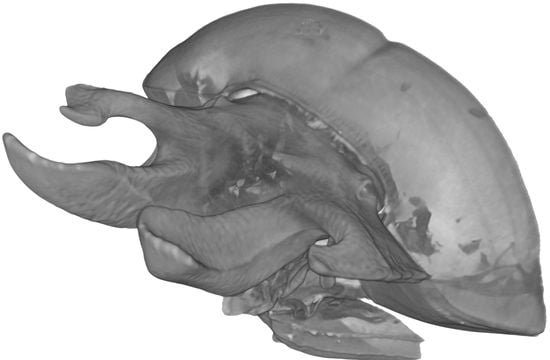
Male Horn Lack of Allometry May be Tied to Food Relocation Behaviour in Lifting Dung Beetles (Coleoptera, Scarabaeidae, Eucraniini)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Traditional Morphometrics Analysis
2.3. Geometric Morphometrics Analysis
3. Results
3.1. Traditional Morphometric Analysis
3.2. Geometric Morphometric Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Holter, P.; Scholtz, C. Re-establishment of biting mouthparts in desert-living Dung Beetles (Scarabaeidae: Scarabaeinae) feeding on plant litter—Old structures reacquired or new ones evolved? J. Morphol. 2011, 272, 1007–1016. [Google Scholar] [CrossRef]
- Ocampo, F.C.; Philips, T.K. Food relocation behavior of the Argentinian dung beetle genus Eucranium Brullé and comparison with the southwest African Scarabaeus (Pachysoma) MacLeay (Coleoptera: Scarabaeidae: Scarabaeinae). Rev. Soc. Entomol. Arg. 2005, 64, 53–59. [Google Scholar]
- Ocampo, F.C. Food relocation behavior and synopsis of the southern South American genus Glyphoderus Westwood (Scarabaeidae: Scarabaeinae: Eucraniini). Coleopts. Bull. 2004, 58, 295–305. [Google Scholar] [CrossRef]
- Ocampo, F.C. Revision of the southern South American endemic genus Anomiopsoides Blackwelder 1944 (Coleoptera: Scarabaeidae: Scarabaeinae: Eucraniini) with description of its food relocation behavior. J. Nat. Hist. 2005, 39, 2537–2557. [Google Scholar] [CrossRef]
- Ocampo, F.C. El género argentino de escarabajos estercoleros Anomiopsoides (Scarabaeidae: Scarabaeinae: Eucraniini): Descripcción de una especie nueva y nuevas sinonimias para A. heteroclyta. Rev. Soc. Entomol. Arg. 2007, 66, 159–168. [Google Scholar]
- Ocampo, F.C. The South American dung beetle genus Ennearabdus Lansberge (Coleoptera: Scarabaeidae: Scarabaeinae: Eucraniini). J. Insect Sci. 2010, 10, 93. [Google Scholar] [CrossRef]
- Ocampo, F.C. A revision of the Argentinean endemic genus Eucranium Brullé (Coleoptera: Scarabaeidae: Scarabaeinae) with description of one new species and new synonymies. J. Insect Sci. 2010, 10, 205. [Google Scholar] [CrossRef]
- Ocampo, F.C.; Hawks, D.C. Molecular phylogenetics and evolution of the food relocation behaviour of the dung beetle tribe Eucraniini (Coleoptera: Scarabaeidae: Scarabaeinae). Invertebr. Syst. 2006, 20, 557–570. [Google Scholar] [CrossRef]
- Phillips, T.K.; Scholtz, C.H.; Ocampo, F.C. A phylogenetic analysis of the Eucraniini (Coleoptera: Scarabaeidae: Scarabaeinae). Insect Syst. Evol. 2002, 33, 241–252. [Google Scholar]
- Löwenberg-Neto, P. Neotropical region: A shapefile of Morrone’s (2014) biogeographical regionalisation. Zootaxa 2014, 3802, 300. [Google Scholar] [CrossRef]
- Morrone, J.J. Biogeographical regionalisation of the Neotropical region. Zootaxa 2014, 3782, 1–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knell, R. Male Contest Competition and the Evolution of Weapons. In Ecology and Evolution of Dung Beetles; Simmons, L.W., Ridsdill-Smith, T.J., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2011; pp. 47–65. [Google Scholar]
- Warren, I.A.; Vera, J.C.; Johns, A.; Zinna, R.; Marden, J.H.; Emlen, D.J.; Dworkin, I.; Lavine, L.C. Insights into the development and evolution of exaggerated traits using de novo transcriptomes of two species of horned scarab beetles. PLoS ONE 2014, 9, e88364. [Google Scholar] [CrossRef] [PubMed]
- Busey, H.A.; Zattara, E.E.; Moczek, A.P. Conservation, innovation, and bias: Embryonic segment boundaries position posterior, but not anterior, head horns in adult beetles. J. Exp. Zool. Part B 2016, 326, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Emlen, D.J. Integrating development with evolution: A case study with beetle horns. BioScience 2000, 50, 403–418. [Google Scholar] [CrossRef]
