Impact of Initial Population Density of the Dubas Bug, Ommatissus lybicus (Hemiptera: Tropiduchidae), on Oviposition Behaviour, Chlorophyll, Biomass and Nutritional Response of Date Palm (Phoenix dactylifera)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects and Experimental Procedures
2.2. Chlorophyll Measurement
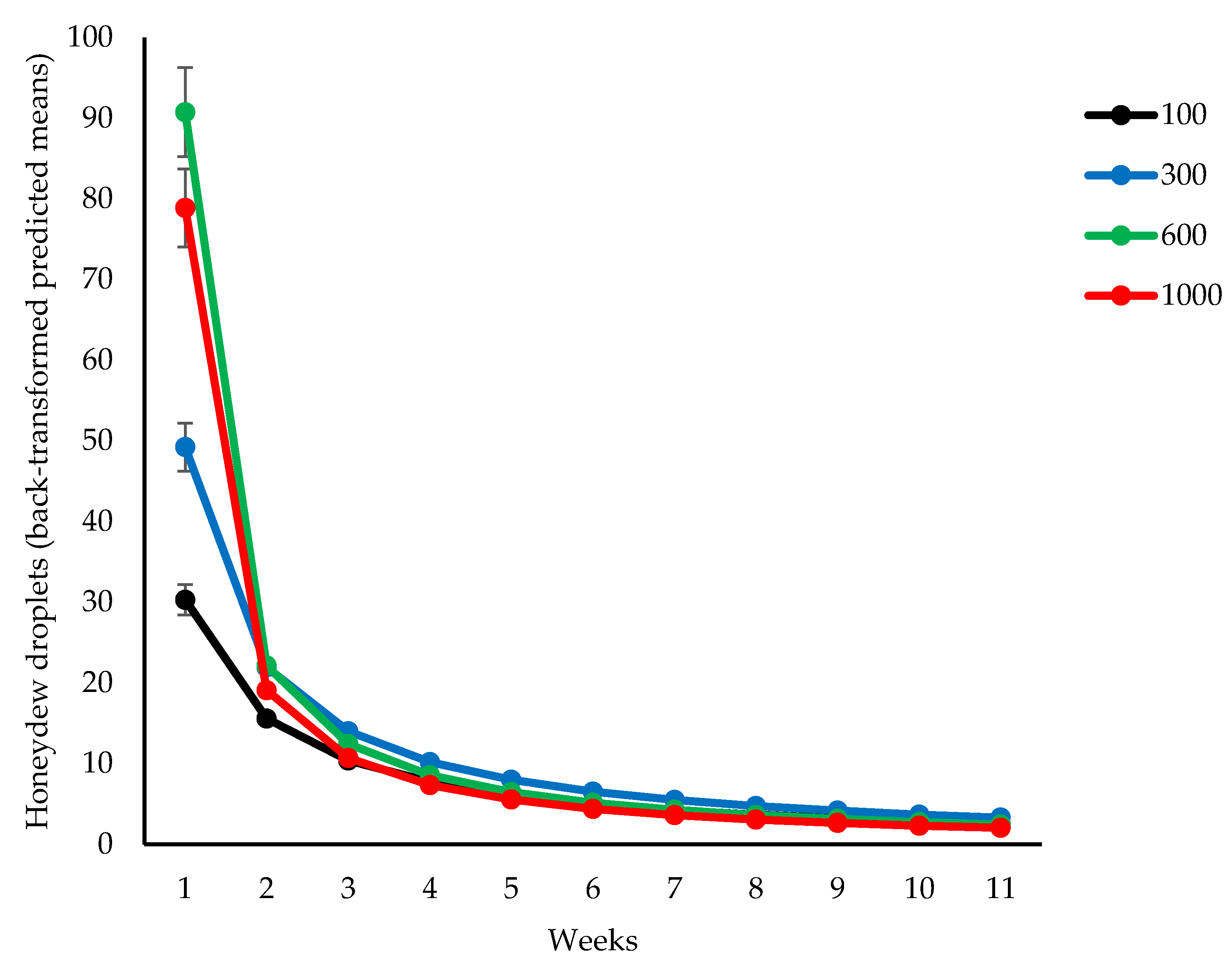
2.3. Honeydew Droplets Count
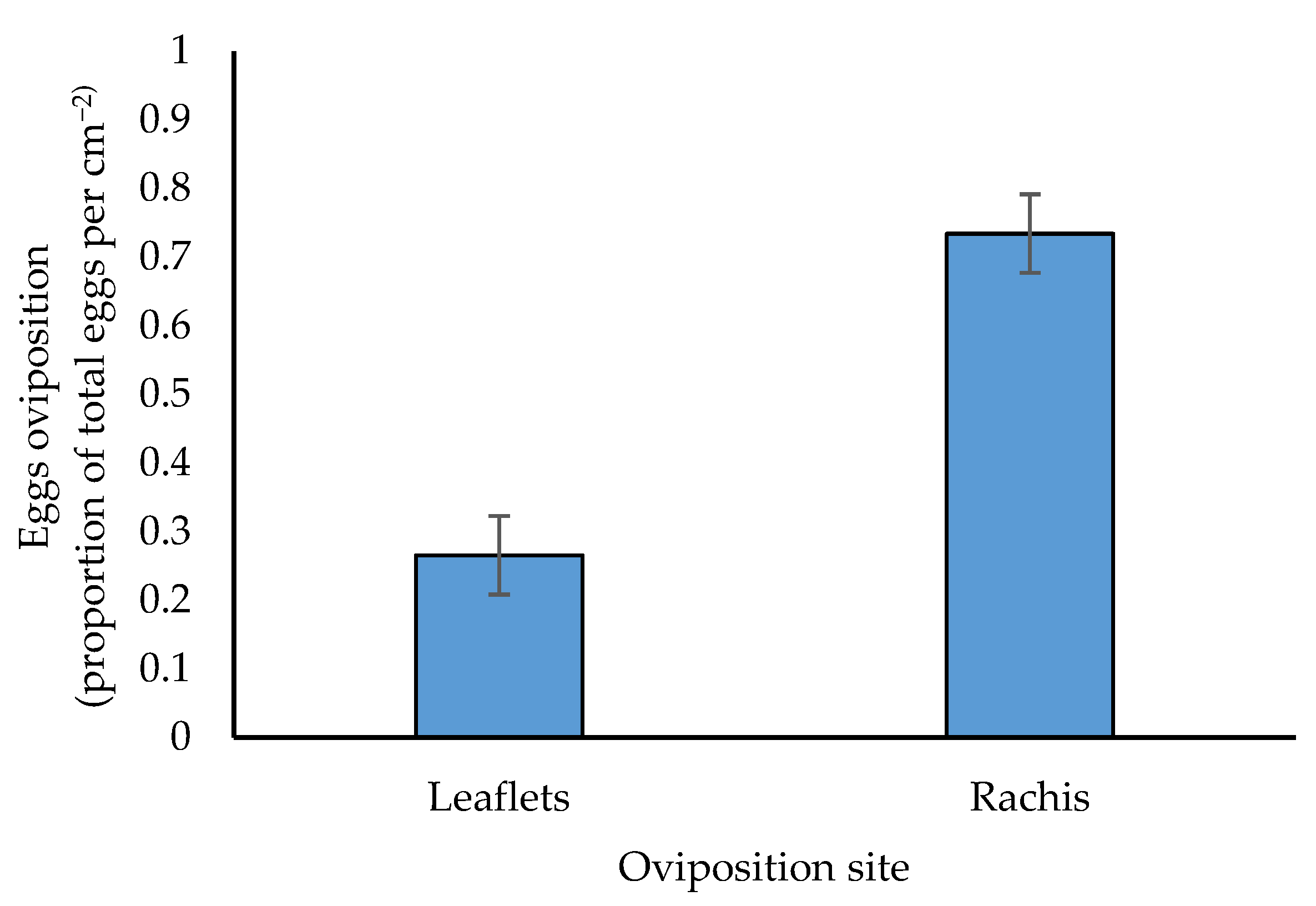
2.4. Ommatissus lybicus Oviposition Density
2.5. Plant Area and Biomass Measurements
2.6. Plant Nutritional Measurements
2.7. Statistical Analysis
3. Results
3.1. Loss of Chlorophyll
3.2. Honeydew Droplets
3.3. Ommatissus lybicus Oviposition
3.4. Plant Biomass
3.5. Nutritional Measurements
3.6. Regression Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yaish, M.W.; Patankar, H.V.; Assaha, D.V.M.; Zheng, Y.; Al-Yahyai, R.; Sunkar, R. Genome-Wide Expression Profiling in Leaves and Roots of Date Palm (Phoenix Dactylifera, L.) Exposed to Salinity. BMC Genom. 2017, 18, 246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Khatri, S.A.H. Biological, Ecological and Phylogenic Studies of Pseudoligosita Babylonica Viggiani, a Native Egg Parasitoid of Dubas Bug Ommatissus Lybicus de Bergevin, the Major Pest of Date Palm in the Sultanate of Oman. Doctoral Thesis, University of Reading, Reading, UK, 2011. [Google Scholar]
- Chao, C.T.; Krueger, R.R. The Date Palm (Phoenix Dactylifera, L.): Overview of Biology, Uses, and Cultivation. HortScience 2007, 42, 1077–1082. [Google Scholar] [CrossRef] [Green Version]
- Capinera, J.L. Encyclopedia of Entomology; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008; ISBN 1402062427. [Google Scholar]
- Wakil, W.; Faleiro, J.R.; Miller, T.A.; Bedford, G.O.; Krueger, R.R. Date Palm Production and Pest Management Challenges. In Sustainable Pest Management in Date Palm: Current Status and Emerging Challenges; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–11. [Google Scholar]
- Franzen, L.D.; Gutsche, A.R.; Heng-Moss, T.M.; Higley, L.G.; Macedo, T.B. Physiological Responses of Wheat and Barley to Russian Wheat Aphid, Diuraphis Noxia (Mordvilko) and Bird Cherry-Oat Aphid, Rhopalosiphum Padi (L.)(Hemiptera: Aphididae). Arthropod Plant Interact. 2008, 2, 227–235. [Google Scholar] [CrossRef]
- Gullan, P.J.; Cranston, P.S. The Insects: An Outline of Entomology; John Wiley & Sons: Hoboken, NJ, USA, 2014; ISBN 111884615X. [Google Scholar]
- Singh, V.; Sood, A.K. Plant Nutrition: A Tool for the Management of Hemipteran Insect-Pests-A Review. Agric. Rev. 2017, 38, 260–270. [Google Scholar] [CrossRef] [Green Version]
