R. Soc. B. 2013, 280, 20130267. [Google Scholar] [CrossRef] [PubMed]
- Chaffiol, A.; Kropf, J.; Barrozo, R.B.; Gadenne, C.; Rospars, J.P.; Anton, S. Plant odour stimuli reshape pheromonal representation in neurons of the antennal lobe macroglomerular complex of a male moth. J. Exp. Biol. 2012, 215, 1670–1680. [Google Scholar] [CrossRef] [Green Version]
- Namiki, S.; Iwabuchi, S.; Kanzaki, R. Representation of pheromone and host plant odor by antennal lobe projection neurons of the silkmoth Bombyx mori. J. Comp. Physiol. A 2008, 194, 501–515. [Google Scholar] [CrossRef]
- Anton, S.; Løfstedt, C.; Hansson, B.S. Central nervous processing of sex pheromones in two strains of the European corn borer Ostrinia nubilalis (Lepidoptera: Pyrilidae). J. Exp. Biol. 1997, 200, 1073–1087. [Google Scholar] [PubMed]
- Seki, Y.; Kanzaki, R. Comprehensive morphological identification and GABA immunocytochemistry of antennal lobe local interneurons in Bombyx mori. J. Comp. Neurol. 2008, 506, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Reisenman, C.E.; Dacks, A.M.; Hildebrand, J.G. Local interneuron diversity in the primary olfactory center of the moth Manduca sexta. J. Comp. Physiol. A 2011, 197, 653–665. [Google Scholar] [CrossRef]
- Pregitzer, P.; Schubert, M.; Breer, H.; Hansson, B.S.; Sachse, S.; Krieger, J. Plant odorants interfere with detection of sex pheromone signals by male Heliothis virescens. Front. Cell. Neurosci. 2012, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Hillier, N.K.; Vickers, N.J. Mixture interactions in moth olfactory physiology: Examining the effects of odorant mixture, concentration, distal stimulation, and antennal nerve transection on sensillar responses. Chem. Senses 2011, 36, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Ochieng, S.A.; Park, K.C.; Baker, T.C. Host plant volatiles synergize responses of sex pheromone-specific olfactory receptor neurons in male Helicoverpa zea. J. Comp. Physiol. A 2002, 188, 325–333. [Google Scholar] [CrossRef]
- Kanzaki, R.; Soo, K.; Seki, Y.; Wada, S. Projections to higher olfactory centers from subdivisions of the antennal lobe macroglomerular complex of the male silkmoth. Chem. Senses 2003, 28, 113–130. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.C.; Berg, B.G. Morphological and physiological characteristics of the serotonin-immunoreactive neuron in the antennal lobe of the male oriental tobacco budworm, Helicoverpa assulta. Chem. Senses 2009, 34, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.C.; Pfuhl, G.; Surlykke, A.; Tro, J.; Berg, B.G. A multisensory centrifugal neuron in the olfactory pathway of heliothine moths. J. Comp. Neurol. 2013, 521, 152–168. [Google Scholar] [CrossRef] [PubMed]
- Baker, T.C.; Cardé, R. Disruption of gypsy moth male sex pheromone behavior by high frequency sound. Environ. Entomol. 1977, 7, 45–52. [Google Scholar]
- Acharya, L.; McNeil, J.N. Predation risk and mating behavior: The responses of moths to bat-like ultrasound. Behav. Ecol. 1998, 6, 552–558. [Google Scholar] [CrossRef]
- Surlykke, A.; Filskov, M.; Fullard, J.H.; Forrest, E. Auditory relationships to size in noctuid moths: Bigger is better. Naturwissenschaften 1999, 86, 238–241. [Google Scholar] [CrossRef]
- Skals, N.; Andersen, P.; Kanneworff, M.; Løfstedt, C.; Surlykke, A. Her odors make him deaf: Crossmodal modulation of olfaction and hearing in a male moth. J. Exp. Biol. 2005, 208, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Svensson, G.P.; Löfstedt, C.; Skals, N. The odour makes the difference: Male moths attracted by sex pheromones ignore the threat by predatory bats. OIKOS 2004, 104, 91–97. [Google Scholar] [CrossRef]
- Anton, S.; Evengaard, K.; Barrozo, R.B.; Anderson, P.; Skals, N. Brief predator sound exposure elicits behavioral and neuronal long-term sensitization in the olfactory system of an insect. Proc. Natl. Acad. Sci. USA 2011, 108, 3401–3405. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berg, B.G.; Zhao, X.-C.; Wang, G. Processing of Pheromone Information in Related Species of Heliothine Moths. Insects 2014, 5, 742-761. https://doi.org/10.3390/insects5040742
Berg BG, Zhao X-C, Wang G. Processing of Pheromone Information in Related Species of Heliothine Moths. Insects. 2014; 5(4):742-761. https://doi.org/10.3390/insects5040742
Chicago/Turabian StyleBerg, Bente G., Xin-Cheng Zhao, and Guirong Wang. 2014. "Processing of Pheromone Information in Related Species of Heliothine Moths" Insects 5, no. 4: 742-761. https://doi.org/10.3390/insects5040742