Shared Ancestry of Symbionts? Sagrinae and Donaciinae (Coleoptera, Chrysomelidae) Harbor Similar Bacteria
Abstract
:1. Introduction
| Species | Taxonomic Affiliation | Mycetomes | Cocoon Formation? |
|---|---|---|---|
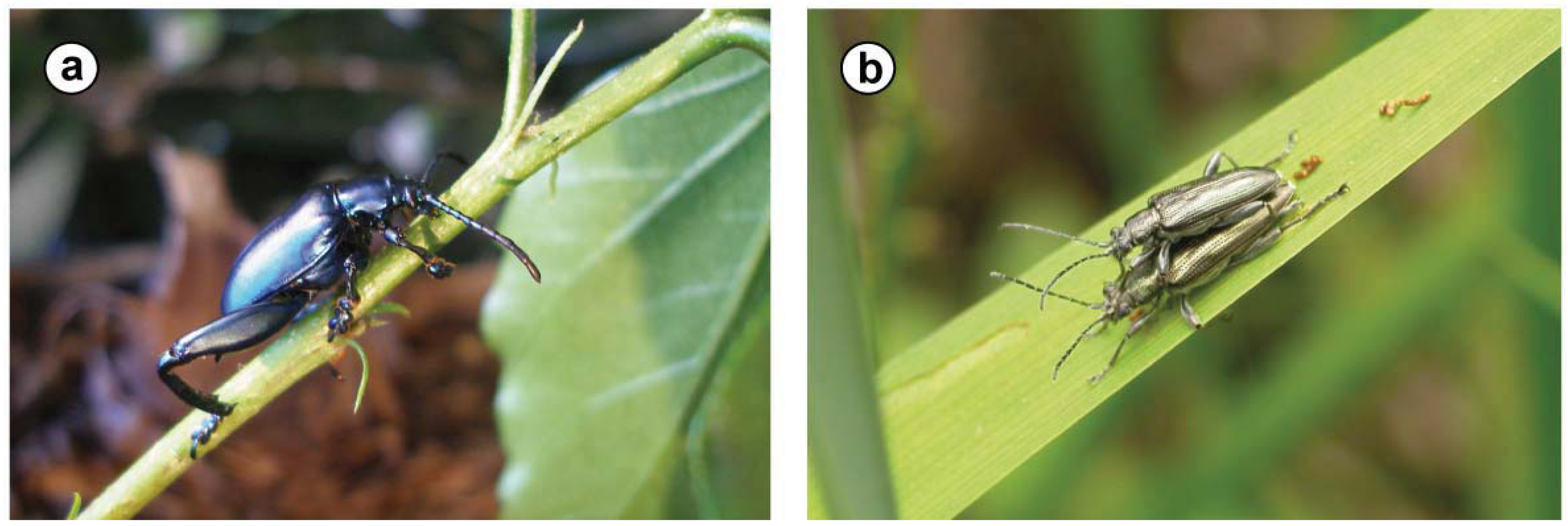
| Sagra femorata(Drury 1773) | Chrysomelidae: Sagrinae; closely related to Donaciinae [20,21] | larvae: four blind sacs ‘at the cranial end of the ventriculus’ [22] | yes, for example within the root of the host plant (Kudzu Pueraria lobata and others; [23]) |
| Bromius obscurus(L. 1758) | Chrysomelidae: Eumolpinae | (a) ‘blind sacs’ differing in morphology between adults and larvae (but containing the same intracellular bacteria) at the beginning of the midgut; (b) short blind ending tubes (‘crypts’) at the midgut, close to the Malpighian tubules, containing bacteria in their lumen [24] | no, larvae are root feeders that pupate in the soil |
| Stegobium paniceum(L. 1758) | Anobiidae | ‘evaginations’ at the cranial end of the midgut with intracellular yeast cells, smaller in the imago than in the larva [5] | yes, largely made up of substrate material |
| Unidentified species | Cerambycidae | mycetomes at the cranial end of the midgut, varying in size between species, and containing yeasts in the cells [5] | no, larvae pupate in the wood they are feeding in |
2. Results and Discussion
2.1. Sequence Statistics
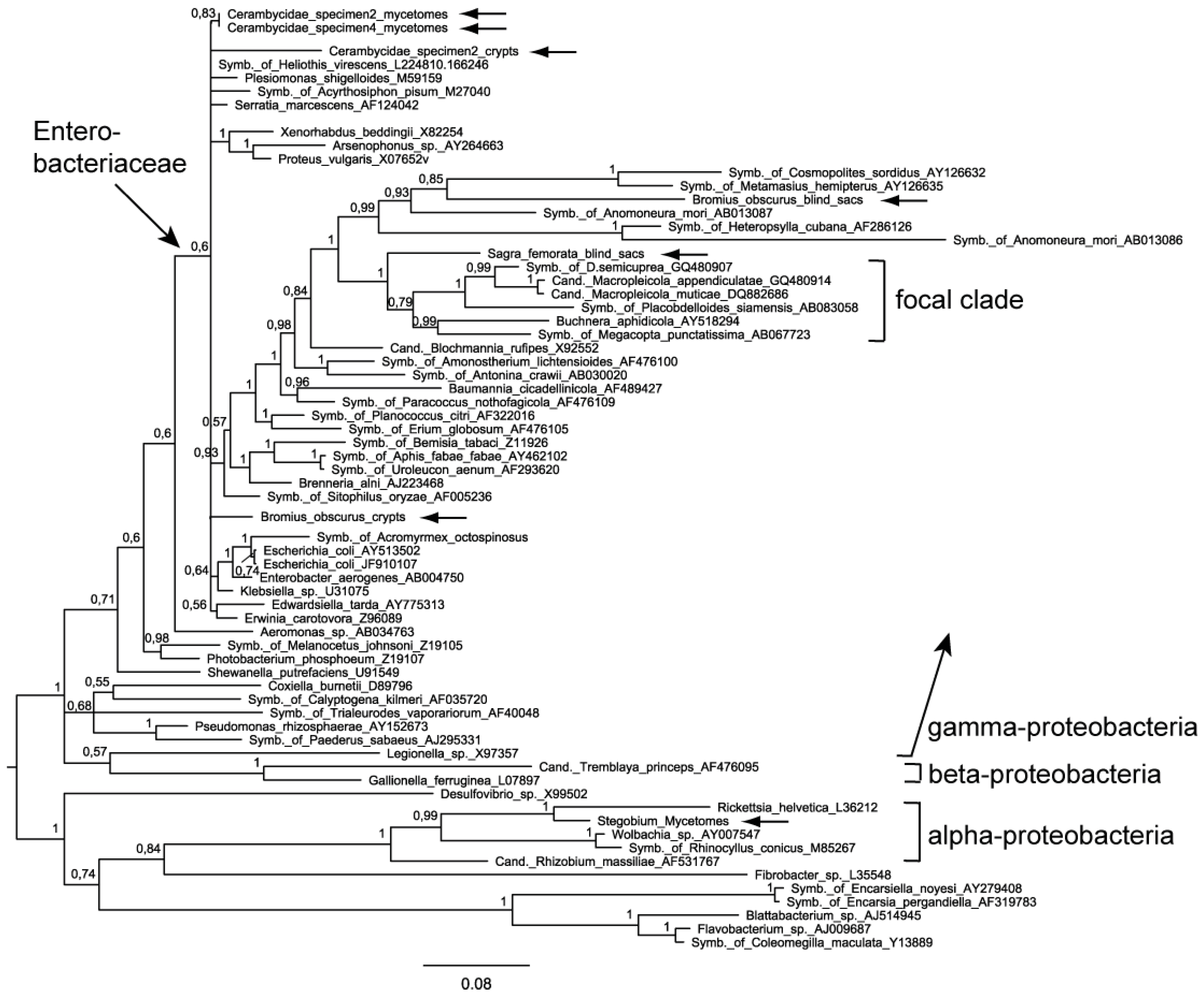
2.2. Phylogenetic Analysis
| Organism | GenBank Accession Number | ||||
|---|---|---|---|---|---|
| Organ/Tissue | Bacteria (16S) | Fungi (18S/28S) | %GC (16S only) | ||
| Cerambycidae specimen 2 | mycetomes | JQ805034 | JQ805028 | 53.2% | |
| crypts | JQ805035 | 52.1% | |||
| Cerambycidae specimen 4 | mycetomes | JQ805036 | 52.3% | ||
| Sagra femorata | blind sacs | JQ805032 | 47.9% | ||
| Bromius obscurus | blind sacs | JQ805030 | 44.9% | ||
| crypts | JQ805033 | 53.7% | |||
| Stegobium paniceum | mycetomes | JQ805029 | 52.1% | ||
| Sequences Analyzed | Chi-squared Value | df | Probability |
|---|---|---|---|
| (a) including Bromius (blind sacs) | 33.40 | 18 | p ≤ 0.05 |
| (b) excluding Bromius (blind sacs) | 11.97 | 15 | not significant |

2.3 Pattern of Nucleotide Substitutions
2.4. Evaluation of the Phylogenetic Trees

| Dataset | Taxa analyzed | Conserved regions | Variable regions | Ratio var./cons. |
|---|---|---|---|---|
| ‘Enterobacteriaceae’ | Average within focal clade excl. Bromius | 3 | 78 | 29.4 |
| Bromius compared to members of the focal clade | 21 | 116 | 5.5 | |
