A Phase I Study of High-Dose Calcitriol in Combination with Temozolomide for Patients with Metastatic Melanoma
Abstract
:1. Introduction
2. Experimental
2.1. Patient Eligibility/Selection
2.2. Study Design
2.3. Patient Evaluation
2.4. Vitamin D Receptor SNP Genotyping
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
| Characteristic | n = 20 |
|---|---|
| Median Age (range) | 58 (30–86) |
| Race, n (%) | |
| Caucasian | 20 (100) |
| Gender, n (%) | |
| Male | 15 (75) |
| Female | 5 (25) |
| Median time from initial diagnosis, years (range) | 2.4 (0.6–10) |
| ECOG performance status, n (%) | |
| 0 | 9 (45) |
| 1 | 10 (50) |
| 2 | 1 (5) |
3.2. Drug Exposure
3.3. Safety/Toxicity
| Toxicity | No. | % |
|---|---|---|
| Thrombocytopenia | 2 | 10 |
| Vascular | 2 | 10 |
| Nausea/vomiting | 1 | 5 |
| Leukopenia | 2 | 10 |
| Fatigue | 1 | 5 |
| Anemia | 2 | 10 |
| Lymphopenia | 2 | 10 |
| Hemorrhage | 1 | 5 |
| Rash | 1 | 5 |
| Anorexia | 1 | 5 |
3.4. Efficacy Evaluation
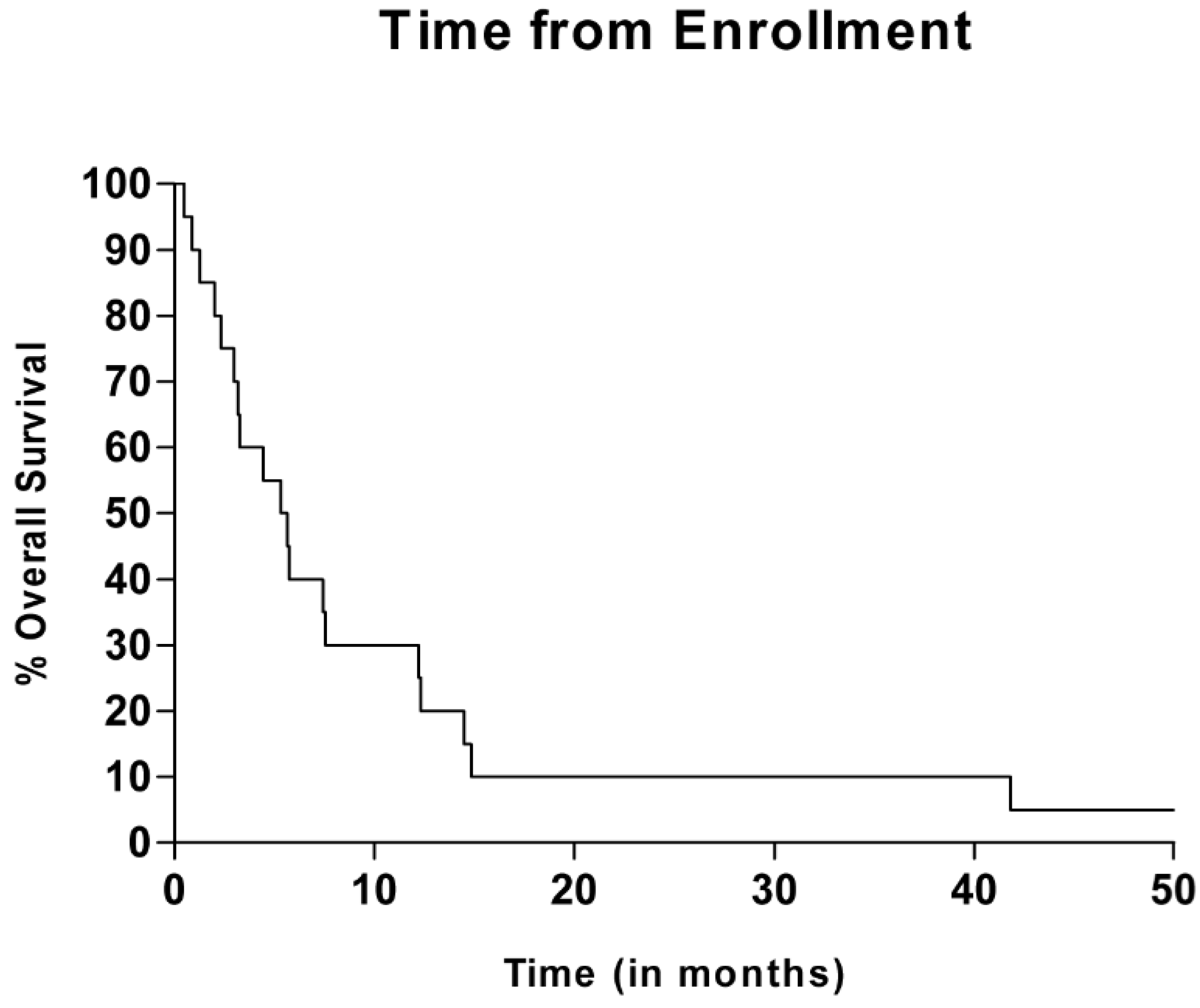
3.5. VDR Polymorphism Evaluation
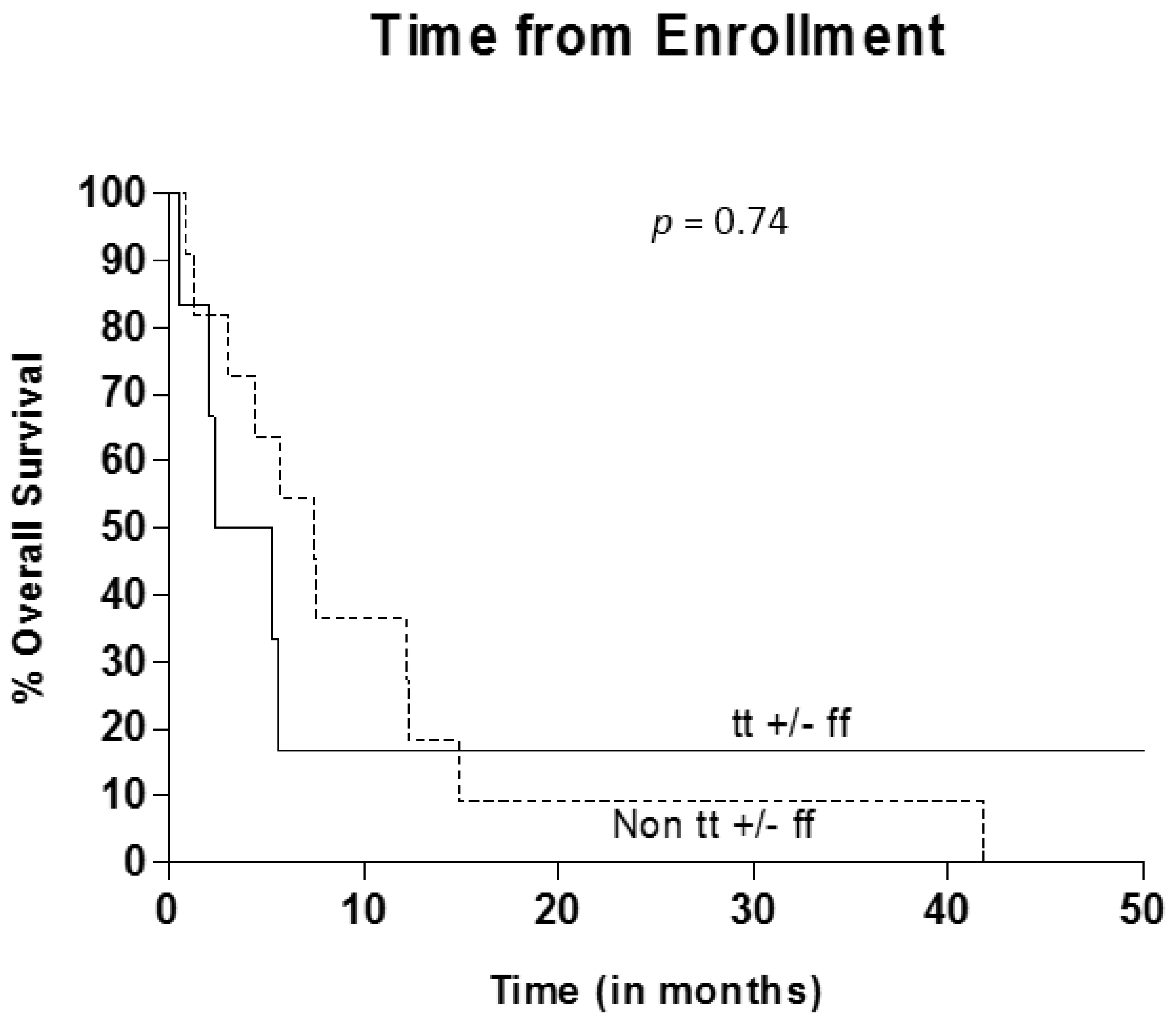
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Anderson, C.M.; Buzaid, A.C.; Legha, S.S. Systemic treatments for advanced cutaneous melanoma. Oncology (Williston Park) 1995, 9, 1149–1158; discussion 1163–1164, 1167–1168. [Google Scholar]
- Chapman, P.B.; Hauschild, A.; Robert, C.; Haanen, J.B.; Ascierto, P.; Larkin, J.; Dummer, R.; Garbe, C.; Testori, A.; Maio, M.; et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 2011, 364, 2507–2516. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.L.; Tomsu, K.; von Eschen, K.B. Duration of survival for disseminated malignant melanoma: Results of a meta-analysis. Melanoma Res. 2000, 10, 81–92. [Google Scholar] [PubMed]
- Middleton, M.R.; Grob, J.J.; Aaronson, N.; Fierlbeck, G.; Tilgen, W.; Seiter, S.; Gore, M.; Aamdal, S.; Cebon, J.; Coates, A.; et al. Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J. Clin. Oncol. 2000, 18, 158–166. [Google Scholar] [PubMed]
