What Is the Carcass-Usage Mode of the Collembola? A Case Study of Entomobrya proxima in the Laboratory
Abstract
:1. Introduction
2. Materials and Methods
2.1. Insect Food and Collembolan Activity
2.2. Feeding Strategies (E. proxima)
2.3. Stable Isotope Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bardgett, R.D.; Whittaker, J.B.; Frankland, J.C. The effect of Collembolan grazing on fungal activity in differently managed upland pastures: A microcosm study. Biol. Fertil. Soils 1993, 16, 255–262. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Chan, K.F. Experimental evidence that soil fauna enhance nutrient mineralization and plant nutrient uptake in montane grassland ecosystems. Soil Biol. Biochem. 1999, 31, 1007–1014. [Google Scholar] [CrossRef]
- Osler, G.H.R.; Sommerkorn, M. Toward a complete soil C and N cycle: Incorporating the soil fauna. Ecology 2007, 88, 1611–1621. [Google Scholar] [CrossRef] [PubMed]
- Schutz, K.; Bonkowski, M.; Scheu, S. Effects of Collembola and fertilizers on plant performance (Triticum aestivum) and aphid reproduction (Rhopalosiphum padi). Basic Appl. Ecol. 2008, 9, 182–188. [Google Scholar] [CrossRef]
- Chamberlain, P.M.; Bull, I.D.; Black, H.I.J.; Ineson, P.; Evershed, R.P. Collembolan trophic preferences determined using fatty acid distributions and compound-specific stable carbon isotope values. Soil Biol. Biochem. 2006, 38, 1275–1281. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Whittaker, J.B.; Frankland, J.C. The diet and food preferences of Onychiurus procampatus (collembola) from upland grassland soils. Biol. Fertil. Soils 1993, 16, 296–298. [Google Scholar] [CrossRef]
- Bokhorst, S.; Ronfort, C.; Huiskes, A.; Convey, P.; Aerts, R. Food choice of antarctic soil arthropods clarified by stable isotope signatures. Polar Biol. 2007, 30, 983–990. [Google Scholar] [CrossRef]
- Buse, T.; Filser, J. Mucilaginous seeds and algal diets attract soil Collembola in preference tests. Eur. J. Soil Biol. 2014, 65, 1–6. [Google Scholar] [CrossRef]
- Rusek, J. Biodiversity of Collembola and their functional role in the ecosystem. Biodivers. Conserv. 1998, 7, 1207–1219. [Google Scholar] [CrossRef]
- Seastedt, T.R.; Mameli, L.; Gridley, K. Arthropod use of invertebrate carrion. Am. Midl. Nat. 1981, 105, 124–129. [Google Scholar] [CrossRef]
- Maynard, E.A. A Monograph of the Collembola or Springtail Insects of New York State. Part I–II; Comstock Publishing Company, Inc.: New York, NY, USA, 1951; p. 339. [Google Scholar]
- Jensen, T.C.; Leinaas, H.P.; Hessen, D.O. Age-dependent shift in response to food element composition in Collembola: Contrasting effects of dietary nitrogen. Oecologia 2006, 149, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Endlweber, K.; Ruess, L.; Scheu, S. Collembola switch diet in presence of plant roots thereby functioning as herbivores. Soil Biol. Biochem. 2009, 41, 1151–1154. [Google Scholar] [CrossRef]
- Heděnec, P.; Radochová, P.; Nováková, A.; Kaneda, S.; Frouz, J. Grazing preference and utilization of soil fungi by Folsomia candida, (Isotomidae: Collembola). Eur. J. Soil Biol. 2013, 55, 66–70. [Google Scholar] [CrossRef]
- Staaden, S.; Milcu, A.; Rohlfs, M.; Scheu, S. Olfactory cues associated with fungal grazing intensity and secondary metabolite pathway modulate Collembola foraging behavior. Soil Biol. Biochem. 2011, 43, 1411–1416. [Google Scholar] [CrossRef]
