Predator-Pest Dynamics of Arthropods Residing in Louisiana Soybean Agroecosystems
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Plots
2.2. Sampling
2.3. Statistical Analysis
3. Results
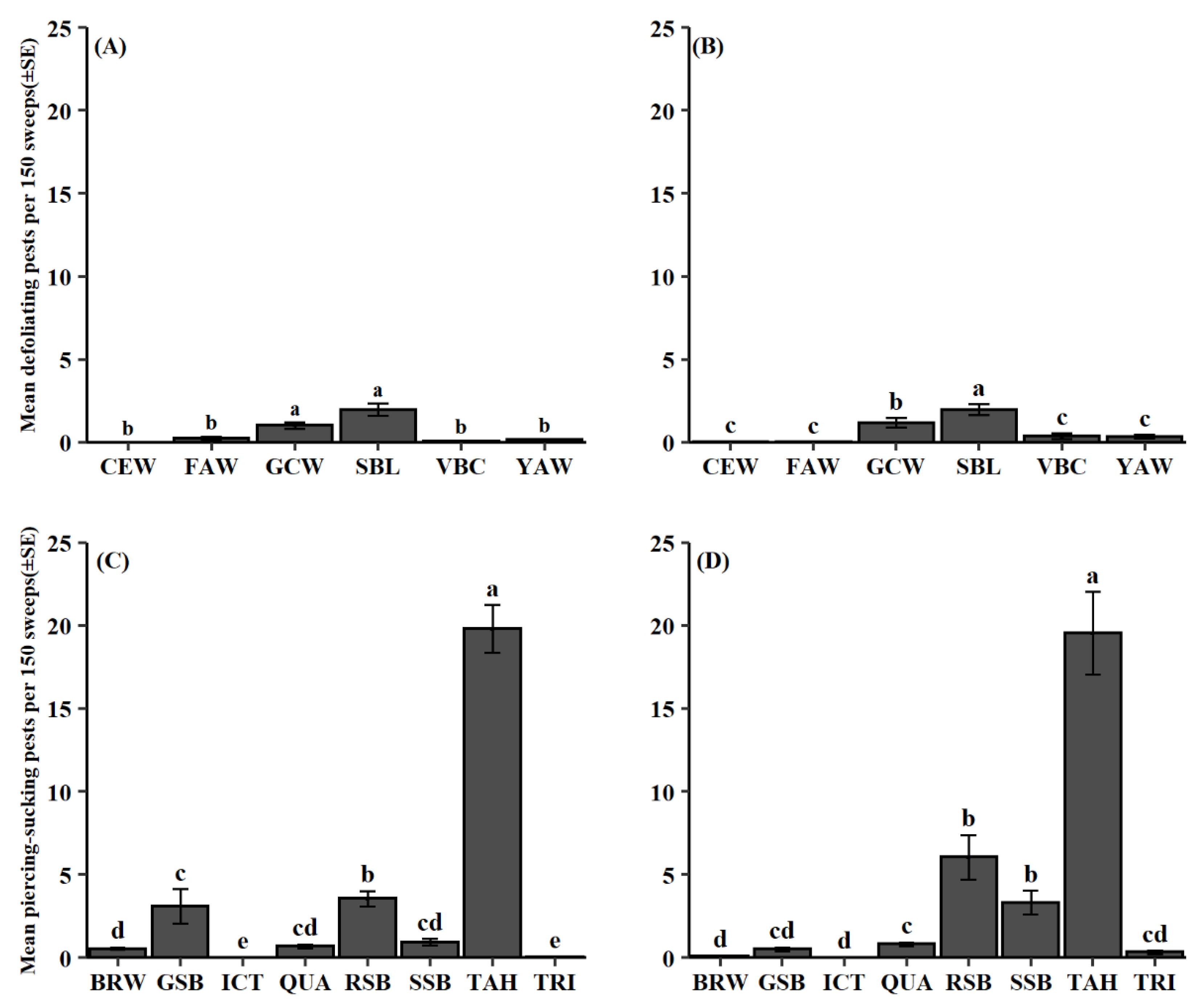
3.1. Pest Composition and Phenology
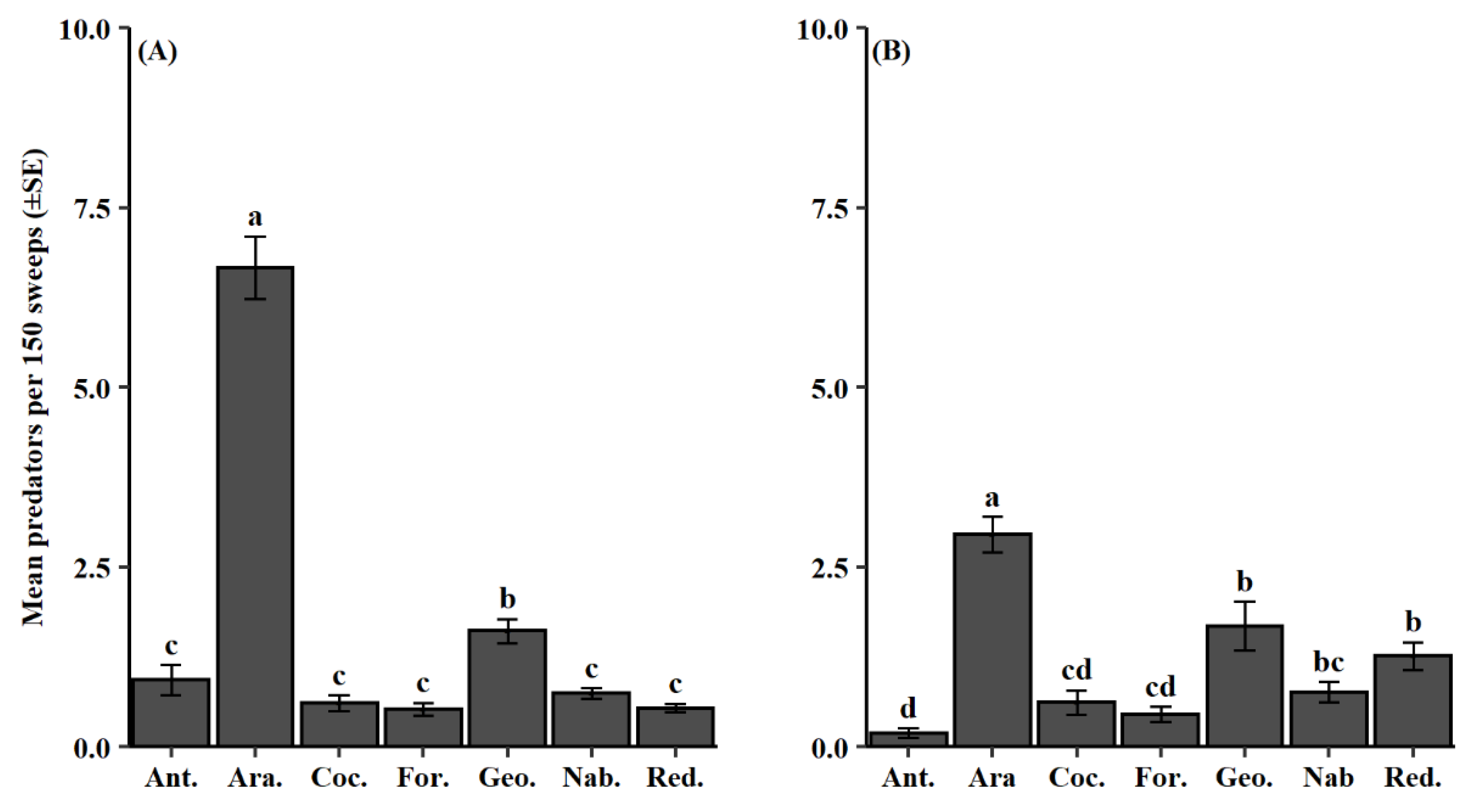
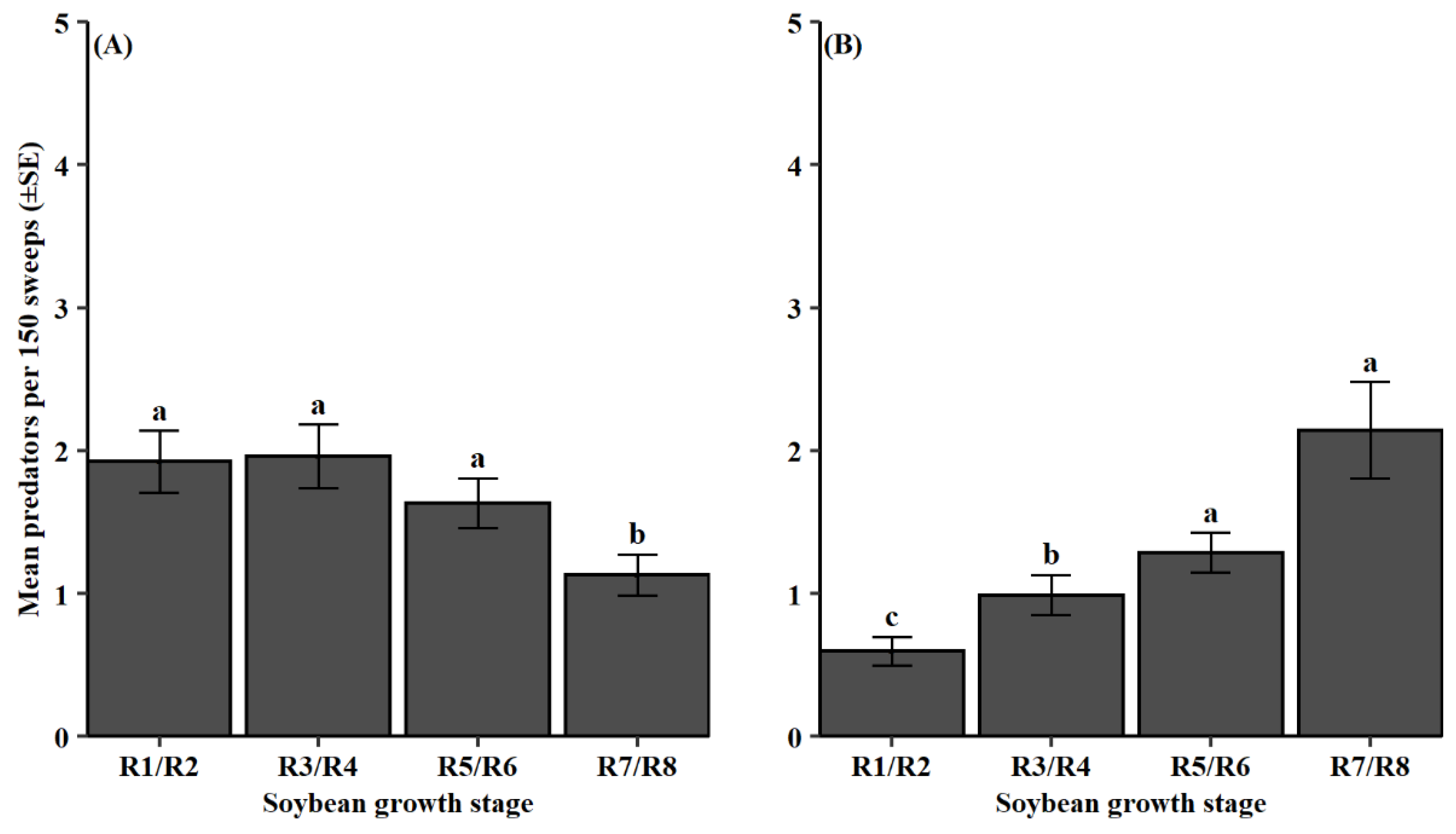
3.2. Natural Enemy Composition and Phenology
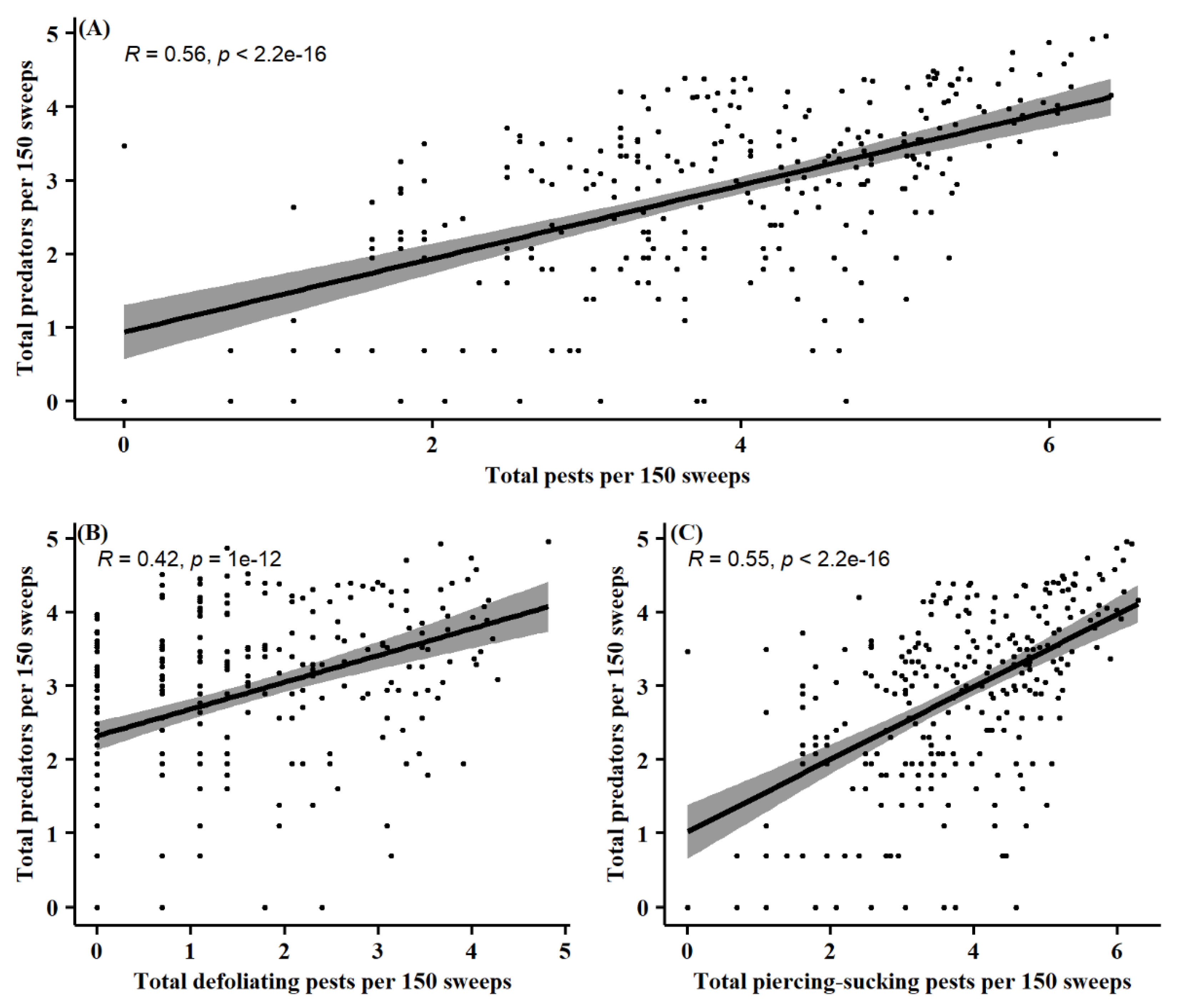
3.3. Pest and Natural Enemy Correlation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, R.F. Integration of biological and chemical control. Bull. Entomol. Soc. Am. 1962, 8, 188–189. [Google Scholar]
- Stern, V.M.; Smith, R.F.; van den Bosch, R.; Hagen, K.S. The integration of chemical and biological control of the spotted alfalfa aphid: The integrated control concept. Hilgardia 1959, 29, 81–101. [Google Scholar] [CrossRef] [Green Version]
- Heinrichs, E.A.; Mochida, O. From secondary to major pest status: The case of insecticide-induced rice brown planthopper, Nilaparvata lugens, resurgence. Prot. Ecol. 1984, 7, 201–218. [Google Scholar]
- Settle, W.H.; Ariawan, H.; Astuti, E.T.; Cahyana, W.; Hakim, A.L.; Hindayana, D.; Lestari, A.S. Managing tropical rice pests through conservation of generalist natural enemies and alternative prey. Ecology 1996, 77, 1975–1988. [Google Scholar] [CrossRef]
- Naranjo, S.E. Conservation and evaluation of natural enemies in IPM systems for Bemisia tabaci. Crop Prot. 2001, 20, 835–852. [Google Scholar] [CrossRef] [Green Version]
- Johnson, K.D.; O’Neal, M.E.; Bradshaw, J.D.; Rice, M.E. Is preventative, concurrent management of the soybean aphid (Hemiptera: Aphididae) and bean leaf beetle (Coleoptera: Chrysomelidae) possible? J. Econ. Entomol. 2008, 101, 801–809. [Google Scholar] [CrossRef]