- Emlen, D.J.; Corley Lavine, L.; Ewen-Campen, B. On the origin and evolutionary diversification of beetle horns. Proc. Natl. Acad. Sci. USA 2007, 104, 8661–8668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerman, K.; Roggero, A.; Rolando, A.; Palestrini, C. Evidence for male horn dimorphism and related pronotal shape variation in Copris lunaris (Linnaeus, 1758) (Coleoptera: Scarabaeidae, Coprini). Insects 2018, 9, A9030108. [Google Scholar] [CrossRef] [PubMed]
- Moczek, A.P. On the Origins of Novelty and diversity in development and evolution: A case study on beetle horns. Cold Spring Harb. Sym. 2009, 74, 1–8. [Google Scholar] [CrossRef]
- Buzatto, B.A.; Tomkins, J.L.; Simmons, L.W. Maternal effects on male weaponry: Female dung beetles produce major sons with longer horns when they perceive higher population density. BMC Evol. Biol. 2012, 12, A118. [Google Scholar] [CrossRef]
- Buzatto, B.A.; Kotiaho, J.S.; Tomkins, J.L.; Simmons, L.W. Intralocus tactical conflict: Genetic correlations between fighters and sneakers of the dung beetle Onthophagus Taurus. J. Evol. Biol. 2015, 28, 730–738. [Google Scholar] [CrossRef]
- McCullough, E.L.; Tobalske, B.W.; Emlen, D.J. Structural adaptations to diverse fighting styles in sexually selected weapons. Proc. Natl. Acad. Sci. USA 2014, 2014, 1–5. [Google Scholar] [CrossRef]
- Simmons, L.W.; Ridsdill-Smith, T.J. Reproductive competition and its impact on the evolution and ecology of dung beetles. In Ecology and Evolution of Dung Beetles; Simmons, L.W., Ridsdill-Smith, T.J., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2011; pp. 1–20. [Google Scholar]
- Moczek, A.P.; Emlen, D.J. Male horn dimorphism in the scarab beetle, Onthophagus taurus: Do alternative reproductive tactics favour alternative phenotypes? Anim. Behav. 2000, 59, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Tarasov, S.; Dimitrov, D. Multigene phylogenetic analysis redefines dung beetles relationships and classification (Coleoptera: Scarabaeidae: Scarabaeinae). BMC Evol. Biol. 2016, 16, A257. [Google Scholar] [CrossRef] [PubMed]
- Vaz-De-Mello, F.Z.; Edmonds, W.D.; Ocampo, F.C.; Schoolmeesters, P. A multilingual key to the genera and subgenera of the subfamily Scarabaeinae of the New World (Coleoptera: Scarabaeidae). Zootaxa 2011, 2854, 1–73. [Google Scholar] [CrossRef] [Green Version]
- Cambefort, Y. From saprophagy to coprophagy. In Dung Beetle Ecology; Hanski, I., Cambefort, Y., Eds.; Princeton University Press: Princeton, NY, USA, 1991; pp. 22–35. [Google Scholar]
- Genise, J.F. Ichnoentomology. Insect Traces in Soils and Paleosoils; Springer: Berlin, Germany, 2017. [Google Scholar]
- Cupello, M.; Vaz-de-Mello, F.Z. Taxonomic revision of the South American dung beetle genus Gromphas Brullé, 1837 (Coleoptera: Scarabaeidae: Scarabaeinae: Phanaeini: Gromphadina). Zootaxa 2013, 3722, 439–482. [Google Scholar] [CrossRef] [PubMed]
- Edmonds, W.D.; Zidek, J. A taxonomic review of the neotropical genus Coprophanaeus Olsoufieff, 1924 (Coleoptera: Scarabaeidae, Scarabaeinae. Insecta Mundi 2010, 649, 129–130. [Google Scholar]
- Price, D. A phylogenetic analysis of the dung beetle genus Phanaeus (Coleoptera: Scarabaeidae) based on morphological data. Insect Syst. Evol. 2007, 38, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Price, D. Phylogeny and biogeography of the dung beetle genus Phanaeus (Coleoptera: Scarabaeidae). Syst. Entomol. 2009, 34, 137–150. [Google Scholar] [CrossRef]