| ‘Bacterial phylogeny’ | Average within focal clade | 2 | 154 | 97.0 |
| Bromius compared to members of the focal clade | 5 | 147 | 29.5 |
| Dataset | Taxa analyzed | Conserved regions | Variable regions | Ratio var./cons. |
|---|---|---|---|---|
| ‘Enterobacteriaceae’ | Average within focal clade | 9 | 81 | 9.2 |
| Sagra compared to members of the focal clade | 6 | 110 | 19.4 | |
| ‘Bacterial phylogeny’ | Average within focal clade | 3 | 190 | 64.3 |
| Sagra compared to members of the focal clade | ‘1’ (0.3) | 122 | 367.0 |
2.5. Details of the Symbioses and the Biology of the Species Involved

3. Experimental Section
3.1. Sample Collection
3.2. Laboratory Procedures
3.3. Phylogenetic Analysis
3.4. Variable and Conserved Regions, Nucleotide Frequencies
4. Conclusions
Acknowledgments
References and Notes
- Sabater, B.; van Ham, R.C.H.J.; Martínez-Torres, D.; Silva, F.; Latorre, A.; Moya, A. Molecular evolution of aphids and their primary (Buchnera sp.) and secondary endosymbionts: Implications for the role of symbiosis in insect evolution. Interciencia 2001, 26, 508–512. [Google Scholar]
- Wernegreen, J.J. Genome evolution in bacterial endosymbionts of insects. Nat. Rev. Genet. 2002, 3, 850–861. [Google Scholar]
- Gibson, C.M.; Hunter, M.S. Extraordinarily widespread and fantastically complex: Comparative biology of endosymbiotic bacterial and fungal mutualists of insects. Ecol. Lett. 2010, 13, 223–234. [Google Scholar]
- Feldhaar, H. Bacterial symbionts as mediators of ecologically important traits of insect hosts. Ecol. Entomol. 2011, 36, 533–543. [Google Scholar]
- Buchner, P. Endosymbiose der Tiere mit pflanzlichen Mikroorganismen; Birkhäuser: Basel, Switzerland, 1953; p. 771. [Google Scholar]
- Richards, A.G.; Brooks, M.A. Internal symbiosis in insects. Annu. Rev. Entomol. 1958, 3, 37–56. [Google Scholar]
- Jurzitza, G. Die symbiose der anobiiden und cerambyciden mit hefeartigen pilzen. Arch. Microbiol. 1962, 43, 412–424. [Google Scholar]
- Silva, F.J.; Latorre, A.; Gómez-Valero, L.; Moya, A. Genomic Changes in Bacteria: From Free-Living to Endosymbiotic Life. In Structural Approaches to Sequence Evolution: Molecules, Networks, Populations; Bastolla, U., Porto, M., Roman, H.E., Vendruscolo, M., Eds.; Springer: New York, NY, USA, 2007; pp. 149–165. [Google Scholar]
- Burke, G.R.; Moran, N.A. Massive genomic decay in Serratia symbiotica, a recently evolved symbiont of aphids. Genome Biol. Evol. 2011, 3, 195–208. [Google Scholar] [CrossRef]