- Hiol, F.H.; Dixon, R.K.; Curl, E.A. The feeding preference of mycophagous Collembola varies with the ectomycorrhizal symbiont. Mycorrhiza 1994, 5, 99–103. [Google Scholar] [CrossRef]
- Jałoszyński, P. Adults of european ant-like stone beetles (Coleoptera: Staphylinidae: Scydmaeninae) Scydmaenus tarsatus Müller & Kunze and Scydmaenus hellwigii (Herbst) prey on soft-bodied arthropods. Entomol. Sci. 2012, 15, 35–41. [Google Scholar]
- Tantawi, T.I.; El-Kady, E.M.; Greenberg, B.; El-Ghaffar, H.A. Arthropod succession on exposed rabbit carrion in Alexandria, Egypt. J. Med. Entomol. 1996, 33, 566–580. [Google Scholar] [CrossRef] [PubMed]
- Van Veen, J.A.; Kuikman, P.J. Soil structural aspects of decomposition of organic matter by microorganisms. Biogeochemistry 1990, 11, 213–233. [Google Scholar] [CrossRef]
- Schultz, P.A. Grazing preferences of two collembolan species, Folsomia candida and Proisotoma minuta, for ectomycorrhizal fungi. Pedobiologia 1991, 35, 313–325. [Google Scholar]
- Jonas, J.L.; Wilson, G.W.T.; White, P.M.; Joern, A. Consumption of mycorrhizal and saprophytic fungi by Collembola in grassland soils. Soil Biol. Biochem. 2007, 39, 2594–2602. [Google Scholar] [CrossRef]
- Jørgensen, H.B.; Elmholt, S.; Petersen, H. Collembolan dietary specialisation on soil grown fungi. Biol. Fertil. Soils 2003, 39, 9–15. [Google Scholar] [CrossRef]
- Ponge, J.F. Vertical distribution of Collembola (Hexapoda) and their food resources in organic horizons of beech forests. Biol. Fertil. Soils 2000, 32, 508–522. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; Snider, R.J.; Snider, R.M. Food preference and effects of food type on the life history of some soil Collembola. Pedobiologia 1995, 39, 496–505. [Google Scholar]
- Bardgett, R.D.; van der Putten, W.H. Belowground biodiversity and ecosystem functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Ladygina, N.; Caruso, T.; Hedlund, K. Dietary switching of Collembola in grassland soil food webs. Soil Biol. Biochem. 2008, 40, 2898–2903. [Google Scholar] [CrossRef]
- Sechi, V.; D’Annibale, A.; Ambus, P.; Sárossy, Z.; Krogh, P.H.; Eriksen, J.; Holmstrup, M. Collembola feeding habits and niche specialization in agricultural grasslands of different composition. Soil Biol. Biochem. 2014, 74, 31–38. [Google Scholar] [CrossRef]
- Klironomos, J.N.; Widden, P.; Deslandes, I. Feeding preferences of the collembolan Folsomia candida in relation to microfungal successions on decaying litter. Soil Biol. Biochem. 1992, 24, 685–692. [Google Scholar] [CrossRef]
- Focken, U.; Becker, K. Metabolic fractionation of stable carbon isotopes: Implications of different proximate compositions for studies of the aquatic food webs using 13C data. Oecologia 1998, 115, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Oelbermann, K.; Scheu, S. Stable isotope enrichment (δ15N and δ13C) in a generalist predator (Pardosa lugubris, Araneae: Lycosidae): Effects of prey quality. Oecologia 2002, 130, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Ponsard, S.; Arditi, R. What can stable isotopes (δ15N and δ13C) tell about the food web of soil macroinvertebrates? Ecology 2000, 81, 852–864. [Google Scholar]
- Okuzaki, Y.; Tayasu, I.; Okuda, N.; Sota, T. Vertical heterogeneity of a forest floor invertebrate food web as indicated by stable-isotope analysis. Ecol. Res. 2009, 24, 1351–1359. [Google Scholar] [CrossRef]
- Gannes, L.Z.; O’Brien, D.M.; Rio, C.M.D. Stable isotopes in animal ecology: Assumptions, caveats, and a call for more laboratory experiments. Ecology 1997, 78, 1271–1276. [Google Scholar] [CrossRef]