- Pedigo, L.P.; Rice, M.E. Entomology and Pest Management, 6th ed.; Waveland Press Inc.: Long Grove, IL, USA, 2009. [Google Scholar]
- Losey, J.E.; Vaughan, M. The economic value of ecological services provided by insects. Bioscience 2006, 56, 311–323. [Google Scholar] [CrossRef] [Green Version]
- Kring, T.J.; Gilstrap, F.E.; Michels, G.J., Jr. Role of indigenous coccinellids in regulating greenbugs (Homoptera: Aphididae) on Texas grain sorghum. J. Econ. Entomol. 1985, 78, 269–273. [Google Scholar] [CrossRef] [Green Version]
- Eubanks, M.D. Estimates of the direct and indirect effects of red imported fire ants on biological control in field crops. Biol. Control 2001, 21, 35–43. [Google Scholar] [CrossRef] [Green Version]
- Rutledge, C.E.; O’Neil, R.J.; Fox, T.B.; Landis, D.A. Soybean aphid predators and their use in integrated pest management. Ann. Entomol. Soc. Am. 2004, 97, 240–248. [Google Scholar] [CrossRef]
- Seagraves, M.P.; McPherson, R.M. Monitoring red imported fire ant (Hymenoptera: Formicidae) foraging behavior and impact on foliage-dwelling arthropods on soybean produced under three ant suppression regimes. J. Entomol. Sci. 2006, 41, 374–384. [Google Scholar] [CrossRef]
- Knight, K.M.; Brier, H.B.; Lucy, M.J.; Kopittke, R.A. Impact of mirid (Creontiades spp.) (Hemiptera: Miridae) pest management on Helicoverpa spp. (Lepidoptera: Noctuidae) outbreaks: The case for conserving natural enemies. Pest Manag. Sci. 2007, 63, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Gontijo, L.M.; Beers, E.H.; Snyder, W.E. Complementary suppression of aphids by predators and parasitoids. Biol. Control 2015, 90, 83–91. [Google Scholar] [CrossRef]