- Casasa, S.; Moczek, A.P. The role of ancestral phenotypic plasticity in evolutionary diversification: Population density effects in horned beetles. Anim. Behav. 2018, 137, 53–61. [Google Scholar] [CrossRef]
- Fusco, G.; Minelli, A. Phenotypic plasticity in development and evolution: Facts and concepts. Philos. T. Roy. Soc. Lond. B 2010, 365, 547–556. [Google Scholar] [CrossRef]
- Kijimoto, T.; Pespeni, M.; Beckers, O.; Moczek, A.P. Beetle horns and horned beetles: Emerging models in developmental evolution and ecology. WIREs Dev. Biol. 2012, 2, 405–418. [Google Scholar] [CrossRef]
- Moczek, A.P. Phenotypic plasticity and the origins of diversity: A case study on horned beetles. In Phenotypic Plasticity of Insects: Mechanisms and Consequences; Whitman, D.W., Ananthakrishnan, T.N., Eds.; Science Publishers: Enfield, CT, USA, 2009; pp. 81–134. [Google Scholar]
- Moczek, A.P. Phenotypic plasticity and diversity in insects. Philos. T. Roy. Soc. Lond. B 2010, 365, 593–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowland, M.J.; Emlen, D.J. Two thresholds, three male forms result in facultative male trimorphism in beetles. Science 2009, 323, 773–776. [Google Scholar] [CrossRef] [PubMed]
- Knell, R.J. On the analysis of non-linear allometries. Ecol. Entomol. 2009, 34, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Mirth, C.K.; Frankino, W.A.; Shingleton, A.W. Allometry and size control: What can studies of body size regulation teach us about the evolution of morphological scaling relationships? Curr. Opin. Insect Sci. 2016, 13, 93–98. [Google Scholar] [CrossRef]
- Pélabon, C.; Hansen, T.F. On the adaptive accuracy of directional asymmetry in insect wing size. Evolution 2008, 62, 2855–2867. [Google Scholar] [CrossRef]
- Brakefield, P.M. Evo-devo and constraints on selection. Trends Ecol. Evol. 2006, 21, 362–368. [Google Scholar] [CrossRef]
- Tucić, B.; Budečević, S.; Manitašević Jovanović, S.; Vuleta, A.; Klingenberg, C.P. Phenotypic plasticity in response to environmental heterogeneity contributes to fluctuating asymmetry in plants: First empirical evidence. J. Evol. Biol. 2018, 31, 197–210. [Google Scholar] [CrossRef]
- Klingenberg, C.P. Analyzing fluctuating asymmetry with geometric morphometrics: Concepts, methods, and applications. Symmetry 2015, 7, 843–934. [Google Scholar] [CrossRef]
- Klingenberg, C.P.; Barluenga, M.; Meyer, A. Shape analysis of symmetric structures: Quantifying variation among individuals and asymmetry. Evolution 2002, 56, 1909–1920. [Google Scholar] [CrossRef]
- McCullough, E.L.; Ledger, K.J.; O’Brien, D.M.; Emlen, D.J. Variation in the allometry of exaggerated rhinoceros beetle horns. Anim. Behav. 2015, 109, 133–140. [Google Scholar] [CrossRef] [Green Version]
- Eberhard, W.G.; Rodríguez, R.L.; Huber, B.A.; Speck, B.; Miller, H.; Buzatto, B.A.; Machado, G. Sexual selection and static allometry: The importance of function. Q. Rev. Biol. 2018, 93, 207–250. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. Past: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Polilov, A.A.; Makarova, A.A. The scaling and allometry of organ size associated with miniaturization in insects: A case study for Coleoptera and Hymenoptera. Sci. Rep. UK 2017, 7, 43095. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.C.; Rohlf, F.J.; Slice, D.E. A field comes of age: Geometric morphometrics in the 21st century. Hystrix 2013, 24, 7–14. [Google Scholar]
- Mitteroecker, P.; Gunz, P. Advances in geometric morphometrics. Evol. Biol. 2009, 36, 235–247. [Google Scholar] [CrossRef]