- Bandi, C.; Sironi, M.; Damiani, G.; Magrassi, L.; Nalepa, C.A.; Laudani, U.; Sacchi, L. The establishment of intracellular symbiosis in an ancestor of cockroaches and termites. Proc. R. Soc. Lond. B 1995, 259, 293–299. [Google Scholar] [CrossRef]
- Moran, N.A.; Baumann, P. Bacterial endosymbionts in animals. Curr. Opin. Microbiol. 2000, 3, 270–275. [Google Scholar]
- Moran, N.A.; Wernegreen, J.J. Lifestyle evolution in symbiotic bacteria: Insights from genomics. Trends Ecol. Evol. 2000, 15, 321–326. [Google Scholar]
- Lo, N.; Bandi, C.; Watanabe, H.; Nalepa, C.; Beninati, T. Evidence for cocladogenesis between diverse Dictyopteran lineages and their intracellular endosymbionts. Mol. Biol. Evol. 2003, 20, 907–913. [Google Scholar]
- Kölsch, G.; Matz-Grund, C.; Pedersen, B.V. Ultrastructural and molecular characterization of endosymbionts of the reed beetle genus Macroplea (Chrysomelidae, Donaciinae), and proposal of “Candidatus Macropleicola appendiculatae” and “Candidatus Macropleicola muticae". Can. J. Microbiol. 2009, 55, 1250–1260. [Google Scholar] [CrossRef]
- Charles, H.; Heddi, A.; Rahbe, Y. A putative insect intracellular endosymbiont stem clade, within the Enterobacteriaceae, infered from phylogenetic analysis based on a heterogenous model of DNA evolution. C. R. Acad. Sci. Paris Ser. III 2001, 324, 489–494. [Google Scholar]
- Stammer, H.-J. Bau und Bedeutung der Malpighischen Gefässe der Coleopteren. Z. Morphol. Ökol. Tiere 1934, 29, 196–217. [Google Scholar] [CrossRef]
- Kölsch, G.; Pedersen, B.V. Can the tight co-speciation between reed beetles (Col., Chrysomelidae, Donaciinae) and their bacterial endosymbionts providing cocoon material clarify the deeper phylogeny of the hosts? Mol. Phylogenet. Evol. 2010, 54, 810–821. [Google Scholar] [CrossRef]
- Douglas, A.E. Nutritional interactions in insect-microbial symbioses: Aphids and their symbiotic bacteria. Buchnera. Annu. Rev. Entomol. 1998, 43, 17–37. [Google Scholar] [CrossRef]
- Moran, N.A.; Plague, G.R.; Sandström, J.P.; Wilcox, J.L. A genomic perspective on nutrient provisioning by bacterial symbionts of insects. Proc. Natl. Acad. Sci. USA 2003, 100, 14543–14548. [Google Scholar]
- Askevold, I.S. Reconstructed phylogeny and reclassification of the genera of Donaciinae (Coleoptera: Chrysomelidae). Quaest. Entomol. 1990, 26, 601–664. [Google Scholar]
- Reid, C.A.M. A Cladistic Analysis of Subfamilial Relationships in the Chrysomelidae sensu lato (Chrysomeloidea). In Biology, Phylogeny, and Classification of Coleoptera: Papers Celebrating the 80th Birthday of Roy A. Crowson; Pakaluk, J., Slipinski, S.A., Eds.; Muzeum i Instytut Zoologii PAN: Warszawa, Poland, 1995; pp. 559–631. [Google Scholar]