- Griffiths, H. Applications of stable isotope technology in physiological ecology. Funct. Ecol. 1991, 5, 254–269. [Google Scholar] [CrossRef]
- McCutchan, J.H.; Lewis, W.M.; Kendall, C.; McGrath, C.C. Variation in trophic shift for stable isotope ratios of carbon, nitrogen, and sulfur. Oikos 2003, 102, 378–390. [Google Scholar] [CrossRef]
- Briones, M.J.I.; Ineson, P.; Sleep, D. Use of δ13C to determine food selection in collembolan species. Soil Biol. Biochem. 1999, 31, 937–940. [Google Scholar] [CrossRef]
- Hopkin, S.P. Biology of the Springtails (Insecta: Collembola); Oxford University Press: Oxford, UK, 1997; pp. 1–330. [Google Scholar]
- Handschin, E. Considérations sur la Position Systématique des Collemboles., Mémoires de la Société Royale d’Entomologie de Belgique; Tome Vingt-Septieme: Brussels, Belgium, 1955; Volume 27, pp. 40–53. [Google Scholar]
- Thibaud, J.M. Biologie et écologie des collemboles Hypogastruidae édaphiques et cavernicoles. Mém. Mus. Natl. d’Hist. Nat. Zool. A 1970, 61, 83–201. [Google Scholar]
- Garrity, G.M.; Bell, J.A.; Lilburn, T.G. Taxonomic outline of the prokaryotes release 5.0. In Bergey’s Manual of Systematic Bacteriology, 2nd ed.; Springer: New York, NY, USA, 2004; pp. 1–399. [Google Scholar]
- Kirk, P.M.; Cannon, P.E.; Minter, D.W.; Stalpers, J.A. Dictionary of the Fungi, 10th ed.; CABI: London, UK, 2008; pp. 1–784. [Google Scholar]
- Janssen, P.H. Identifying the dominant soil bacterial taxa in libraries of 16S rRNA and 16S rRNA genes. Appl. Environ. Microbiol. 2006, 72, 1719–1728. [Google Scholar] [CrossRef] [PubMed]
- Kõljalg, U.; Nilsson, R.H.; Abarenkov, K.; Tedersoo, L.; Taylor, A.F.; Bahram, M.; Bates, S.T.; Bruns, T.D.; Bengtsson-Palme, J.; Callaghan, T.M.; et al. Towards a unified paradigm for sequence-based identification of fungi. Mol. Ecol. 2013, 22, 5271–5277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavy, D.; Nedved, O.; Verhoef, H.A. Effects of starvation on body composition and cold tolerance in the collembolan Orchesella cincta and the isopod Porcellio scaber. J. Insect Physiol. 1997, 10, 973–978. [Google Scholar] [CrossRef]
- Fountain, M.T.; Hopkin, S.P. Folsomia candida (Collembola): A “standard” soil arthropod. Annu. Rev. Entomol. 2005, 50, 201–222. [Google Scholar] [CrossRef] [PubMed]
- OECD. Guidelines for the Testing of Chemicals. Collembola Reproduction Test. Version 3.4; OECD: Paris, France, 2008; p. 232. [Google Scholar]
- Scheu, S. The soil food web: Structure and perspectives. Eur. J. Soil Biol. 2002, 38, 11–20. [Google Scholar] [CrossRef]
- Anslan, S.; Bahram, M.; Tedersoo, L. Temporal changes in fungal communities associated with guts and appendages of Collembola as based on culturing and high-throughput sequencing. Soil Biol. Biochem. 2016, 96, 152–159. [Google Scholar] [CrossRef]
- Tiunov, A.V. Stable isotopes of carbon and nitrogen in soil ecological studies. Biol. Bull. 2007, 34, 395–407. [Google Scholar] [CrossRef]
- Phillips, D.L.; Gregg, J.W. Source partitioning using stable isotopes: Coping with too many sources. Oecologia 2003, 136, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.L.; Newsome, S.D.; Gregg, J.W. Combining sources in stable isotope mixing models: Alternative methods. Oecologia 2005, 144, 520–527. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria. Available online: http://www.R-project.org/ (accessed on 20 December 2018).