- Palestrini, C.; Roggero, A.; Hernandez Nova, L.K.; Giachino, P.M.; Rolando, A. On the evolution of shape and size divergence in Nebria (Nebriola) ground beetles (Coleoptera, Carabidae). Syst. Biodivers. 2012, 10, 147–157. [Google Scholar] [CrossRef]
- Roggero, A.; Giachino, P.M.; Palestrini, C. A new cryptic ground beetle species from the Alps characterised via geometric morphometrics. Contrib. Zool. 2013, 82, 171–183. [Google Scholar] [CrossRef] [Green Version]
- Tocco, C.; Roggero, A.; Rolando, A.; Palestrini, C. Inter-specific shape divergence in Aphodiini dung beetles: The case of Amidorus obscurus and A. immaturus. Org. Divers. Evol. 2011, 11, 263–273. [Google Scholar] [CrossRef]
- Webster, M.; Sheets, H.D. Introduction to landmark-based geometric morphometrics. Paleontol. Soc. Pap. 2010, 16, 163–188. [Google Scholar] [CrossRef]
- Wrozyna, C.; Neubauer, T.A.; Meyer, J.; Piller, W.E. Shape variation in Neotropical Cytheridella (Ostracoda) using semilandmarks-based geometric morphometrics: A methodological approach and possible biogeographical implications. PLoS ONE 2016, 11, e0168438. [Google Scholar] [CrossRef]
- tpsDig version 2.31. Free Software, Available at the Stony Brook New York State University URL. Available online: http://life.bio.sunysb.edu/morph/ (accessed on 4 September 2019).
- tpsUtil version 1.79. Free Software, Available at the Stony Brook New York State University URL. Available online: http://life.bio.sunysb.edu/morph/ (accessed on 4 September 2019).
- tpsRelw version 1.70. Free Software, Available at the Stony Brook New York State University URL. Available online: http://life.bio.sunysb.edu/morph/ (accessed on 4 September 2019).
- tpsRegr version 1.45. Free Software, Available at the Stony Brook New York State University URL. Available online: http://life.bio.sunysb.edu/morph/ (accessed on 4 September 2019).
- tpsPLS version 1.23. Free Software, Available at the Stony Brook New York State University URL. Available online: http://life.bio.sunysb.edu/morph/ (accessed on 4 September 2019).
- Shingleton, A.W.; Mirth, C.K.; Bates, P.W. Developmental model of static allometry in holometabolous insects. P. Roy. Soc. B-Biol. Sci. 2008, 275, 1875–1885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tobler, A.; Nijhout, H.F. Developmental constraints on the evolution of wing-body allometry in Manduca sexta. Evol. Dev. 2010, 12, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Armbruster, W.S.; Pélabon, C.; Bolstad, G.H.; Hansen, T.F. Integrated phenotypes: Understanding trait covariation in plants and animals. Philos. Trans. R. Soc. Lond. B 2014, 369, 20130245. [Google Scholar] [CrossRef] [PubMed]
- Klingenberg, C.P. Studying morphological integration and modularity at multiple levels: Concepts and analysis. Philos. Trans. R. Soc. Lond. B 2014, 369, A20130249. [Google Scholar] [CrossRef] [PubMed]
- Conner, J.K.; Cooper, I.A.; La Rosa, R.J.; Pérez, S.G.; Royer, A.M. Patterns of phenotypic correlations among morphological traits across plants and animals. Philos. Trans. R. Soc. Lond. B 2014, 369, A20130246. [Google Scholar] [CrossRef] [PubMed]
- Voje, K.L.; Hansen, T.F.; Egset, C.; Bolstad, G.H.; Pélabon, C. Allometric constraints and the evolution of allometry. Evolution 2014, 68, 866–885. [Google Scholar] [CrossRef]
- Perl, C.D.; Rossoni, S.; Niven, J.E. Conservative whole-organ scaling contrasts with highly labile suborgan scaling differences among compound eyes of closely related Formica ants. Ecol. Evol. 2017, 7, 1663–1673. [Google Scholar] [CrossRef]
- Nijhout, H.F.; Riddiford, L.M.; Mirth, C.; Shingleton, A.W.; Suzuki, Y.; Callier, V. The developmental control of size in insects. WIREs Dev. Biol. 2014, 3, 113–134. [Google Scholar] [CrossRef]