- Tayade, D.S.; Rawat, R.R.; Chunduwar, R.D. First record of caecal diverticula in Sagra femorata Drury. (Sagrinae: Coleoptera). Curr. Sci. 1975, 44, 812. [Google Scholar]
- Kimoto, S.; Gressitt, J.L. Chrysomelidae (Coleoptera) of Thailand, Cambodia, Laos and Vietnam. I. Sagrinae, Donaciinae, Zeugophorinae, Megalopodinae and Criocerinae. Pac. Insects 1979, 20, 191–256. [Google Scholar]
- Stammer, H.-J. Studien an Symbiosen zwischen Käfern und Mikroorganismen. Zoomorphology 1936, 31, 682–697. [Google Scholar]
- Fukatsu, T.; Hosokawa, T. Capsule-transmitted gut symbiotic bacterium of the Japanese common plataspid stinkbug, Megacopta punctatissima. Appl. Environ. Microbiol. 2002, 68, 389–396. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Fukatsu, T. Endosymbiotic bacteria in the esophageal organ of glossiphoniid leeches. Appl. Environ. Microbiol. 2002, 68, 4637–4641. [Google Scholar]
- Kölsch, G.; Pedersen, B.V. Molecular phylogeny of reed beetles (Col., Chrysomelidae, Donaciinae): The signature of ecological specialization and geographical isolation. Mol. Phylogenet. Evol. 2008, 48, 936–952. [Google Scholar] [CrossRef]
- Schmitt, M. The phylogenetic system of the Chrysomelidae - history of ideas and present state of knowledge. In Chrysomelidae Biology, vol. 1: The Classification, Phylogeny and Genetics; Jolivet, P.H.A., Cox, M.L., Eds.; SPB Academic Publishing: Amsterdam, The Netherlands, 1996; pp. 57–96. [Google Scholar]
- Vogt, G.B.; McGuire, J.U., Jr.; Cushman, A.D. Probable Evolution and Morphological Variation in South American Disonychine Flea Beetles (Coleoptera: Chrysomelidae) and their Amaranthaceous Hosts; USDA Techn. Bull. No. 1593; United States Department of Agriculture: Washington, WA, USA, 1979; p. 148. [Google Scholar]
- Böving, A.G. Natural history of the larvae of Donaciinae. Int. Revue ges. Hydrobiol. Biol. Suppl. 1910, 3, 1–108. [Google Scholar]
- Kleinschmidt, B.; Kölsch, G. Adopting bacteria in order to adapt to water—How reed beetles colonized the wetlands (Coleoptera, Chrysomelidae, Donaciinae). Insects 2011, 2, 540–554. [Google Scholar] [CrossRef]
- Vencl, F.V.; Aiello, A. A new species of leaf-mining Oulema from Panama (Coleoptera: Chrysomelidae; Criocerinae). J. N. Y. Entomol. Soc. 1997, 105, 40–44. [Google Scholar]
- Schomann, H. Die Symbiose der Bockkäfer. Zoomorphology 1937, 32, 542–611. [Google Scholar]
- Grünwald, S.; Pilhofer, M.; Höll, W. Microbial associations in gut systems of wood- and bark-inhabiting longhorned beetles [Coleoptera: Cerambycidae]. Syst. Appl. Microbiol. 2010, 33, 25–34. [Google Scholar]