- Peterson, R.K.D.; Higley, L.G. Arthropod Injury and Plant Gas Exchange: Current Understandings and Approaches for Synthesis. Trends Agric. Sci. Entomol. 1993, 1, 93–100. [Google Scholar]
- Ecale, C.L.; Backus, E.A. Mechanical and Salivary Aspects of Potato Leafhopper Probing in Alfalfa Stems. Entomol. Exp. Appl. 1995, 77, 121–132. [Google Scholar] [CrossRef]
- Haile, F.J.; Higley, L.G.; Ni, X.; Quisenberry, S.S. Physiological and Growth Tolerance in Wheat to Russian Wheat Aphid (Homoptera: Aphididae) Injury. Environ. Entomol. 1999, 28, 787–794. [Google Scholar] [CrossRef]
- Kranz, J.; Schmutterer, H.; Koch, W. Diseases, Pests, and Weeds in Tropical Crops. Soil Sci. 1978, 125, 272. [Google Scholar] [CrossRef]
- Mokhtar, A.M.; AI-Mjeni, A.M. A Novel Approach to Determine the Efficacy of Control Measures Against Dubas Bug, Ommatissus Lybicus de Berg, on Date Palms. J. Agric. Mar. Sci. 1999, 4, 1–4. [Google Scholar]
- Khan; Al-Ghafri, T.H.A.; Al-Khatri, S.A.H.; Al-Mazidi, I.S.S.; Al-Rawahi, F.G. Resistance to Deltamethrin and Fenitrothion in Dubas Bug, Ommatissus Lybicus de Bergevin (Homoptera: Tropiduchidae) and Possible Biochemical Mechanisms. Sci. Rep. 2020, 10, 13220. [Google Scholar] [CrossRef]
- Hussain, A.A. Biology and Control of the Dubas Bug, Ommatissus binotatus lybicus De Berg.(Homoptera, Tropiduchidae), Infesting Date Palms in Iraq. Bull. Entomol. Res. 1963, 53, 737–745. [Google Scholar] [CrossRef]
- Al Shidi, R.; Kumar, L.; Al-Khatri, S.A.H. Humid-thermal Index for a New Management Approach of Ommatissus Lybicus. Pest Manag. Sci. 2019, 75, 3060–3069. [Google Scholar] [CrossRef] [PubMed]
- Kinawi, M.M. Date Palm and Date Pests in Sultanate of Oman; Royal Court Affairs, Sultanate of Oman: Muscat, Oman, 2005. [Google Scholar]
- Shah, A.; Zia, A.; Rafi, M.A.; Mehmood, S.A.; Aslam, S.; Chaudhry, M.T. Quantification of Honeydew Production Caused by Dubas Bug on Three Date Palm Cultivars. J. Entomol. Zool. Stud. 2016, 4, 478–484. [Google Scholar]
- Thacker, J.; Al-Mahmooli, I.; Deadman, M. Population Dynamics and Control of the Dubas Bug Ommatissus Lybicus in the Sultanate of Oman. In Proceedings of the BCPC International Congress–Crop Science and Technology, Glasgow, UK, 10–12 November 2003; pp. 987–992. [Google Scholar]
- Elwan, A.A.; Al-Tamimi, S.S. Life Cycle of Dubas Bug Ommatissus binotatus lybicus De Berg.(Homoptera: Tropiduchidae) in Sultanate of Oman. Egypt. J. Agric. Res. 1999, 77, 1547–1553. [Google Scholar]
- Arbabtafti, R.; Pezhman, H.; Fassihi, M.T.; Assari, M.; Noori, H.; Hosseini Gharalari, A. Determination of Economic Injury Level of Ommatissus lybicus (Hemiptera: Tropiduchidae) on Two Commercial Date Cultivars in Southern Iran. J. Entomol. Soc. Iran 2020, 40, 123–133. [Google Scholar]