- Hegi, M.E.; Liu, L.; Herman, J.G.; Stupp, R.; Wick, W.; Weller, M.; Mehta, M.P.; Gilbert, M.R. Correlation of O6-methylguanine methyltransferase (MGMT) promotor methylation with clinical outcomes in glioblastoma and clinical strategies to modulate MGMT activity. J. Clin. Oncol. 2008, 26, 4189–4199. [Google Scholar] [CrossRef] [PubMed]
- Chinot, O.L.; Barrie, M.; Fuentes, S.; Eudes, N.; Lancelot, S.; Metellus, P.; Muracciole, X.; Braquer, D.; Ouafik, L.; Martin, P.M.; et al. Correlation between O6-methylguanine-DNA methyltransferase and survival in inoperable newly diagnosed glioblastoma patients treated with neoadjuvant temozolomide. J. Clin. Oncol. 2007, 25, 1470–1475. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.M.; Suciu, S.; Mortier, L.; Kruit, W.H.; Robert, C.; Schadendorf, D.; Trefzer, U.; Punt, C.J.; Dummer, R.; Davidson, N.; et al. Extended schedule, escalated dose temozolomide versus dacarbazine in stage IV melanoma: Final results of a randomised phase III study (EORTC 18032). Eur. J. Cancer 2011, 47, 1476–1483. [Google Scholar] [CrossRef]
- Bikle, D.D.; Ng, D.; Tu, C.L.; Oda, Y.; Xie, Z. Calcium- and vitamin D-regulated keratinocyte differentiation. Mol. Cell. Endocrinol. 2001, 177, 161–171. [Google Scholar] [CrossRef]
- Lehmann, B. Role of the vitamin D3 pathway in healthy and diseased skin—Facts, contradictions and hypotheses. Exp. Dermatol. 2009, 18, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Colston, K.; Colston, M.J.; Feldman, D. 1,25-Dihydroxyvitamin D3 and malignant melanoma: The presence of receptors and inhibition of cell growth in culture. Endocrinology 1981, 108, 1083–1086. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.S.; Pryke, A.M.; Ranson, M.; Thomas, H.E.; Posen, S. Human melanoma cells: Functional modulation by calciotropic hormones. J. Investig. Dermatol. 