Use of Flowering Plants to Enhance Parasitism and Predation Rates on Two Squash Bug Species Anasa tristis and Anasa armigera (Hemiptera: Coreidae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Experiment
2.2. Statistical Analysis
3. Results
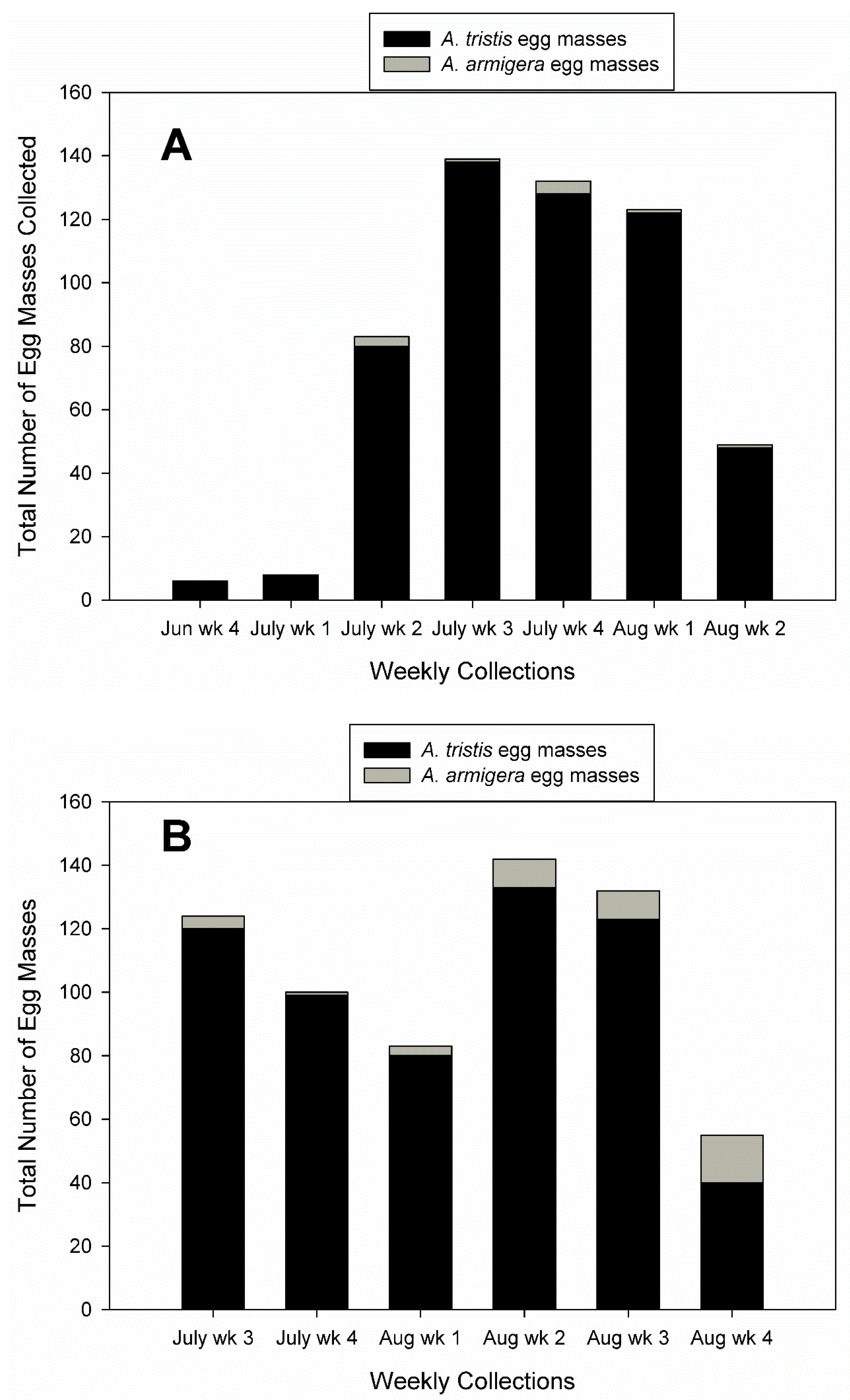
3.1. Field Collections of Egg Masses and Adults
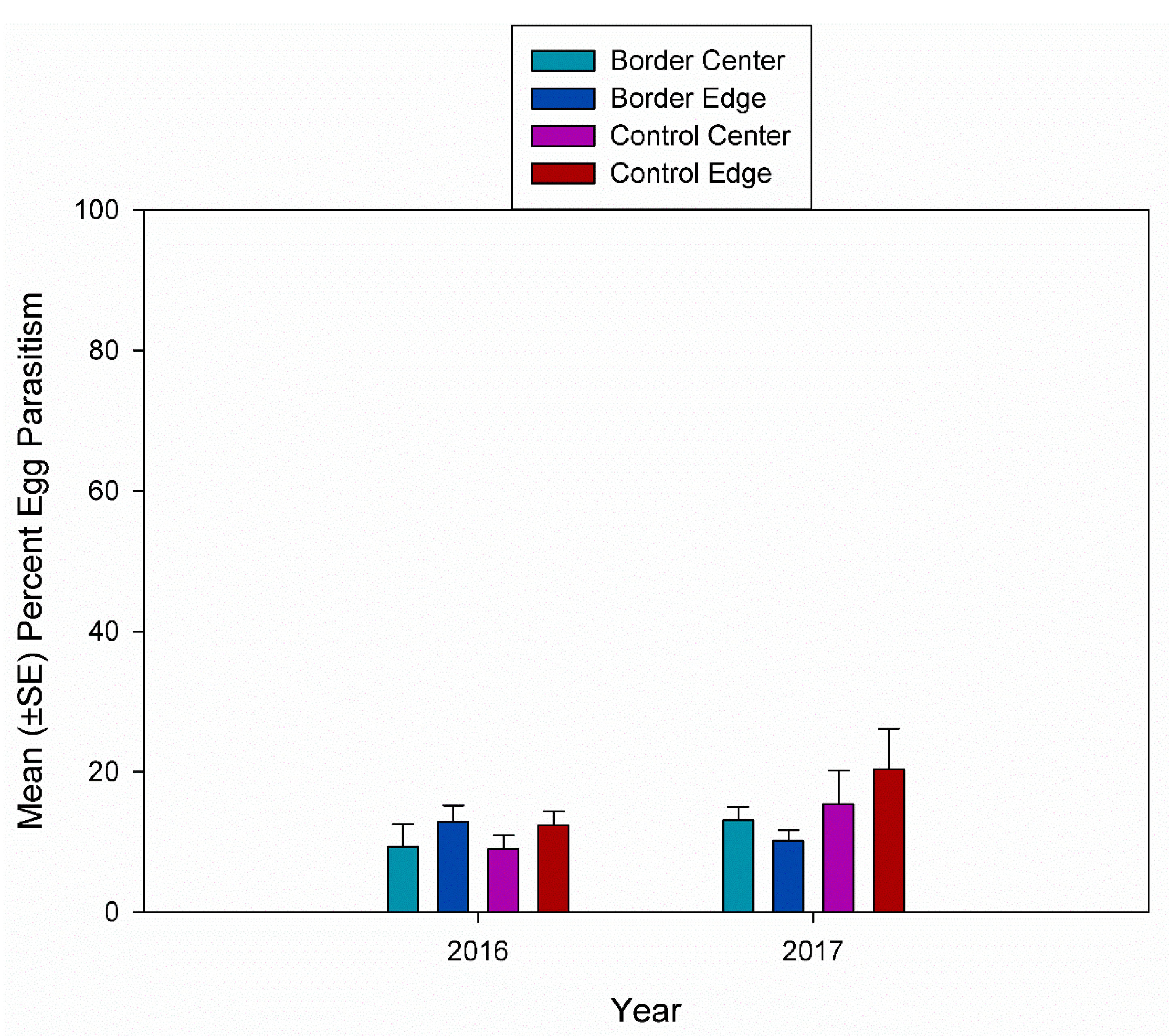
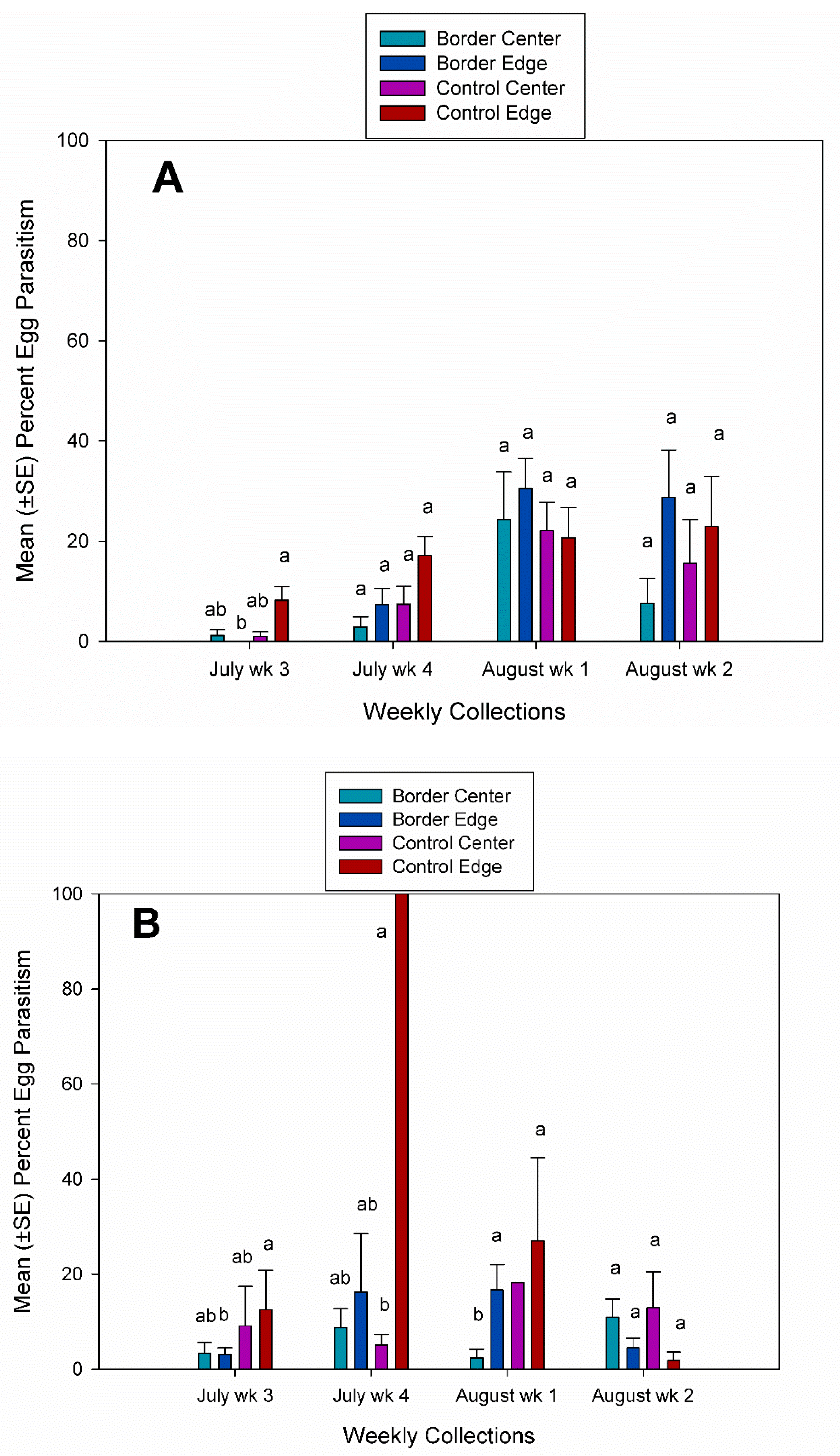
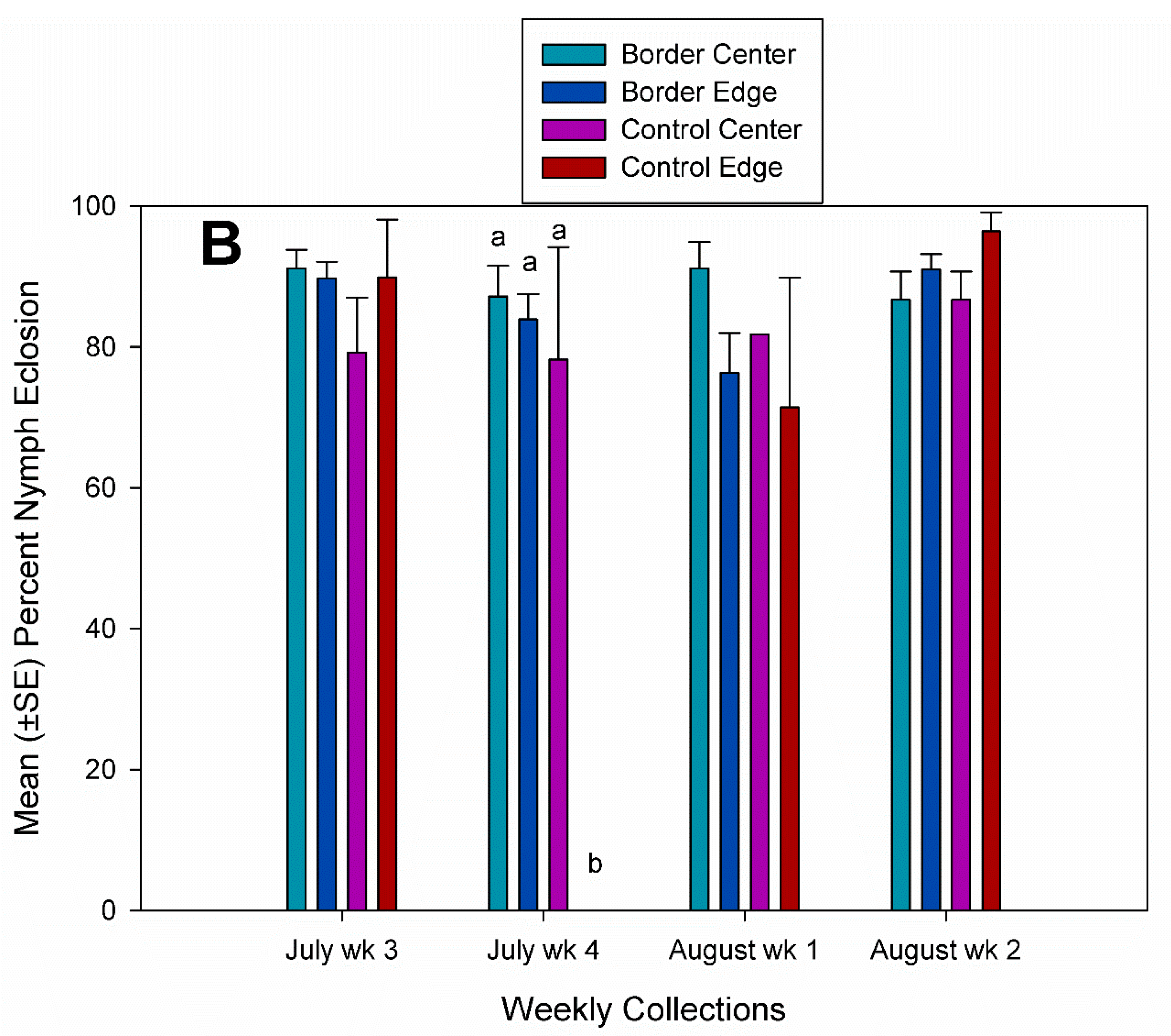
3.2. Egg Parasitism, Egg Predation, and Nymph Eclosion
3.3. Adult Parasitism
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sánchez-Bayo, F.; Wyckhuys, A.G. Worldwide decline of the entomofauna: A review of its drivers. Biol. Conserv. 2019, 232, 8–27. [Google Scholar] [CrossRef]
- Lin, B.B.; Philpott, S.M.; Jha, S. The future of urban agriculture and biodiversity ecosystem services: Challenges and next steps. Basic Appl. Ecol. 2015, 16, 189–201. [Google Scholar] [CrossRef]
- Landis, D.A.; Menalled, F.D.; Costamagna, A.C.; Wilkinson, T.K. Manipulating plant resources to enhance beneficial arthropods in agricultural landscapes. Weed Sci. 2005, 53, 902–908. [Google Scholar] [CrossRef]
- Buhk, C.; Oppermann, R.; Schanowski, A.; Bleil, R.; Lüdemann, J.; Maus, C. Flower strip networks offer promising long term effects on pollinator species richness in intensively cultivated agricultural areas. BMC Ecol. 2018, 18, 55. [Google Scholar] [CrossRef] [PubMed]
- Idris, A.B.; Grafius, E. Wildflowers as nectar sources for Diadegma insulare (Hymenoptera: Ichneumonidae), a parasitoid of diamondback moth (Lepidoptera: Yponomeutidae). Environ. Entomol. 1995, 24, 1726–1735. [Google Scholar] [CrossRef]