- Potapov, A.M.; Semenina, E.E.; Kurakov, A.V. Large 13C/12C and small 15N/14N isotope fractionation in an experimental detrital foodweb (litter–fungi–collembolans). Ecol. Res. 2013, 28, 1069–1079. [Google Scholar] [CrossRef]
- Martínez del Rio, C.; Wolf, N.; Carleton, S.A.; Gannes, L.Z. Isotopic ecology ten years after a call for more laboratory experiments. Biol. Rev. 2008, 84, 91–111. [Google Scholar] [CrossRef] [PubMed]
- Thimm, T.; Larink, O. Grazing preferences of some Collembola for endomycorrhizal fungi. Biol. Fertil. Soils 1995, 19, 266–268. [Google Scholar] [CrossRef]
- Kitaysky, A.S.; Piatt, J.F.; Hatch, S.A.; Kitaiskaia, E.V.; Benowitz-Fredericks, Z.M.; Shultz, T.; Wingfield, J.C. Food availability and population processes: Severity of nutritional stress during reproduction predicts survival of long-lived seabirds. Funct. Ecol. 2009, 24, 625–637. [Google Scholar] [CrossRef]
- Berg, M.P.; Bengtsson, J. Temporal and spatial variability in soil food web structure. Oikos 2007, 116, 1789–1804. [Google Scholar] [CrossRef]