- Asiimwe, P.; Ellsworth, P.C.; Naranjo, S.E. Natural enemy impacts on Bemisia tabaci (MEAM1) dominate plant quality effects in the cotton system. Ecol. Entomol. 2016, 41, 642–652. [Google Scholar] [CrossRef] [Green Version]
- Fox, T.B.; Landis, D.A.; Cardoso, F.F.; Difonzo, C.D. Predators suppress Aphis glycines Matsumura population growth in soybean. Environ. Entomol. 2004, 33, 608–618. [Google Scholar] [CrossRef] [Green Version]
- Funderburk, J.; Stavisky, J.; Olson, S. Predation of Frankliniella occidentalis (Thysanoptera: Thripidae) in field peppers by Orius insidiosus (Hemiptera: Anthocoridae). Environ. Entomol. 2000, 29, 376–382. [Google Scholar] [CrossRef]
- Boyd, M.L.; Boethel, D.J.; Leonard, B.R.; Habetz, R.J.; Brown, L.P.; Hallmack, W.B. Seasonal Abundance of Arthropod Populations on Selected Soybean Varieties Grown in Early Season Production Systems in Louisiana; Louisiana Agricultural Experiment Station: Baton Rouge, LA, USA, 1997; Volume 860. [Google Scholar]
- Baur, M.E.; Boethel, D.J.; Boyd, M.L.; Bowers, G.R.; Way, M.O.; Heatherly, L.G.; Rabb, J.; Ashlock, L. Arthropod populations in early soybean production systems in the Mid-South. Environ. Entomol. 2000, 29, 312–328. [Google Scholar] [CrossRef]
- Temple, J.H.; Davis, J.A.; Micinski, S.; Hardke, J.T.; Price, P.; Leonard, B.R. Species composition and seasonal abundance of stink bugs (Hemiptera: Pentatomidae) in Louisiana soybean. Environ. Entomol. 2013, 42, 648–657. [Google Scholar] [CrossRef] [Green Version]
- Temple, J.H.; Davis, J.A.; Hardke, J.; Moore, J.; Leonard, B.R. Susceptibility of southern green stink bug and redbanded stink bug to insecticides in soybean field experiments and laboratory bioassays. Southwest. Entomol. 2013, 38, 393–406. [Google Scholar] [CrossRef]