- Witting-Bissinger, B.E.; Orr, D.B.; Linker, H.M. Effects of floral resources on fitness of the parasitoids Trichogramma exiguum (Hymenoptera: Trichogrammatidae) and Cotesia congregata (Hymenoptera: Braconidae). Biol. Control 2008, 47, 180–186. [Google Scholar] [CrossRef]
- Díaz, M.F.; Ramírez, A.; Poveda, K. Efficiency of different egg parasitoids and increased floral diversity for the biological control of noctuid pests. Biol. Control 2012, 60, 182–191. [Google Scholar] [CrossRef]
- Aduba, O.L.; Olson, D.M.; Ruberson, J.R.; Hartel, P.G.; Potter, T.L. Flowering plant effects on adults of the stink bug parasitoid Aridelus rufotestaceus (Hymenoptera: Braconidae). Biol. Control 2013, 67, 344–349. [Google Scholar] [CrossRef]
- Balmer, O.; Géneau, C.E.; Belz, E.; Weishaupt, B.; Förderer, G.; Moos, S.; Ditner, N.; Juric, I.; Luka, H. Wildflower companion plants increase pest parasitation and yield in cabbage fields: Experimental demonstration and call for caution. Biol. Control 2014, 76, 19–27. [Google Scholar] [CrossRef]
- Araj, S.-E.; Wratten, S.D. Comparing existing weeds and commonly used insectary plants as floral resources for a parasitoid. Biol. Control 2015, 81, 15–20. [Google Scholar] [CrossRef]
- Tillman, P.G.; Carpenter, J.E. Milkweed (Gentianales: Apocynaceae): A farmscape resource for increasing parasitism of stink bugs (Hemiptera: Pentatomidae) and providing nectar to insect pollinators and monarch butterflies. Environ. Entomol. 2014, 43, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Landis, D.A.; Wratten, S.D.; Gurr, G.M. Habitat management to conserve natural enemies of arthropod pests in agriculture. Annu. Rev. Entomol. 2000, 45, 175–201. [Google Scholar] [CrossRef] [PubMed]