- Noda, H.; Kodama, K. Phylogenetic position of yeastlike endosymbionts of anobiid beetles. Appl. Environ. Microbiol. 1996, 62, 162–167. [Google Scholar]
- Karawaiew, W. Über Anatomie und Metamorphose des Darmkanals der Larve von Anobium paniceum. Biol. Zentralbl. 1899, 19, 122-130, 161-171, 196-202. [Google Scholar]
- Küchler, S.M.; Kehl, S.; Dettner, K. Characterization and localization of Rickettsia sp. in water beetles of genus Deronectes (Coleoptera: Dytiscidae). FEMS Microbiol. Ecol. 2009, 68, 201–211. [Google Scholar] [CrossRef]
- Pant, N.C.; Fraenkel, G. The function of the symbiotic yeasts of two insect species, Lasioderma serricorne F. and Stegobium (Sitodrepa) paniceum L. Science 1950, 112, 498–500. [Google Scholar]
- Lefkovitch, L.P.; Currie, J.E. Factors affecting adult survival and fecundity in Lasioderma serricorne (F.) (Coleoptera, Anobiidae). J. Stored Prod. Res. 1967, 3, 199–212. [Google Scholar]
- Gamalie, G.; Mustata, M. The attack of Anobiids on books from the ecclesiastic patrimony. Eur. J. Sci. Theol. 2006, 2, 69–81. [Google Scholar]
- Rojas-Rousse, D.; Grille, G.; Basso, C. A natural refuge for an Anobiidae species (Tricorynus sp.) in persistent pods of Acacia caven (Mol.) in Uruguay. Bol. San. Veg. Plagas 2009, 35, 423–428. [Google Scholar]
- Heitz, E. Über intrazelluläre Symbiose bei holzfressenden Käferlarven I. Zoomorphology 1927, 7, 279–305. [Google Scholar] [CrossRef]
- Jurzitza, G.; Kühlwein, H.; Kreger-van Rij, N.J.W. Zur Systematik einiger Cerambycidensymbionten. Arch. Microbiol. 1960, 36, 229–243. [Google Scholar]
- Kukor, J.J.; Cowan, D.P.; Martin, M.M. The role of ingested fungal enzymes in cellulose digestion in the larvae of cerambycid beetles. Physiol. Zool. 1988, 61, 364–371. [Google Scholar]
- Jurzitza, G. Physiologische Untersuchungen an Cerambycidensymbionten. Arch. Microbiol. 1959, 33, 305–332. [Google Scholar]
- Ohkuma, M.; Kudo, T. Phylogenetic diversity of the intestinal bacterial community in the termite Reticulitermes speratus. Appl. Environ. Microbiol. 1996, 62, 461–468. [Google Scholar]
- Egert, M.; Wagner, B.; Lemke, T.; Brune, A.; Friedrich, M.W. Microbial community structure in midgut and hindgut of the humus-feeding larva of Pachnoda ephippiata (Coleoptera: Scarabaeidae). Appl. Environ. Microbiol. 2003, 69, 6659–6668. [Google Scholar] [CrossRef]
- Broderick, N.A.; Raffa, K.F.; Goodman, R.M.; Handelsman, J. Census of the bacterial community of the gypsy moth larval midgut by using culturing and culture-independent methods. Appl. Environ. Microbiol. 2004, 70, 293–300. [Google Scholar]