- Wool, D.; Hendrix, D.L.; Shukry, O. Seasonal Variation in Honeydew Sugar Content of Galling Aphids (Aphidoidea: Pemphigidae: Fordinae) Feeding on Pistacia: Host Ecology and Aphid Physiology. Basic Appl. Ecol. 2006, 7, 141–151. [Google Scholar] [CrossRef]
- Askari, M.; Bagheri, A. Study on the Effect of Imidachloprid on Date Palm Hopper by Soil Application and Injection. In Proceedings of the International Conference on Mango and date palm: Culture and Export, University of Agriculture, Faisalabad, Pakistan, 20–23 June 2005; pp. 20–23. [Google Scholar]
- Shah, A. Biology and Management of Dubas Bug, Ommatissus Lybicus on Date Palm in Balochistan (Pakistan). Doctoral Thesis, Pir Mehr Ali Shah Arid Agriculture University, Rawalpindi, Pakistan, 2014. [Google Scholar]
- Khan, A.L.; Asaf, S.; Khan, A.; Khan, A.; Imran, M.; Al-Harrasi, A.; Lee, I.-J.; Al-Rawahi, A. Transcriptomic Analysis of Dubas Bug (Ommatissus lybicus Bergevin) Infestation to Date Palm. Sci. Rep. 2020, 10, 11505. [Google Scholar] [CrossRef]
- Al Shidi, R.H.; Kumar, L.; Al-Khatri, S.A.H.; Al-Ajmi, N.A. Ommatissus lybicus Infestation in Relation to Spatial Characteristics of Date Palm Plantations in Oman. Agriculture 2019, 9, 50. [Google Scholar] [CrossRef] [Green Version]
- Khan, R.R.; Al-Khatri, S.A.H.; Al-Ghafri, T.H.A.; Al-Mazidi, I.S.S.; Al-Rawahi, F.G.; Al-Jabri, S.S.; Hussain, M.H. Susceptibility Survey of Ommatissus lybicus (de Bergevin) Populations against Deltamethrin and Fenitrothion in Oman. Sci. Rep. 2019, 9, 11690. [Google Scholar] [CrossRef] [Green Version]
- Auclair, J.L. Aphid Feeding and Nutrition. Annu. Rev. Entomol. 1963, 8, 439–490. [Google Scholar] [CrossRef]
- Way, M.J. Mutualism between Ants and Honeydew-Producing Homoptera. Annu. Rev. Entomol. 1963, 8, 307–344. [Google Scholar] [CrossRef]
- Hackman, R.H.; Trikojus, V.M. The Composition of the Honeydew Excreted by Australian Coccids of the Genus Ceroplastes. Biochem. J. 1952, 51, 653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delabie, J.H.C. Trophobiosis between Formicidae and Hemiptera (Sternorrhyncha and Auchenorrhyncha): An Overview. Neotrop. Entomol. 2001, 30, 501–516. [Google Scholar] [CrossRef] [Green Version]
- Burrack, H.J.; Fornell, A.M.; Connell, J.H.; O’Connell, N.V.; Phillips, P.A.; Vossen, P.M.; Zalom, F.G. Intraspecific Larval Competition in the Olive Fruit Fly (Diptera: Tephritidae). Environ. Entomol. 2009, 38, 1400–1410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klomp, H. Intraspecific Competition and the Regulation of Insect Numbers. Annu. Rev. Entomol. 1964, 9, 17–40. [Google Scholar] [CrossRef]