- Gontijo, L.M.; Beers, E.H.; Snyder, W.E. Flowers promote aphid suppression in apple orchards. Biol. Control 2013, 66, 8–15. [Google Scholar] [CrossRef]
- Tschumi, M.; Albrecht, M.; Collatz, J.; Dubsky, V.; Entling, M.H.; Najar-Rodriguez, A.J.; Jacot, K. Tailored flower strips promote natural enemy biodiversity and pest control in potato crops. J. Appl. Ecol. 2016, 53, 1169–1176. [Google Scholar] [CrossRef] [Green Version]
- Balmer, O.; Pfiffner, L.; Schied, J.; Willareth, M.; Leimgruber, A.; Luka, H.; Traugott, M. Noncrop flowering plants restore top-down herbivore control in agricultural fields. Ecol. Evol. 2013, 3, 2634–2646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, B.W.; Gardiner, M.M. Does local habitat management or large-scale landscape composition alter the biocontrol services provided to pumpkin agroecosystems? Biol. Control 2016, 92, 181–194. [Google Scholar] [CrossRef]
- Fair, C.G.; Braman, S.K. Assessment of habitat modification and varied planting dates to enhance potential natural enemies of Anasa tristis (Hemiptera: Coreidae) in squash. Environ. Entomol. 2017, 46, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Kahn, B.A.; Rebek, E.J.; Brandenberger, L.P.; Reed, K.; Payton, M.E. Companion planting with white yarrow or with feverfew for squash bug, Anasa tristis (Hemiptera: Coreidae), management on summer squash. Pest Manag. Sci. 2017, 73, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Quinn, N.F.; Brainard, D.C.; Szendreii, Z. Floral strips attract beneficial insects but do not enhance yield in cucumber fields. J. Econ. Entomol. 2017, 110, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Belz, E.; Kölliker, M.; Balmer, O. Olfactory attractiveness of flowering plants to the parasitoid Microplitis mediator: Potential implications for biological control. BioControl 2013, 58, 163–173. [Google Scholar] [CrossRef]