- Castaño-Meneses, G.; Palacios-Vargas, J.G.; Cutz-Pool, L.Q. Feeding habits of Collembola and their ecological niche. An. Inst. Biol. Ser. Zool. 2004, 75, 135–142. [Google Scholar]
- Maraun, M.; Martens, H.; Migge, S.; Theenhaus, A.; Scheu, S. Adding to ‘the enigma of soil animal diversity’: Fungal feeders and saprophagous soil invertebrates prefer similar food substrates. Eur. J. Soil Biol. 2003, 39, 85–95. [Google Scholar] [CrossRef]
- Shelomi, M.; Matern, L.M.; Dinstell, J.M.; Harris, D.W.; Kimsey, R.B. DEET (N,N-Diethyl-meta-toluamide) induced delay of blowfly landing and oviposition rates on treated pig carrion (Sus scrofa L.). J. Forensic Sci. 2012, 57, 1507–1511. [Google Scholar] [CrossRef] [PubMed]
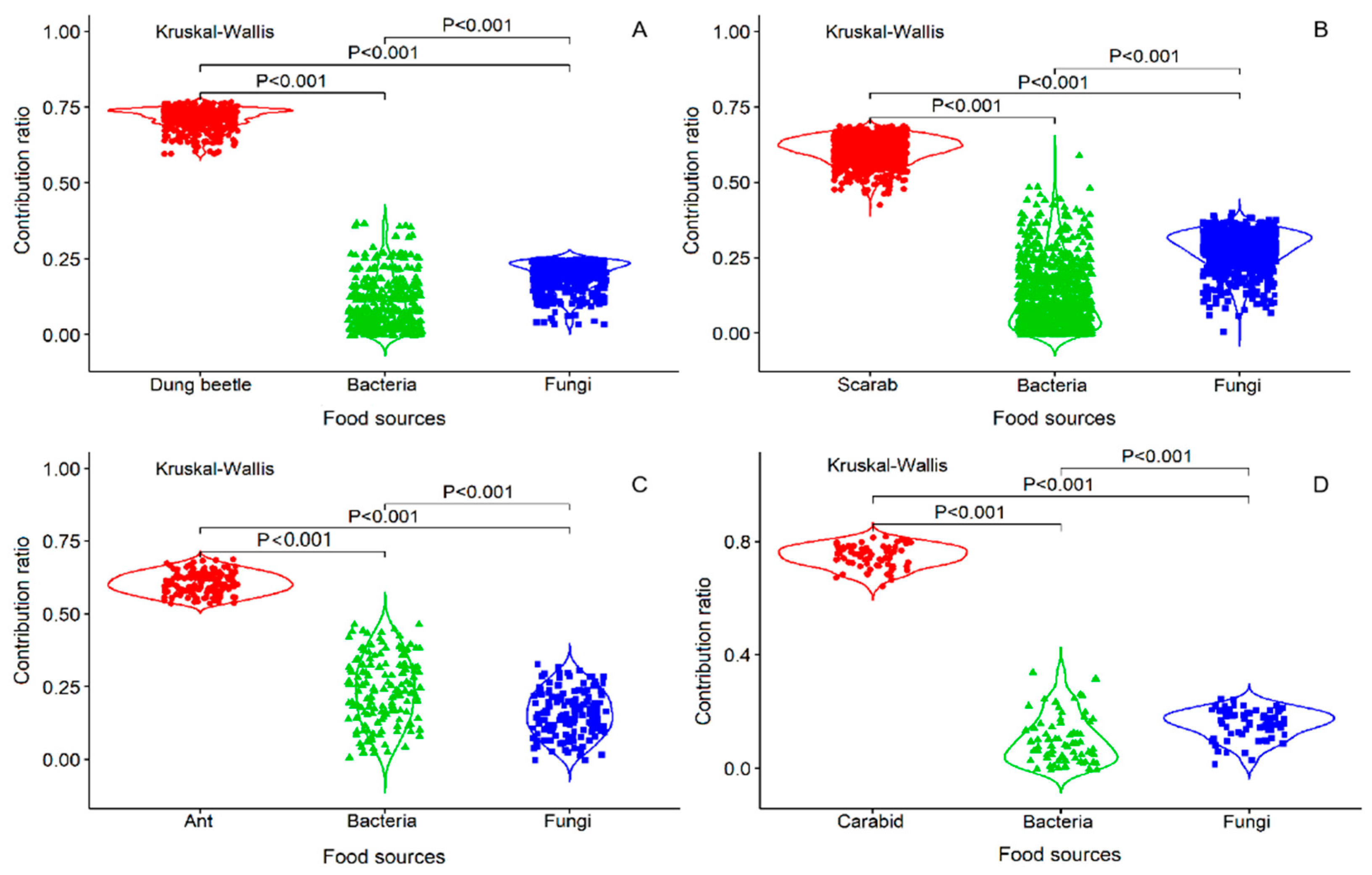

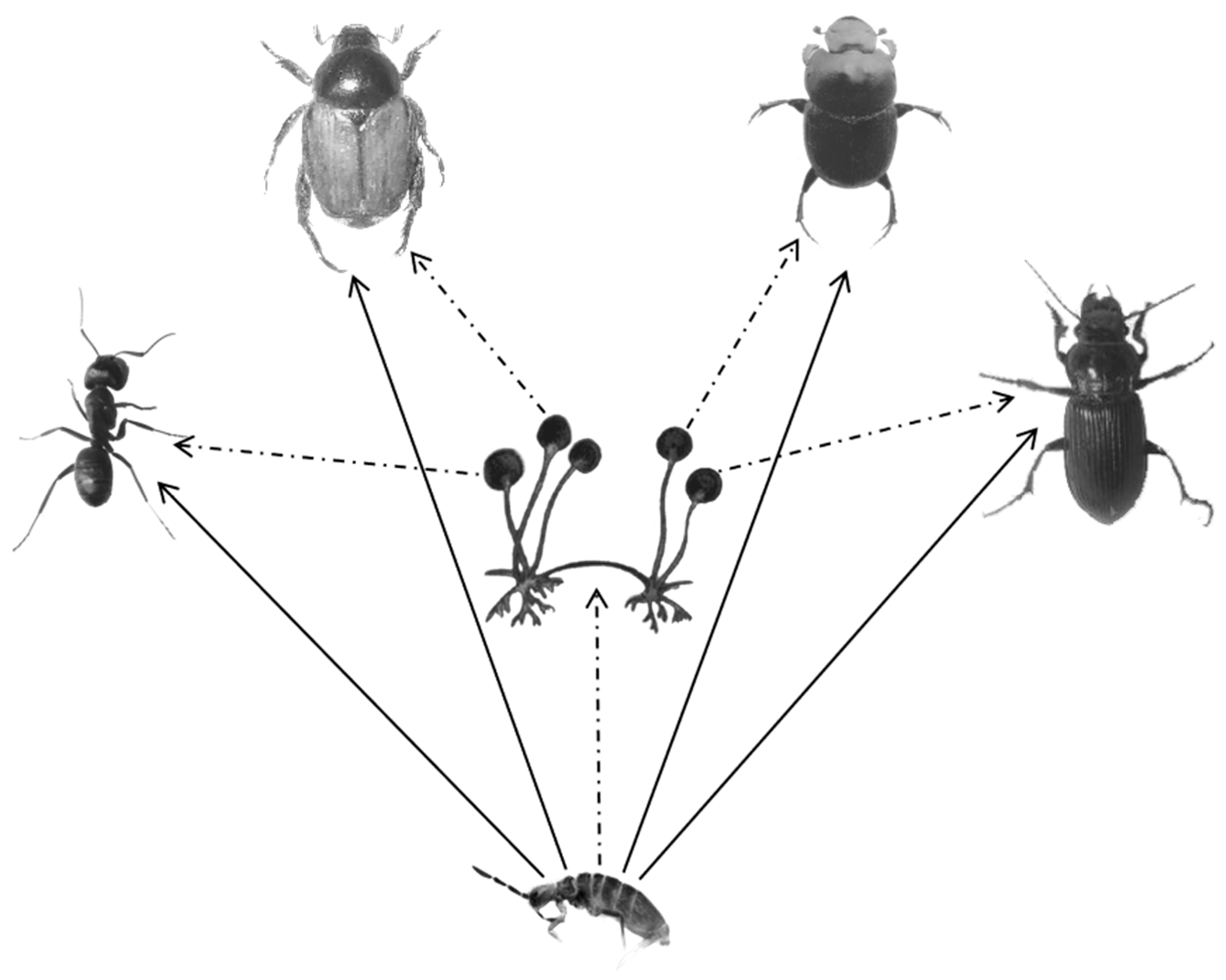
| Insect Carcasses | Food Sources | Stable Isotope Value (δ13C) after Feeding by E. proxima (‰) | δm Value | Median Value of of Each Dietary Aggregate Distribution (%) | Mean Percent Frequencies of Each Dietary Aggregate Distribution |
|---|---|---|---|---|---|
| Dung beetle | Body | −22.0530 | −19.9473 | 72 | 43 |
| Mixed fungi | −13.5415 | 20 | 35 | ||
| Mixed bacteria | −17.1413 | 8 | 26 | ||