- Nicholson, A.J. An Outline of the Dynamics of Animal Populations. Aust. J. Zool. 1954, 2, 9–65. [Google Scholar] [CrossRef]
- Arcese, P.; Smith, J.N.M. Effects of Population Density and Supplemental Food on Reproduction in Song Sparrows. J. Anim. Ecol. 1988, 57, 119–136. [Google Scholar] [CrossRef]
- Dreiss, A.N.; Cote, J.; Richard, M.; Federici, P.; Clobert, J. Age-and Sex-Specific Response to Population Density and Sex Ratio. Behav. Ecol. 2010, 21, 356–364. [Google Scholar] [CrossRef] [Green Version]
- Mamai, W.; Bimbile-Somda, N.S.; Maiga, H.; Juarez, J.G.; Muosa, Z.A.I.; Ali, A.B.; Lees, R.S.; Gilles, J.R.L. Optimization of Mosquito Egg Production under Mass Rearing Setting: Effects of Cage Volume, Blood Meal Source and Adult Population Density for the Malaria Vector, Anopheles Arabiensis. Malar. J. 2017, 16, 41. [Google Scholar] [CrossRef] [Green Version]
- Rafi, M.M.; Zemetra, R.S.; Quisenberry, S.S. Interaction between Russian Wheat Aphid (Homoptera: Aphididae) and Resistant and Susceptible Genotypes of Wheat. J. Econ. Entomol. 1996, 89, 239–246. [Google Scholar] [CrossRef]
- Amezaga, I.; Garbisu, C. Effect of Intraspecific Competition on Progeny Production of Tomicus Piniperda (Coleoptera: Scolytidae). Environ. Entomol. 2000, 29, 1011–1017. [Google Scholar] [CrossRef] [Green Version]
- Morales-Ramos, J.A.; Rojas, M.G.; Kay, S.; Shapiro-Ilan, D.I.; Tedders, W.L. Impact of Adult Weight, Density, and Age on Reproduction of Tenebrio Molitor (Coleoptera: Tenebrionidae). J. Entomol. Sci. 2012, 47, 208–220. [Google Scholar] [CrossRef]
- Luft, P.A.; Paine, T.D.; Redak, R.A. Limiting the Potential for Intraspecific Competition: Regulation of Trioza Eugeniae Oviposition on Unexpanded Leaf Tissue. Ecol. Entomol. 2001, 26, 395–403. [Google Scholar] [CrossRef]
- Shah, A.; Mohsin, A.U.; Hafeez, Z.; Naeem, M.; Haq, M.I.U. Eggs Distribution Behaviour of Dubas Bug (Ommatissus lybicus: Homoptera: Tropiduchidae) in Relation to Seasons and Some Physico-Morphic Characters of Date Palm Leaves. J. Insect Behav. 2013, 26, 371–386. [Google Scholar] [CrossRef]
- Cabrera, H.M.; Argandona, V.H.; Corcuera, L.J. Metabolic Changes in Barley Seedlings at Different Aphid Infestation Levels. Phytochemistry 1994, 35, 317–319. [Google Scholar] [CrossRef]
- Goławska, S.; Krzyzanowski, R.; Łukasik, I. Relationship between Aphid Infestation and Chlorophyll Content in Fabaceae Species. Acta Biol. Crac. Ser. Bot. 2010, 52, 76–80. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, P.-J.; Zhang, J.; Lu, Y.-B.; Huang, F.; Li, M.-J. Chlorophyll Content and Chlorophyll Fluorescence in Tomato Leaves Infested with an Invasive Mealybug, Phenacoccus Solenopsis (Hemiptera: Pseudococcidae). Environ. Entomol. 2013, 42, 973–979. [Google Scholar] [CrossRef]