- Sigsgaard, L.; Betzer, C.; Naulin, C.; Eilenberg, J.; Enkegaard, A.; Kristensen, K. The effect of floral resources on parasitoid and host longevity: Prospects for conservation biological control in strawberries. J. Insect Sci. 2013, 13, 1–17. [Google Scholar] [CrossRef] [PubMed]
- James, D.G.; Seymour, L.; Lauby, G.; Buckley, K. Beneficial insects attracted to native flowering buckwheats (Eriogonum Michx) in Central Washington. Environ. Entomol. 2014, 43, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.W.; Irvin, A.; Irvin, H.; Stanley-Stahr, C.; Ellis, J.D. Insect visitors to flowering buckwheat, Fagopyrum esculentum (Polygonales: Polygonaceae), in north–central Florida. Fla. Entomol. 2016, 99, 264–268. [Google Scholar] [CrossRef]
- Lahiri, S.; Orr, D.; Yasmin, J.; Sorenson, C. Longevity and fecundity of the egg parasitoid Telenomus podisi provided with different carbohydrate diets. Entomol. Exp. Appl. 2017, 162, 178–187. [Google Scholar] [CrossRef]
- Tillman, P.G.; Khrimian, A.; Cottrell, T.E.; Luo, X.; Mizell, R.F., III; Johnson, J. Trap cropping systems and a physical barrier for suppression of stink bug (Hemiptera: Pentatomidae) in cotton. J. Econ. Entomol. 2015, 108, 2324–2334. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Heimpel, G.E. Impact of flowering buckwheat on Lepidopteran cabbage pests and their parasitoids at two spatial scales. Biol. Control 2005, 34, 290–301. [Google Scholar] [CrossRef]