- Easterbrook, M.A. A field assessment of the effects of insecticides on the beneficial fauna of strawberry. Crop Prot. 1997, 16, 147–152. [Google Scholar] [CrossRef]
- Michaud, J.P.; Grant, A.K. IPM-compatibility of foliar insecticides for citrus: Indices derived from toxicity to beneficial insects from four orders. J. Insect Sci. 2003, 3, 1–10. [Google Scholar] [CrossRef]
- Seagraves, M.P.; Lundgren, J.G. Effects of neonicitinoid seed treatments on soybean aphid and its natural enemies. J. Pest Sci. 2012, 85, 125–132. [Google Scholar] [CrossRef]
- Fernandes, M.E.; Alves, F.M.; Pereira, R.C.; Aquino, L.A.; Fernandes, F.L.; Zanuncio, J.C. Lethal and sublethal effects of seven insecticides on three beneficial insects in laboratory assays and field trials. Chemosphere 2016, 156, 45–55. [Google Scholar] [CrossRef]
- Prabhaker, N.; Naranjo, S.E.; Perring, T.; Castle, S.J. Comparative toxicities of newer and conventional insecticides: Against four generalist predator species. J. Econ. Entomol. 2017, 110, 2630–2636. [Google Scholar] [CrossRef] [PubMed]
- Douglas, M.R.; Tooker, J.F. Large-scale deployment of seed treatments has driven rapid increase in use of neonicotinoid insecticides and preemptive pest management in US field crops. Environ. Sci. Technol. 2015, 49, 5088–5097. [Google Scholar] [CrossRef]
- Douglas, M.R.; Tooker, J.F. Meta-analysis reveals that seed-applied neonicotinoids and pyrethroids have similar negative effects on abundance of arthropod natural enemies. PeerJ 2016, 4, e2776. [Google Scholar] [CrossRef]