- Hongoh, Y.; Deevong, P.; Inoue, T.; Moriya, S.; Trakulnaleamsai, S.; Ohkuma, M.; Vongkaluang, C.; Noparatnaraporn, N.; Kudo, T. Intra- and interspecific comparisons of bacterial diversity and community structure support coevolution of gut microbiota and termite host. Appl. Environ. Microbiol. 2005, 71, 6590–6599. [Google Scholar]
- Shinzato, N.; Muramatsu, M.; Matsui, T.; Watanabe, Y. Molecular phylogenetic diversity of the bacterial community in the gut of the termite Coptotermes formosanus. Biosci. Biotechnol. Biochem. 2005, 69, 1145–1155. [Google Scholar] [CrossRef]
- Schloss, P.D.; Delalibera, I.; Handelsman, J.; Raffa, K.F. Bacteria associated with the guts of two wood-boring beetles: Anoplophora glabripennis and Saperda vestita (Cerambycidae). Environ. Entomol. 2006, 35, 625–629. [Google Scholar] [CrossRef]
- Vasanthakumar, A.; Delalibera, I.; Handelsman, J.; Klepzig, K.D.; Schloss, P.D.; Raffa, K.F. Characterization of gut-associated bacteria in larvae and adults of the southern pine beetle, Dendroctonus frontalis Zimmermann. Environ. Entomol. 2006, 35, 1710–1717. [Google Scholar] [CrossRef]
- Park, D.-S.; Oh, H.-W.; Jeong, W.-J.; Kim, H.; Park, H.-Y.; Bae, K.S. A culture-based study of the bacterial communities within the guts of nine longicorn beetle species and their exo-enzyme producing properties for degrading xylan and pectin. J. Microbiol. 2007, 45, 394–401. [Google Scholar]
- Geib, S.M.; Jimenez-Gasco, M.D.M.; Carlson, J.E.; Tien, M.; Hoover, K. Effect of host tree species on cellulase activity and bacterial community composition in the gut of larval Asian longhorned beetle. Environ. Entomol. 2009, 38, 686–699. [Google Scholar]
- Reid, N.M.; Addison, S.L.; Macdonald, L.J.; Lloyd-Jones, G. Biodiversity of active and inactive bacteria in the gut flora of wood-feeding huhu beetle larvae (Prionoplusreticularis). Appl. Environ. Microbiol. 2011, 77, 7000–7006. [Google Scholar] [CrossRef]
- Gräbner, K.-E. Vergleichend morphologische und physiologische Studien an Anobiiden- und Cerambyciden-Symbionten. Zoomorphology 1954, 42, 471–528. [Google Scholar]
- White, T.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M., Gelfand, D., Shinsky, J., White, T., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Hall, T.A. Bioedit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl. Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar]