- Berndt, L.A.; Wratten, S.D.; Scarratt, S.L. The influence of floral resource subsidies on parasitism rates of leafrollers (Lepidoptera: Tortricidae) in New Zealand vineyards. Biol. Control 2006, 37, 50–55. [Google Scholar] [CrossRef]
- Hooks, C.R.R.; Valenzuela, H.R.; Defrank, J. Incidence of pests and arthropod natural enemies in zucchini grown with living mulches. Agric. Ecosys. Environ. 1998, 69, 217–231. [Google Scholar] [CrossRef]
- Frank, D.L.; Liburd, O.E. Effects of living and synthetic mulch on the population dynamics of whiteflies and aphids, their associated natural enemies and insect-transmitted plant diseases in zucchini. Environ. Entomol. 2005, 34, 857–865. [Google Scholar] [CrossRef]
- Nyoike, T.W.; Liburd, O.E. Effect of living (buckwheat) and UV reflective mulches with and without imidacloprid on whiteflies, aphids and marketable yields of zucchini squash. Int. J. Pest Manag. 2010, 56, 31–39. [Google Scholar] [CrossRef]
- Pair, S.D.; Bruton, B.D.; Mitchell, F.; Fletcher, J.; Wayadande, A.; Melcher, U. Overwintering squash bugs harbor and transmit the causal agent of cucurbit yellow vine disease. J. Econ. Entomol. 2004, 97, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Bruton, B.D.; Mitchell, F.; Fletcher, J.; Pair, S.D.; Wayadande, A.; Melcher, U.; Brady, J.; Bextine, B.; Popham, T.W. Serratia marcescens, a phloem-colonizing, squash bug-transmitted bacterium: Causal agent of cucurbit yellow vine disease. Plant Dis. 2003, 87, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Wayadande, A.; Bruton, B.; Fletcher, J.; Pair, S.; Mitchell, F. Retention of cucurbit yellow vine disease bacterium Serratia marcescens through transstadial molt of vector Anasa tristis (Hemiptera: Coreidae). Ann. Entomol. Soc. Am. 2005, 98, 770–774. [Google Scholar] [CrossRef]