- Heatherly, L.G.; Bowers, G.R. Early Soybean Production System Handbook; USB 6009-09; United Soybean Board: St. Louis, MO, USA, 1998. [Google Scholar]
- Buschman, L.L.; Pitre, H.N.; Hodges, H.F. Soybean cultural practices: Effects on populations of geocorids, nabids, and other soybean arthropods. Environ. Entomol. 1984, 13, 305–317. [Google Scholar] [CrossRef]
- McPherson, R.M.; Layton, R.C.; McLaurin, W.J.; Mills III, W.A. Influence of irrigation and maturity group on the seasonal abundance of soybean arthropods. J. Entomol. Sci. 1998, 33, 378–392. [Google Scholar] [CrossRef]
- Teare, I.D.; Hodges, H.F. Soybean ecology and physiology. In Handbook of Soybean Insect Pests; Higley, L.G., Boethel, D.J., Eds.; Entomological Society of America: Lanham, MD, USA, 1994; pp. 4–7. [Google Scholar]
- QGIS Development Team. QGIS Geographic Information System, Open Source Geospatial Foundation Project, Version 3.16.0. 2020. Available online: https://qgis.osgeo.org (accessed on 26 May 2021).
- Mosely, D.; Padgett, B.; Price, T.; Harrell, D.; Kongchum, M.; Stephenson, D.; Gentry, G.; Buckley, B.; Parvej, R.; Brown, S.; et al. 2021 Soybean Variety Yields and Production Practices; LSU AgCenter: Baton Rouge, LA, USA, 2020; Volume 2269. [Google Scholar]
- Fehr, W.R.; Caviness, C.E.; Burmood, D.T.; Pennington, J.S. Stage of development descriptions for soybeans, Glycine max (L.) Merrill. Crop Sci. 1971, 11, 929–931. [Google Scholar] [CrossRef]
- Triplehorn, C.A.; Johnson, N.F. Borror and DeLong’s Introduction to the Study of Insects, 7th ed.; Thomson Brooks/Cole: Belmont, CA, USA, 2005. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002; Available online: https://www.stats.ox.ac.uk/pub/MASS4 (accessed 25 March 2021).