- Edelson, J.V.; Duthie, J.; Roberts, W. Watermelon Growth, Fruit Yield and Plant Survival as Affected by Squash Bug (Hemiptera: Coreidae) Feeding. J. Econ. Entomol. 2003, 96, 64–70. [Google Scholar] [CrossRef]
- Pierson, L.M.; Heng-Moss, T.M.; Hunt, T.E.; Reese, J.C. Categorizing the Resistance of Soybean Genotypes to the Soybean Aphid (Hemiptera: Aphididae). J. Econ. Entomol. 2010, 103, 1405–1411. [Google Scholar] [CrossRef] [Green Version]
- Hosseini, A.; Hosseini, M.; Katayama, N.; Mehrparvar, M. Effect of Ant Attendance on Aphid Population Growth and above Ground Biomass of the Aphid’s Host Plant. Eur. J. Entomol. 2017, 114, 106. [Google Scholar] [CrossRef] [Green Version]
- Rubia-Sanchez, E.; Suzuki, Y.; Miyamoto, K.; Watanabe, T. The Potential for Compensation of the Effects of the Brown Planthopper Nilaparvata Lugens Stal (Homoptera: Delphacidae) Feeding on Rice. Crop Prot. 1999, 18, 39–45. [Google Scholar] [CrossRef]
- Wang, P.; Wu, J.-C.; Xue, S.; Wang, F.; Liu, J.-L.; Yu, Y.-S.; Gu, H. Responses in Nutrient Uptake in Rice Roots to Infestation of Brown Planthopper, Nilaparvata Lugens (Stål)(Homoptera: Delphacidae). Int. J. Pest Manag. 2006, 52, 97–107. [Google Scholar] [CrossRef]
- Wu, J.-C.; Qiu, H.-M.; Yang, G.-Q.; Dong, B.; Gu, H. Nutrient Uptake of Rice Roots in Response to Infestation of Nilaparvata Lugens (Stål)(Homoptera: Delphacidae). J. Econ. Entomol. 2003, 96, 1798–1804. [Google Scholar] [CrossRef] [PubMed]
- Nagaraj, N.; Reese, J.C.; Kirkham, M.B.; Kofoid, K.; Campbell, L.R.; Loughin, T.M. Relationship between Chlorophyll Loss and Photosynthetic Rate in Greenbug (Homoptera: Aphididae) Damaged Sorghum. J. Kans. Entomol. Soc. 2002, 75, 101–109. [Google Scholar]
- Al-Yahyai, R.; Al-Khanjari, S. Biodiversity of Date Palm in the Sultanate of Oman. Afr. J. Agric. Res. 2008, 3, 389–395. [Google Scholar]
- Simpson, K.L.S.; Jackson, G.E.; Grace, J. The Response of Aphids to Plant Water Stress–the Case of Myzus Persicae and Brassica Oleracea Var. Capitata. Entomol. Exp. Appl. 2012, 142, 191–202. [Google Scholar] [CrossRef] [Green Version]
- Deol, G.S.; Reese, J.C.; Gill, B.S. A Rapid, Nondestructive Technique for Assessing Chlorophyll Loss from Greenbug (Homoptera: Aphididae) Feeding Damage on Sorghum Leaves. J. Kans. Entomol. Soc. 1997, 70, 305–312. [Google Scholar]
- Yue, X.; Hu, Y.; Zhang, H.; Schmidhalter, U. Evaluation of Both SPAD Reading and SPAD Index on Estimating the Plant Nitrogen Status of Winter Wheat. Int. J. Plant Prod. 2020, 14, 67–75. [Google Scholar] [CrossRef]