| Scarab | Body | −24.7320 | −20.2697 | 62 | 59 |
| Mixed Fungi | −11.6806 | 28 | 36 | ||
| Mixed bacteria | −17.0270 | 10 | 31 | ||
| Ant | Body | −26.3250 | −21.9593 | 60 | 18 |
| Mixed fungi | −12.6193 | 16 | 8 | ||
| Mixed bacteria | −17.0197 | 24 | 6 | ||
| Carabid | Body | −20.7560 | −19.2826 | 75 | 7 |
| Mixed fungi | −13.4896 | 17 | 7 | ||
| Mixed bacteria | −16.7463 | 8 | 5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, L.; Chang, L.; Zhang, S.; Zhu, X.; Adl, S.; Wu, D. What Is the Carcass-Usage Mode of the Collembola? A Case Study of Entomobrya proxima in the Laboratory. Insects 2019, 10, 67. https://doi.org/10.3390/insects10030067
Feng L, Chang L, Zhang S, Zhu X, Adl S, Wu D. What Is the Carcass-Usage Mode of the Collembola? A Case Study of Entomobrya proxima in the Laboratory. Insects. 2019; 10(3):67. https://doi.org/10.3390/insects10030067
Chicago/Turabian StyleFeng, Lichao, Liang Chang, Shaoqing Zhang, Xinyu Zhu, Sina Adl, and Donghui Wu. 2019. "What Is the Carcass-Usage Mode of the Collembola? A Case Study of Entomobrya proxima in the Laboratory" Insects 10, no. 3: 67. https://doi.org/10.3390/insects10030067