- Lenth, R.V. Least-Squares Means: The R Package lsmeans. J. Stat. Softw. 2016, 69, 1–33. [Google Scholar] [CrossRef] [Green Version]
- Turnipseed, S.G.; Kogan, M. Soybean entomology. Ann. Rev. Entomol. 1976, 21, 247–282. [Google Scholar] [CrossRef]
- Leonard, B.R.; Boethel, D.J.; Sparks, A.N., Jr.; Layton, B.M.; Mink, J.S.; Pavloff, A.M.; Burris, E.; Graves, J.B. Variations in response of soybean looper (Lepidoptera: Noctuidae) to selected insecticides in Louisiana. J. Econ. Entomol. 1990, 83, 27–34. [Google Scholar] [CrossRef]
- Shepard, M.; Carner, G.R.; Turnipseed, S.G. Colonization and resurgence of insect pests of soybean in response to insecticides and field isolation. Environ. Entomol. 1977, 6, 501–506. [Google Scholar] [CrossRef]
- McPherson, R.M.; Newsom, L.D. Trap crops for control of stink bugs (Hemiptera, Pentatomidae) in soybean. J. Georg. Entomol. Soc. 1984, 19, 470–480. [Google Scholar]
- McPherson, R.M. Relationship between soybean maturity group and the phenology and abundance of stink bugs (Heteroptera: Pentatomidae): Impact on yield and quality. J. Entomol Sci. 1996, 31, 199–208. [Google Scholar] [CrossRef]
- McPherson, R.M.; Wells, M.L.; Bundy, C.S. Impact of the early soybean production system on arthropod pest populations in Georgia. Environ. Entomol. 2001, 30, 76–81. [Google Scholar] [CrossRef]
- Baur, M.; Baldwin, J. Redbanded stink bugs trouble Louisiana. Louis. Agric. 2006, 48, 9–10. [Google Scholar]
- Panizzi, A.R.; Slansky, F., Jr. Review of phytophagous pentatomids (Hemiptera: Pentatomidae) associated with soybean in the Americas. Fl. Entomol. 1985, 184–214. [Google Scholar] [CrossRef]
- McPherson, J.E.; McPherson, R. Stink Bugs of Economic Importance in America North of Mexico; CRC Press: Boca Raton, FL, USA, 2000; pp. 177–179. [Google Scholar]
- Bastola, A.; Davis, J.A. Cold tolerance and supercooling capacity of the redbanded stink bug (Hemiptera: Pentatomidae). Environ. Entomol. 2018, 47, 133–139. [Google Scholar] [CrossRef]
- Tillman, P.G.; Greenstone, M.H.; Hu, J.S. Predation of stinkbugs (Hemiptera: Pentatomidae) by a complex of predators in cotton and adjoining soybean habitats in Georgia, USA. Fla. Entomol. 2015, 98, 1114–1126. [Google Scholar] [CrossRef] [Green Version]
- Athey, K.J.; Ruberson, J.R.; Olson, D.M.; Hardwood, J.D. Predation on stink bugs (Hemiptera: Pentatomidae) in cotton and soybean agroecosystems. PLoS ONE 2019, 14, e0214325. [Google Scholar] [CrossRef] [PubMed]
- Nyffler, M.; Dean, D.A.; Sterling, W.L. Evaluation of the importance of the striped lynx spider, Oxyopes salticus (Araneae, Oxyopidae), as a predator in Texas cotton. Environ. Entomol. 1987, 16, 114–1123. [Google Scholar] [CrossRef]
- Pfannenstiel, R.S. Spider predators of lepidopteran eggs in south Texas. Biol. Control 2008, 46, 202–208. [Google Scholar] [CrossRef]
- Carner, G.R.; Shepard, M.; Turnipseed, S.G. Seasonal abundance of insect pests of soybeans. J. Econ. Entomol. 1974, 67, 487–493. [Google Scholar] [CrossRef]
- Kogan, M.; Cope, D. Feeding and nutrition of insects associated with soybeans. 3. Food intake, utilization, and growth in the soybean looper, Pseudoplusia includens. Ann. Entomol. Soc. Am. 1974, 67, 66–72. [Google Scholar] [CrossRef]
- McPherson, R.M.; Todd, J.W.; Yeargan, K.V. Stink bugs. In Handbook of Soybean Insect Pests; Higley, L.G., Boethel, D.J., Eds.; Entomological Society of America: Lanham, MD, USA, 1994; p. 136. [Google Scholar]
- Shepard, M.; Carner, G.R.; Turnipseed, S.G. Seasonal abundance of predaceous arthropods in soybeans. Environ. Entomol. 1974, 3, 985–988. [Google Scholar] [CrossRef]
- Price, P.W.; Denno, R.F.; Eubanks, M.D.; Finke, D.L.; Kaplan, I. Insect Ecology: Behavior, Populations and Communities; Cambridge University Press: New York, NY, USA, 2011. [Google Scholar]
- Musser, F.R.; Catchot, A.L., Jr.; Conley, S.P.; Davis, J.A.; DiFonzo, C.; Graham, S.H.; Greene, J.K.; Koch, R.L.; Lorenz, G.M.; Owens, D.; et al. 2020 soybean insect losses in the United States. Midsouth Entomol. 2021, 14, 1–25. [Google Scholar]