- Pélabon, C.; Bolstad, G.H.; Egset, C.K.; Cheverud, J.M.; Pavlicev, M.; Rosenqvist, G. On the relationship between ontogenetic and static allometry. Am. Nat. 2013, 181, 195–212. [Google Scholar] [CrossRef]
- Shingleton, A.W.; Frankino, W.A.; Flatt, T.; Nijhout, H.F.; Emlen, D.J. Size and shape: The developmental regulation of static allometry in insects. BioEssays 2007, 29, 536–548. [Google Scholar] [CrossRef]
- Koyama, T.; Mirth, C.K. Unravelling the diversity of mechanisms through which nutrition regulates body size in insects. Curr. Opin. Insect Sci. 2018, 25, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Emlen, D.J. Alternative reproductive tactics and male-dimorphism in the horned beetle Onthophagus acuminatus (Coleoptera: Scarabaeidae). Behav. Ecol. Sociobiol. 1997, 41, 335–341. [Google Scholar] [CrossRef]
- Monteresino, E.M.; Zunino, M. Sobre el comportamiento de la alimentación de Eucraniini (Coleoptera: Scarabaeidae: Scarabaeinae). In Escarabeidos de Latinoamerica. Estado Actual del Conocimiento; Onore, G., Reyes Castillo, P., Zunino, M., Eds.; Boletin SEA: Zaragoza, Spain, 2003; pp. 75–80. [Google Scholar]
- Zunino, M.; Barbero, E.; Luzzatto, M. Food relocation behavior in Eucraniina beetles (Scarabaeidae) and the constraints of xeric environment. Trop. Zool. 1989, 2, 235–240. [Google Scholar] [CrossRef]
- Holló, G. Demystification of animal symmetry: Symmetry is a response to mechanical forces. Biol. Direct 2017, 12, 11. [Google Scholar] [CrossRef]





| Range | Min | Max | Mean | Std Error | Std Dev | Variance | ||
|---|---|---|---|---|---|---|---|---|
| cavifrons | W_pronotum | 3.664 | 4.958 | 8.622 | 6.472 | 0.092 | 0.886 | 0.786 |
| L_horn 1 | 1.124 | 1.115 | 2.239 | 1.658 | 0.028 | 0.272 | 0.074 | |
| Ls_horn 1 | 1.573 | 1.653 | 3.226 | 2.409 | 0.040 | 0.383 | 0.147 | |
| L_horn 2 | 0.860 | 1.375 | 2.235 | 1.748 | 0.022 | 0.213 | 0.045 | |
| L_tibia | 1.880 | 2.357 | 4.237 | 3.201 | 0.047 | 0.448 | 0.201 | |
| heteroclyta | W_pronotum | 3.769 | 8.961 | 12.730 | 10.585 | 0.262 | 1.049 | 1.101 |
| L_horn 1 | 1.409 | 2.383 | 3.792 | 3.020 | 0.119 | 0.477 | 0.227 | |
| Ls_horn 1 | 2.135 | 3.276 | 5.411 | 4.440 | 0.163 | 0.654 | 0.427 | |
| L_horn 2 | 1.350 | 2.844 | 4.194 | 3.415 | 0.095 | 0.382 | 0.146 | |
| L_tibia | 2.048 | 4.394 | 6.442 | 5.396 | 0158 | 0.633 | 0.401 |
| Name of the Specie | Cross Set Analysis | r | Statistics for Shape1 Projections | Statistics for Shape2 Projections | Permutation Tests | ||||
|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Std Dev | Min | Max | Std Dev | % of Correlations ≥ Observed | |||
| cavifrons | 77.363 | 0.998 | −0.077 | 0.053 | 0.028 | −0.077 | 0.052 | 0.027 | 0.10 |
| heteroclyta | 69.536 | 0.999 | −0.047 | 0.066 | 0.034 | −0.047 | 0.066 | 0.034 | 0.10 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palestrini, C.; Barbero, E.; Roggero, A. Male Horn Lack of Allometry May be Tied to Food Relocation Behaviour in Lifting Dung Beetles (Coleoptera, Scarabaeidae, Eucraniini). Insects 2019, 10, 359. https://doi.org/10.3390/insects10100359
Palestrini C, Barbero E, Roggero A. Male Horn Lack of Allometry May be Tied to Food Relocation Behaviour in Lifting Dung Beetles (Coleoptera, Scarabaeidae, Eucraniini). Insects. 2019; 10(10):359. https://doi.org/10.3390/insects10100359
Chicago/Turabian StylePalestrini, Claudia, Enrico Barbero, and Angela Roggero. 2019. "Male Horn Lack of Allometry May be Tied to Food Relocation Behaviour in Lifting Dung Beetles (Coleoptera, Scarabaeidae, Eucraniini)" Insects 10, no. 10: 359. https://doi.org/10.3390/insects10100359