1988, 90, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Yudoh, K.; Matsuno, H.; Kimura, T. 1alpha,25-dihydroxyvitamin D3 inhibits in vitro invasiveness through the extracellular matrix and in vivo pulmonary metastasis of B16 mouse melanoma. J. Lab. Clin. Med. 1999, 133, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Beer, T.M.; Ryan, C.W.; Venner, P.M.; Petrylak, D.P.; Chatta, G.S.; Ruether, J.D.; Redfern, C.H.; Fehrenbacher, L.; Saleh, M.N.; Waterhouse, D.M.; et al. Double-blinded randomized study of high-dose calcitriol plus docetaxel compared with placebo plus docetaxel in androgen-independent prostate cancer: A report from the ASCENT investigators. J. Clin. Oncol. 2007, 25, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Scher, H.I.; Jia, X.; Chi, K.; de Wit, R.; Berry, W.R.; Albers, P.; Henick, B.; Waterhouse, D.; Ruether, D.J.; Rosen, P.J.; et al. Randomized, open-label phase III trial of docetaxel plus high-dose calcitriol versus docetaxel plus prednisone for patients with castration-resistant prostate cancer. J. Clin. Oncol. 2011, 29, 2191–2198. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, P.E.; Osborne, J.E.; Lear, J.T.; Smith, A.G.; Bowers, P.W.; Morris, P.N.; Jones, P.W.; York, C.; Strange, R.C.; Fryer, A.A. Vitamin D receptor polymorphisms are associated with altered prognosis in patients with malignant melanoma. Clin. Cancer Res. 2000, 6, 498–504. [Google Scholar] [PubMed]
- Santonocito, C.; Capizzi, R.; Concolino, P.; Lavieri, M.M.; Paradisi, A.; Gentileschi, S.; Torti, E.; Rutella, S.; Rocchetti, S.; di Carlo, A.; et al. Association between cutaneous melanoma, breslow thickness and vitamin D receptor Bsml polymorphism. Br. J. Dermatol. 2007, 156, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Halsall, J.A.; Osborne, J.E.; Potter, L.; Pringle, J.H.; Hutchinson, P.E. A novel polymorphism in the 1A promoter region of the vitamin D receptor is associated with altered susceptibility and prognosis in malignant melanoma. Br. J. Cancer 2004, 91, 765–770. [Google Scholar] [PubMed]