- Boyd, M.L.; Boethel, D.J. Susceptibility of predaceous hemipteran species to selected insecticides on soybean in Louisiana. J. Econ. Entomol. 1998, 91, 401–409. [Google Scholar] [CrossRef]
- Elzen, G.W. Lethal and sublethal effects of insecticide residues on Orius insidiosus (Hemiptera: Anthocoridae) and Geocoris punctipes (Hemiptera: Lygaeidae). J. Econ. Entomol. 2001, 94, 55–59. [Google Scholar] [CrossRef]
- Baur, M.E.; Ellis, J.; Hutchinson, K.; Boethel, D.J. Contact toxicity of selective insecticides for non-target predaceous hemipterans in soybeans. J. Entomol. Sci. 2003, 38, 269–277. [Google Scholar] [CrossRef]
- Galvan, T.L.; Koch, R.L.; Hutchison, W.D. Toxicity of commonly used insecticides in sweet corn and soybean to multicolored Asian lady beetle (Coleoptera: Coccinellidae). J. Econ. Entomol. 2005, 98, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Cutler, G.C.; Scott-Dupree, C.D.; Tolman, J.H.; Harris, C.R. Toxicity of the insect growth regulator novaluron to the non-target predatory bug Podisus maculiventris (Heteroptera: Pentatomidae). Biol. Control 2006, 38, 196–204. [Google Scholar] [CrossRef] [Green Version]
- Ohnesorg, W.J.; Johnson, K.D.; O’Neal, M.E. Impact of reduced-risk insecticides on soybean aphid and associated natural enemies. J. Econ. Entomol. 2009, 102, 1816–1826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varenhorst, A.J.; O’Neal, M.E. The response of natural enemies to selective insecticides applied to soybean. Environ. Entomol. 2012, 41, 1565–1574. [Google Scholar] [CrossRef] [Green Version]
- Hanna, C.; Hanna, C. The lethal and sublethal effects of three pesticides on the striped lynx spider (Oxyopes salticus Hentz). J. Appl. Entomol. 2013, 137, 68–76. [Google Scholar] [CrossRef]
- Whalen, R.A.; Herbert, D.A.; Malone, S.; Kuhar, T.P.; Brewster, C.C.; Reisig, D.D. Effects of diamide insecticides on predators in soybean. J. Econ. Entomol. 2016, 109, 2014–2019. [Google Scholar] [CrossRef]
- Crocker, R.L.; Whitcomb, W.H. Feeding niches of the big-eyed bugs, Geocoris bullatus, G. punctipes, and G. uliginosus (Hemiptera: Lygaeidae: Geocorinae). Environ. Entomol. 1980, 9, 508–513. [Google Scholar] [CrossRef]
- Prabhaker, N.; Castle, S.J.; Naranjo, S.E.; Toscano, N.C.; Morse, J.G. Compatibility of two systemic neonicotinoids, imidacloprid and thiamethoxam, with various natural enemies of agricultural pests. J. Econ. Entomol. 2011, 104, 773–781. [Google Scholar] [CrossRef]
- Halaj, J.; Cady, A.B.; Uetz, G.W. Modular habitat refugia enhance generalist predators and lower plant damage in soybeans. Environ. Entomol. 2000, 29, 383–393. [Google Scholar] [CrossRef]
- Lee, J.C.; Heimpel, G.E. Floral resources impact longevity and oviposition rate of a parasitoid in the field. J. Anim. Ecol. 2008, 77, 565–572. [Google Scholar] [CrossRef]
- Lee, J.C.; Heimpel, G.E. Effect of floral nectar, water, and feeding frequency on Cotesia glomerata longevity. Biocontrol 2008, 53, 289–294. [Google Scholar] [CrossRef]
- Blaauw, B.R.; Isaacs, R. Larger wildflower plantings increase natural enemy density, diversity, and biological control of sentinel prey, without increasing herbivore density. Ecol. Entomol. 2012, 37, 386–394. [Google Scholar] [CrossRef]
- Schmidt, M.H.; Thewes, U.; Thies, C.; Tscharntke, T. Aphid suppression by natural enemies in mulched cereals. Entomol. Exp. Appl. 2004, 113, 87–93. [Google Scholar] [CrossRef]
- Kahl, H.M.; Leslie, A.W.; Hooks, C.R.R. Effects of red clover living mulch on arthropod herbivores and natural enemies, and cucumber yield. Ann. Entomol. Soc. Am. 2019, 112, 356–364. [Google Scholar] [CrossRef]
- Karp, D.S.; Chaplin-Kramer, R.; Meehan, T.D.; Martin, E.A.; DeClerck, F.; Grab, H.; Gratton, C.; Hunt, L.; Larsen, A.E.; Martinez-Salinas, A.; et al. Crop pests and predators exhibit inconsistent responses to surrounding landscape composition. Proc. Natl. Acad. Sci. USA 2018, 115, E7863–E7870. [Google Scholar] [CrossRef] [Green Version]