- Stocsits, R.R.; Letsch, H.; Hertel, J.; Misof, B.; Stadler, P.F. Accurate and efficient reconstruction of deep phylogenies from structured RNAs. Nucleic Acids Res. 2009, 37, 6184–6193. [Google Scholar]
- Cannone, J.J.; Subramanian, S.; Schnare, M.N.; Collett, J.R.; D’Souza, L.M.; Du, Y.; Feng, B.; Lin, N.; Madabusi, L.V.; Müller, K.M.; et al. The comparative RNA web (CRW) site: An online database of comparative sequence and structure information for ribosomal, intron, and other RNAs. BMC Bioinform. 2002, 3. [Google Scholar]
- Swofford, D.L. PAUP*. Phylogenetic Analysis Using Parsimony (*and Other Methods); Sinauer Ass.: Sunderland, MA, USA, 1998. [Google Scholar]
- Posada, D.; Crandall, K.A. Modeltest: Testing the model of DNA substitution. Bioinformatics 1998, 14, 817–818. [Google Scholar]
- Huelsenbeck, J.P.; Ronquist, F. MrBayes: Bayesian inference of phylogenetic trees. Bioinformatics 2001, 17, 754–755. [Google Scholar]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef]
- Xia, X.; Xie, Z. DAMBE: Software package for data analysis in molecular biology and evolution. J. Hered. 2001, 92, 371–373. [Google Scholar]
- Gevers, D.; Cohan, F.M.; Lawrence, J.G.; Sprat, B.G.; Coenye, T.; Feil, E.J.; Stackebrandt, E.; Van de Peer, Y.; Vandamme, P.; Thompson, F.L.; et al. Re-evaluating prokaryotic species. Nat. Rev. Microbiol. 2005, 3, 733–739. [Google Scholar] [CrossRef]
- Venturi, F. La construzione del bozzolo da parte della larva matura di Lema melanopa L. (Coleoptera, Chrysomelidae) ed elementi di anatomia del suo tubo digerente. Redia Florence 1949, 34, 217–231. [Google Scholar]
- Vats, L.K. Alimentary canal in bruchid larvae (Bruchidae: Coleoptera). Res. Bull. Panjab Univ. (Sci.) 1976, 27, 103–106. [Google Scholar]
- Mann, J.S.; Crowson, R.A. On the occurrence of mid-gut caeca, and organs of symbiont transmission, in leaf-beetles (Coleoptera: Chrysomelidae). Coleopt. Bull. 1983, 37, 1–15. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kölsch, G.; Synefiaridou, D. Shared Ancestry of Symbionts? Sagrinae and Donaciinae (Coleoptera, Chrysomelidae) Harbor Similar Bacteria. Insects 2012, 3, 473-491. https://doi.org/10.3390/insects3020473
Kölsch G, Synefiaridou D. Shared Ancestry of Symbionts? Sagrinae and Donaciinae (Coleoptera, Chrysomelidae) Harbor Similar Bacteria. Insects. 2012; 3(2):473-491. https://doi.org/10.3390/insects3020473
Chicago/Turabian StyleKölsch, Gregor, and Dimitra Synefiaridou. 2012. "Shared Ancestry of Symbionts? Sagrinae and Donaciinae (Coleoptera, Chrysomelidae) Harbor Similar Bacteria" Insects 3, no. 2: 473-491. https://doi.org/10.3390/insects3020473