- Formisano, L.; Pannico, A.; El-Nakhel, C.; Starace, G.; Poledica, M.; Pascale, S.D.; Rouphael, Y. Improved Porosity of Insect Proof Screens Enhances Quality Aspects of Zucchini Squash without Compromising the Yield. Plants 2020, 9, 1264. [Google Scholar] [CrossRef]
- Donnelly, A.; Yu, R.; Rehberg, C.; Meyer, G.; Young, E.B. Leaf Chlorophyll Estimates of Temperate Deciduous Shrubs during Autumn Senescence Using a SPAD-502 Meter and Calibration with Extracted Chlorophyll. Ann. For. Sci. 2020, 77, 30. [Google Scholar] [CrossRef]
- Coppedge, B.R. Twig Morphology and Host Effects on Reproductive Success of the Twig Girdler Oncideres Cingulata (Say)(Coleoptera: Cerambycidae). Coleopt. Bull. 2011, 65, 405–410. [Google Scholar] [CrossRef]
- Paro, C.M.; Arab, A.; Vasconcellos-Neto, J. Specialization of Atlantic Rain Forest Twig-Girdler Beetles (Cerambycidae: Lamiinae: Onciderini): Variation in Host–Plant Use by Microhabitat Specialists. Arthropod Plant Interact. 2014, 8, 557–569. [Google Scholar] [CrossRef]
- Rice, M.E. Branch Girdling and Oviposition Biology of Oncideres Pustulatus (Coleoptera: Cerambycidae) on Acacia Farnesiana. Ann. Entomol. Soc. Am. 1989, 82, 181–186. [Google Scholar] [CrossRef]
- Estefan, G.; Sommer, R.; Ryan, J. Methods of Soil, Plant, and Water Analysis. In A Manual for the West Asia and North Africa Region; International Center for Agricultural Research in the Dry Areas: Beirut, Lebanon, 2013; Volume 3. [Google Scholar]
- Schall, R. Estimation in Generalized Linear Models with Random Effects. Biometrika 1991, 78, 719–727. [Google Scholar] [CrossRef]
- Diaz-Montano, J.; Reese, J.C.; Schapaugh, W.T.; Campbell, L.R. Chlorophyll Loss Caused by Soybean Aphid (Hemiptera: Aphididae) Feeding on Soybean. J. Econ. Entomol. 2007, 100, 1657–1662. [Google Scholar] [CrossRef]
- Moloinyane, S.; Nchu, F. The Effects of Endophytic Beauveria Bassiana Inoculation on Infestation Level of Planococcus Ficus, Growth and Volatile Constituents of Potted Greenhouse Grapevine (Vitis Vinifera, L.). Toxins 2019, 11, 72. [Google Scholar] [CrossRef] [Green Version]
- Demarty, M.; Morvan, C.; Thellier, M. Calcium and the Cell Wall. Plant Cell Environ. 1984, 7, 441–448. [Google Scholar] [CrossRef]
- Raghothama, K.G. Phosphorus and Plant Nutrition: An Overview. Phosphorus: Agric. Environ. 2005, 46, 353–378. [Google Scholar]
- Wydrzynski, T.; Gross, E.L. Effects of Sodium and Magnesium Cations on the “Dark-” and Light-Induced Chlorophyll a Fluorescence Yields in Sucrose-Washed Spinach Chloroplasts. Biochim. Biophys. Acta (BBA) Bioenerg. 1975, 376, 151–161. [Google Scholar] [CrossRef]
- Zörb, C.; Senbayram, M.; Peiter, E. Potassium in Agriculture–Status and Perspectives. J. Plant Physiol. 2014, 171, 656–669. [Google Scholar] [CrossRef]