- Moore, L.C.; Leslie, A.W.; Hooks, C.R.R.; Dively, G.P. Can plantings of partridge pea (Chamaecrista fasciculata) enhance beneficial arthropod communities in neighboring soybeans? Biol. Control 2019, 128, 6–16. [Google Scholar] [CrossRef]
- Whigham, D.K.; Minor, H.C. Agronomic characteristics and environmental stress. In Soybean Physiology, Agronomy, and Utilization, 1st ed.; Norman, A.G., Ed.; Academic Press Inc.: New York, NY, USA, 1978; pp. 78–116. [Google Scholar]
- Costamagna, A.C.; Landis, D.A. Predators exert top-down control of soybean aphid across a gradient of agricultural management systems. Ecol. Appl. 2006, 16, 1619–1628. [Google Scholar] [CrossRef] [Green Version]
- Bowers, C.; Toews, M.; Liu, Y.X.; Schmidt, J.M. Cover crops improve early season natural enemy recruitment and pest management in cotton production. Biol. Control 2020, 141, 104149. [Google Scholar] [CrossRef]



| Classification | Relative Abundance (Total Count) | |||
|---|---|---|---|---|
| Order | Family (Abbr.) | 2012–2014 | 2015–2018 | Total |
| Coleoptera | Coccinellidae (Coc.) | 4.8 (267) | 6.7 (164) | 5.4 (431) |
| Hemiptera | Anthocoridae (Ant.) | 7.6 (426) | 2.1 (51) | 5.9 (477) |
| Hemiptera | Geocoridae (Geo.) | 14.7 (827) | 18.1 (441) | 15.8 (1268) |
| Hemiptera | Nabidae (Nab.) | 7.1 (400) | 10.4 (252) | 8.1 (652) |
| Hemiptera | Pentatomidae | 0.9 (48) | 1.2 (30) | 1.0 (78) |
| Hemiptera | Reduviidae (Red.) | 4.9 (273) | 16.1 (391) | 8.3 (664) |
| Hymenoptera | Formicidae (For.) | 4.0 (225) | 4.4 (107) | 4.1 (332) |
| Neuroptera | Chrysopidae | 0.8 (42) | 1.4 (33) | 0.9 (75) |
| Araneae | NA (Ara.) | 55.3 (3104) | 39.7 (965) | 50.6 (4069) |
| Defoliating Herbivorous Pests | ||||
|---|---|---|---|---|
| Classification | Relative Abundance (Total Count) | |||
| Species | Common Name (Abbr.) | 2012–2014 | 2015–2018 | Total |
| Anticarsia gemmatalis | Velvetbean caterpillar (VBC) | 1.3 (22) | 7.9 (83) | 3.8 (105) |
| Chrysodeixis includens | Soybean looper (SBL) | 49.7 (853) | 50.3 (528) | 50.0 (1381) |
| Helicoverpa zea | Corn earworm (CEW) | 0.2 (3) | 0.5 (5) | 0.3 (8) |
| Hypena scabra | Green cloverworm (GCW) | 32.7 (560) | 31.7 (333) | 32.3 (893) |
| Spodoptera frugiperda | Fall armyworm (FAW) | 11.8 (203) | 1.9 (20) | 8.1 (223) |
| Spodoptera ornithogalli | Yellowstriped armyworm (YAW) | 4.3 (74) | 7.6 (80) | 5.6 (154) |
| Piercing-Sucking Herbivorous Pests | ||||
| Classification | Relative Abundance (Total Count) | |||
| Species | Common Name (Abbr.) | 2012–2014 | 2015–2018 | Total |
| Chinavia hilaris | Green stink bug (GSB) | 10.0 (1383) | 2.0 (158) | 7.0 (273) |
| Euschistus ictericus | NA (ICT) | 0.1 (10) | >0.1 (2) | 0.1 (12) |
| Euschistus quadrator | NA (QUA) | 2.5 (344) | 2.7 (221) | 2.6 (565) |
| Euschistus servus | Brown stink bug (BRW) | 1.8 (248) | 0.3 (25) | 1.2 (273) |
| Euschistus tristigmus | Dusky stink bug (TRI) | 0.3 (37) | 0.9 (75) | 0.5 (112) |
| Nezara viridula | Southern green stink bug (SSB) | 4.2 (586) | 10.2 (824) | 6.4 (1410) |
| Piezodorus guildinii | Redbanded stink bug (RSB) | 12.5 (1734) | 21.8 (1758) | 15.9 (3484) |
| Spissistilus festinus | Threecornered alfalfa hopper (TAH) | 68.7 (9531) | 62.1 (5013) | 66.3 (14544) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.T.; Li, C.; Davis, J.A. Predator-Pest Dynamics of Arthropods Residing in Louisiana Soybean Agroecosystems. Insects 2022, 13, 154. https://doi.org/10.3390/insects13020154
Lee ST, Li C, Davis JA. Predator-Pest Dynamics of Arthropods Residing in Louisiana Soybean Agroecosystems. Insects. 2022; 13(2):154. https://doi.org/10.3390/insects13020154
Chicago/Turabian StyleLee, Scott T., Chaoyang Li, and Jeffrey A. Davis. 2022. "Predator-Pest Dynamics of Arthropods Residing in Louisiana Soybean Agroecosystems" Insects 13, no. 2: 154. https://doi.org/10.3390/insects13020154