- Doughty, H.B.; Wilson, J.M.; Schultz, P.B.; Kuhar, T.P. Squash bug (Hemiptera: Coreidae): Biology and management in cucurbitaceous crops. J. Integr. Pest Manag. 2016, 7, 1–8. [Google Scholar] [CrossRef]
- Drake, C.J.; Harris, H.M. Insect enemies of melons and cucumbers in Iowa. Bull. Iowa Agric. Exp. Stn. 1926, 90, 1–12. [Google Scholar]
- Britton, W.E. Connecticut State Entomologist, Thirty-sixth Report 1936; Connecticut Agricultural Experiment Station Bulletin; Forgotten Books: London, UK, 1937; pp. 289–415. [Google Scholar]
- Gould, G.E. Insect pests of cucurbit crops in Indiana. Proc. Indiana Acad. Sci. 1944, 53, 165–171. [Google Scholar]
- Cornelius, M.L.; Hu, J.S.; Vinyard, B.T. Comparative study of egg parasitism by Gryon pennsylvanicum (Hymenoptera: Scelionidae) on two squash bug species Anasa tristis and Anasa armigera (Hemiptera: Coreidae). Environ. Entomol. 2018, 47, 1451–1458. [Google Scholar] [CrossRef]
- Cornelius, M.L. Ovipositional preferences of two squash bug species, the common squash bug, Anasa tristis, and the horned squash bug, Anasa armigera (Heteroptera: Coreidae) for different cultivars and species of Cucurbitaceae. J. Insect Sci. 2018, 18, 30. [Google Scholar] [CrossRef]
- Nechols, J.R.; Tracy, J.L.; Vogt, E.A. Comparative ecological studies of indigenous egg parastoids (Hymenoptera: Scelionidae; Encyrtidae) of the squash bug Anasa tristis (Hemiptera: Coreidae). J. Kans. Entomol. Soc. 1989, 62, 177–188. [Google Scholar]
- Wilson, J.M.; Kuhar, T.P. A survey of the species of squash bug (Hemiptera: Coreidae) egg parasitoids in Virginia and their distribution. J. Econ. Entomol. 2017, 110, 2727–2730. [Google Scholar] [CrossRef]