- Han, J.; Colditz, G.A.; Hunter, D.J. Polymorphisms in the MTHFR and VDR genes and skin cancer risk. Carcinogenesis 2007, 28, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Beer, T.M.; Munar, M.; Henner, W.D. A Phase I trial of pulse calcitriol in patients with refractory malignancies: Pulse dosing permits substantial dose escalation. Cancer 2001, 91, 2431–2439. [Google Scholar] [CrossRef] [PubMed]
- Therasse, P.; Arbuck, S.G.; Eisenhauer, E.A.; Wanders, J.; Kaplan, R.S.; Rubinstein, L.; Verweij, J.; van Glabbeke, M.; van Oosterom, A.T.; Christian, M.C.; et al. New guidelines to evaluate the response to treatment in solid tumors. J. Natl. Cancer Inst. 2000, 92, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Clarke, J.L.; Iwamoto, F.M.; Sul, J.; Panageas, K.; Lassman, A.B.; DeAngelis, L.M.; Hormigo, A.; Nolan, C.P.; Gavrilovic, I.; Karimi, S.; et al. Randomized phase II trial of chemoradiotherapy followed by either dose-dense or metronomic temozolomide for newly diagnosed glioblastoma. J. Clin. Oncol. 2009, 27, 3861–3867. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pettijohn, E.; Martone, B.; Rademaker, A.; Kuzel, T. A Phase I Study of High-Dose Calcitriol in Combination with Temozolomide for Patients with Metastatic Melanoma. J. Pers. Med. 2014, 4, 448-458. https://doi.org/10.3390/jpm4040448
Pettijohn E, Martone B, Rademaker A, Kuzel T. A Phase I Study of High-Dose Calcitriol in Combination with Temozolomide for Patients with Metastatic Melanoma. Journal of Personalized Medicine. 2014; 4(4):448-458. https://doi.org/10.3390/jpm4040448
Chicago/Turabian StylePettijohn, Erin, Brenda Martone, Alfred Rademaker, and Timothy Kuzel. 2014. "A Phase I Study of High-Dose Calcitriol in Combination with Temozolomide for Patients with Metastatic Melanoma" Journal of Personalized Medicine 4, no. 4: 448-458. https://doi.org/10.3390/jpm4040448