- Mengel, K. Effect of Potassium on the Assimilate Conduction to Storage Tissue. Ber. Dtsch. Bot. Ges. 1980, 93, 353–362. [Google Scholar]
- Amtmann, A.; Troufflard, S.; Armengaud, P. The Effect of Potassium Nutrition on Pest and Disease Resistance in Plants. Physiol. Plant 2008, 133, 682–691. [Google Scholar] [CrossRef] [PubMed]
- Huber, D.M.; Jones, J.B. The Role of Magnesium in Plant Disease. Plant Soil 2013, 368, 73–85. [Google Scholar] [CrossRef]
- Ferrigo, D.; Raiola, A.; Causin, R. Fusarium Toxins in Cereals: Occurrence, Legislation, Factors Promoting the Appearance and Their Management. Molecules 2016, 21, 627. [Google Scholar] [CrossRef] [PubMed]
- Al-Rawi, A.A.H. Fertilization of Date Palm Tree (Phoenix Dactylifera, L.) in Iraq. IPA Agric. Res. Cent. 1998, 1, 320–328. [Google Scholar]
- Zabar, A.F.; Borowy, A. Cultivation of Date Palm in Iraq. Ann. Univ. Mariae Curie-Skłodowska. Sect. EEE Hortic. 2012, 22, 39–54. [Google Scholar]
- Adlan, H.A. Date Palm Culture in Sudan. In Horticulture Department Report; Ministry of Agriculture: Khartoum, Sudan, 1994. [Google Scholar]




| N Nymphs | Fresh Weight (g) | Dry Weight (g) | ||||||
|---|---|---|---|---|---|---|---|---|
| Leaflets | Rachis | Roots | Leaflets | Rachis | Roots | |||
| E1 | E2 | E1 | E2 | |||||
| 0 | 53.4 | 87.1 | 51.4 | 91.8 | 16.4 | 16.1 | 9.6 | 22.3 |
| 100 | 50.2 | 73.0 | 38.1 | 104.7 | 15.7 | 15.8 | 11.7 | 28.6 |
| 300 | 37.9 | 57.2 | 33.7 | 69.2 | 13.7 | 13.2 | 6.5 | 16.8 |
| 600 | 41.6 | 60.7 | 36.3 | 57.6 | 15.4 | 14.5 | 10.8 | 16.0 |
| 1000 | 48.8 | 69.1 | 33.1 | 66.4 | 15.7 | 15.7 | 9.4 | 17.5 |
| p-value (LSD) | ||||||||
| T | 0.02 (9.9) | 0.002 (14.2) | 0.008 (17.2) | 0.62 (3.5) | 0.49 (3.8) | 0.03 (5.2) | ||
| E | 0.2 (6.2) | 0.27 (9.0) | <0.001 (10.9) | 0.18 (2.2) | 0.25 (2.4) | <0.001 (3.3) | ||
| T × E | 0.58 (14.0) | 0.55 (20.0) | 0.123 (24.3) | 0.69 (5.0) | 0.55 (5.4) | 0.22 (7.4) | ||
| CV% | 20.7 | 19.9 | 28.8 | 22.3 | 24.5 | 34.2 | ||
| Number of Nymphs | Nutrient Content (mg/kg of Dry Weight) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leaflets | Rachises | Roots | ||||||||||
| 0 | 1000 | p-Value | SE± | 0 | 1000 | p-Value | SE± | 0 | 1000 | p-Value | SE± | |
| Magnesium (Mg) | 3.85 | 3.48 | 0.01 | 0.099 | 5.65 | 5.78 | 0.88 | 0.759 | 5.73 | 4.53 | 0.02 | 0.371 |
| Phosphorus (P) | 1.38 | 1.00 | 0.01 | 0.095 | 2.78 | 2.08 | 0.02 | 0.207 | 1.18 | 0.98 | 0.29 | 0.172 |
| Potassium (K) | 9.73 | 7.53 | 0.02 | 0.696 | 18.33 | 16.50 | 0.53 | 2.734 | 12.25 | 9.73 | 0.01 | 0.689 |
| N Nymphs | Nutrient Content (mg/kg of Dry Weight) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leaflets | Rachis | Roots | ||||||||||||
| Ca | K | Mg | P | P | K | Mg | ||||||||