- Worthley, H.N. The biology of Trichopoda pennipes Fab. (Diptera, Tachinidae), a parasite of the common squash bug. Psyche 1924, 31, 57–77. [Google Scholar] [CrossRef]
- Beard, R.L. The Biology of Anasa tristis DeGeer, with Particular Reference to the Tachinid Parasite Trichopoda pennipes Fabr; Bulletin 440; Connecticut Agricultural Experiment Station: New Haven, CT, USA, 1940. [Google Scholar]
- Cornelius, M.L.; Buffington, M.L.; Talamas, E.J.; Gates, M.W. Impact of the egg parasitoid, Gryon pennsylvanicum (Hymenoptera: Scelionidae), on sentinel and wild egg masses of the squash bug (Hemiptera: Coreidae) in Maryland. Environ. Entomol. 2016, 45, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Decker, K.B.; Yeargan, K.V. Seasonal phenology and natural enemies of the squash bug (Hemiptera: Coreidae) in Kentucky. Environ. Entomol. 2008, 37, 670–678. [Google Scholar] [CrossRef]
- Schmidt, J.M.; Barney, S.K.; Williams, M.A.; Bessin, R.T.; Coolong, T.W.; Hardwood, J.D. Predator–prey trophic relationships in response to organic management practices. Mol. Ecol. 2014, 23, 3777–3789. [Google Scholar] [CrossRef] [PubMed]
- Heraty, J.; Hawks, D. Hexamethyldisilazane—A chemical alternative for drying insects. Entomol. News 1998, 109, 369–374. [Google Scholar]
- Gibson, G.A.P.; Huber, J.T.; Woolley, J.B. (Eds.) Annotated Keys to the Genera of Nearctic Chalcidoidea (Hymenoptera); NRC Research Press: Ottawa, ON, Canada, 1997; p. 794. [Google Scholar]
- Noyes, J.S. Universal Chalcidoidea Database. World Wide Web Electronic Publication. 2009. Available online: http://www.nhm.ac.uk/chalcidoids (accessed on 20 March 2019).
- SAS. SAS v9.4, SAS/STAT v15.1, PROC GLIMMIX; SAS Institute Inc.: Cary, NC, USA, 2018. [Google Scholar]
- Saxton, A.M. A macro for converting mean separation output to letter groupings in Proc Mixed. In Proceedings of the 23rd SAS Users Group International, Nashville, TN, USA, 22–25 March 1998; SAS Institute: Cary, NC, USA; pp. 1243–1246. [Google Scholar]
- Olson, D.L.; Nechols, J.R. Effects of squash leaf trichome exudates and honey on adult feeding, survival, and fecundity of the squash bug (Heteroptera: Coreidae) egg parasitoid Gryon pennsylvanicum (Hymenoptera: Scelionidae). Environ. Entomol. 1995, 24, 454–458. [Google Scholar] [CrossRef]
- Liu, H.; Mottern, J. An old remedy for a new problem? Identification of Ooencyrtus kuvanae (Hymenoptera: Encyrtidae), an egg parasitoid of Lycorma delicatula (Hemiptera: Fulgoridae) in North America. J. Insect Sci. 2017, 17, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Tillman, P.G. Stink bugs (Heteroptera: Pentatomidae) and their natural enemies in alfalfa in South Georgia. J. Entomol. Sci. 2013, 48, 1–8. [Google Scholar] [CrossRef]
- Gren, A.; Andersson, E. Being efficient and green by rethinking the urban-rural divide—Combining urban expansion and food production by integrating an ecosystem service perspective into urban planning. Sustain. Cities Soc. 2018, 40, 75–82. [Google Scholar] [CrossRef]





| Variable | Effect | F Statistic | df a | p Value * |
|---|---|---|---|---|
| Egg Parasitism | Treatment | 0.4 | 1 | 0.5 |
| Plot Location | 3.7 | 1 | 0.09 | |
| Year | 0.6 | 1 | 0.47 | |
| Week | 21.5 | 3 | <0.0001 * | |
| Treatment × Plot Location | 1.6 | 1 | 0.24 | |
| Year × Treatment | 0.3 | 1 | 0.63 | |
| Year × Plot Location | 0.7 | 1 | 0.44 | |
| Year × Week | 11.4 | 3 | <0.0001 | |
| Treatment × Week | 8.7 | 3 | <0.0001 * | |
| Plot Location × Week | 3.7 | 3 | 0.01 * | |
| Treatment × Year × Week | 2.5 | 3 | 0.06 | |
| Plot Location × Year × Week | 15.1 | 3 | <0.0001 * | |
| Treatment × Plot Location × Week | 2.9 | 3 | 0.03 * | |
| Treatment × Plot Location × Year | 5.1 | 1 | 0.05 * | |
| Treatment × Plot Location × Year × Week | 12.8 | 2 | <0.0001 * | |
| Nymph Eclosion | Treatment | 11.0 | 1 | 0.001 * |
| Plot Location | 12.0 | 1 | 0.0006 * | |
| Year | 2.2 | 1 | 0.14 | |
| Week | 7.5 | 3 | <0.0001 * | |
| Treatment × Plot Location | 7.2 | 1 | 0.008 * | |
| Year × Treatment | 9.3 | 1 | 0.002 * | |
| Year × Plot Location | 5.4 | 1 | 0.02 * | |
| Year × Week | 6.5 | 3 | 0.0002 * | |
| Treatment × Week | 7.5 | 3 | <0.0001 * | |
| Plot Location × Week | 6.0 | 3 | 0.0005 * | |
| Treatment × Year × Week | 5.2 | 3 | 0.002 * | |
| Plot Location × Year × Week | 7.4 | 3 | <0.0001 * | |
| Treatment × Plot Location × Week | 5.9 | 3 | 0.0005 * | |
| Treatment × Plot Location × Year | 7.5 | 1 | 0.006 * | |
| Treatment × Plot Location × Year × Week | 6.7 | 3 | 0.0002 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cornelius, M.L.; Vinyard, B.T.; Gates, M.W. Use of Flowering Plants to Enhance Parasitism and Predation Rates on Two Squash Bug Species Anasa tristis and Anasa armigera (Hemiptera: Coreidae). Insects 2019, 10, 318. https://doi.org/10.3390/insects10100318
Cornelius ML, Vinyard BT, Gates MW. Use of Flowering Plants to Enhance Parasitism and Predation Rates on Two Squash Bug Species Anasa tristis and Anasa armigera (Hemiptera: Coreidae). Insects. 2019; 10(10):318. https://doi.org/10.3390/insects10100318
Chicago/Turabian StyleCornelius, Mary L., Bryan T. Vinyard, and Michael W. Gates. 2019. "Use of Flowering Plants to Enhance Parasitism and Predation Rates on Two Squash Bug Species Anasa tristis and Anasa armigera (Hemiptera: Coreidae)" Insects 10, no. 10: 318. https://doi.org/10.3390/insects10100318