| E1 | E2 | E1 | E2 | E1 | E2 | E1 | E2 | E1 | E2 | E1 | E2 | E1 | E2 | |
| 0 | 5.5 | 6.8 | 9.7 | 9.1 | 3.9 | 4.1 | 1.4 | 1.1 | 2.8 | 1.9 | 12.3 | 11.9 | 5.7 | 6.4 |
| 100 | 4.1 | 5.9 | 12.2 | 9.6 | 4.3 | 3.5 | 1.5 | 0.7 | 3 | 1.1 | 13 | 11.1 | 5.7 | 5.8 |
| 300 | 4.2 | 6 | 12.6 | 9.4 | 4.3 | 3.7 | 1.4 | 0.8 | 2.9 | 1.2 | 14.1 | 11.1 | 5.9 | 6.2 |
| 600 | 3.6 | 4.8 | 12.8 | 10.4 | 3.8 | 3.1 | 1.3 | 0.9 | 2.7 | 1.2 | 14.2 | 11.5 | 4.8 | 6.3 |
| 1000 | 4.7 | 6.2 | 7.5 | 10.4 | 3.5 | 3.9 | 1 | 0.9 | 2.1 | 1.5 | 9.7 | 11.1 | 4.5 | 6.4 |
| p-value (LSD) | ||||||||||||||
| T | 0.004 (0.8) | <0.001 (1.0) | 0.14 (0.5) | 0.2 (0.2) | 0.09 (0.4) | 0.01 (1.3) | 0.5 (0.9) | |||||||
| E | <0.001 (0.6) | 0.03 (1.0) | 0.07 (0.3) | <0.001 (0.1) | <0.001 (0.3) | 0.04 (1.2) | <0.001 (0.4) | |||||||
| T × E | 0.95 (1.2) | 0.008 (1.9) | 0.05 (0.6) | 0.04 (0.3) | 0.05 (0.6) | 0.15 (2.2) | 0.07 (1.1) | |||||||
| CV% | 17.3 | 14.7 | 12 | 18.5 | 21.3 | 15 | 11.3 | |||||||
| Response Variate (Y) | Equation | R2 | p-Value |
|---|---|---|---|
| Chlorophyll content at 11 weeks | Y (100) = 66.38 − 0.305 (HD1) | 0.48 | <0.001 |
| Y (300) = 62.28 − 0.305 (HD1) | |||
| Y (600) = 59.64 − 0.305 (HD1) | |||
| Y (1000) = 62.39 − 0.305 (HD1) | |||
| Chlorophyll content at 11 weeks | Y (0) = 1.47 (Mg) + 60.36 | 0.62 | <0.001 |
| Y (100) = 1.47 (Mg) + 59.49 | |||
| Y (300) = 1.47 (Mg) + 54.78 | |||
| Y (600) = 1.47 (Mg) + 52.28 | |||
| Y (1000) = 1.47 (Mg) + 54.55 | |||
| Rachis fresh weight | Y = 87.57 − 5.62 (ER) | 0.38 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Abri, N.; Al-Raqami, S.; Al-Hashemi, M.; Al-Shidi, R.; Al-Khatri, S.; Ray, R.V. Impact of Initial Population Density of the Dubas Bug, Ommatissus lybicus (Hemiptera: Tropiduchidae), on Oviposition Behaviour, Chlorophyll, Biomass and Nutritional Response of Date Palm (Phoenix dactylifera). Insects 2023, 14, 12. https://doi.org/10.3390/insects14010012
Al-Abri N, Al-Raqami S, Al-Hashemi M, Al-Shidi R, Al-Khatri S, Ray RV. Impact of Initial Population Density of the Dubas Bug, Ommatissus lybicus (Hemiptera: Tropiduchidae), on Oviposition Behaviour, Chlorophyll, Biomass and Nutritional Response of Date Palm (Phoenix dactylifera). Insects. 2023; 14(1):12. https://doi.org/10.3390/insects14010012
Chicago/Turabian StyleAl-Abri, Nasser, Suad Al-Raqami, Maryam Al-Hashemi, Rashid Al-Shidi, Salim Al-Khatri, and Rumiana V. Ray. 2023. "Impact of Initial Population Density of the Dubas Bug, Ommatissus lybicus (Hemiptera: Tropiduchidae), on Oviposition Behaviour, Chlorophyll, Biomass and Nutritional Response of Date Palm (Phoenix dactylifera)" Insects 14, no. 1: 12. https://doi.org/10.3390/insects14010012






