Diagnostics for Developing Countries
Abstract
:1. Introduction
2. The Diagnostics Pipeline
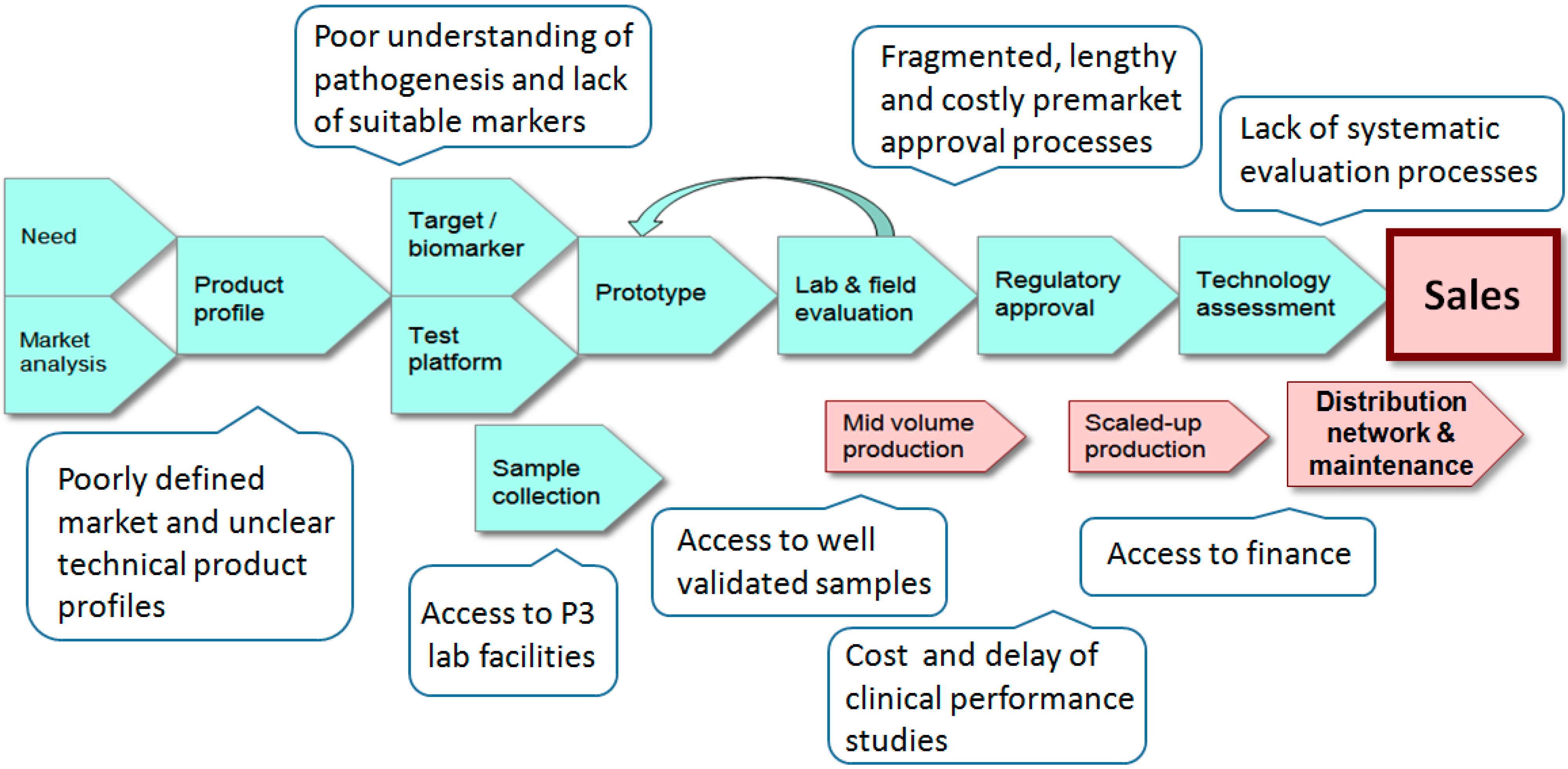
3. Product Profiles
3.1. Technological Considerations
3.2. Epidemiological Considerations
4. Logistical Challenges
5. IVD Regulation in Developing Countries
Prequalification
6. Conclusions
Conflicts of Interest
References
- World Health Organisation. World Health Statistics; World Health Organisation: Geneva, Switzerland, 2014. [Google Scholar]
- Wang, H.; Dwyer-Lindgren, L.; Lofgren, K.T.; Rajaratnam, J.K.; Marcus, J.R.; Levin-Rector, A.; Levitz, C.E.; Lopez, A.D.; Murray, C.J. Age-specific and sex-specific mortality in 187 countries, 1970–2010: A systematic analysis for the global burden of disease study 2010. Lancet 2012, 380, 2071–2094. [Google Scholar] [CrossRef] [PubMed]
- The Joint United Nations Programme on HIV/AIDS (UNAIDS). 90-90-90 An Ambitious Treatment Target to Help End the Aids Epidemic; UNAIDS: Geneva, Switzerland, 2014. [Google Scholar]
- World Health Organisation. Global Tuberculosis Report 2014; WHO: Geneva, Switzerland, 2014. [Google Scholar]
- Newell, M.L.; Coovadia, H.; Cortina-Borja, M.; Rollins, N.; Gaillard, P.; Dabis, F.; Ghent International AIDS Society (IAS) Working Group on HIV Infection in Women and Children. Mortality of infected and uninfected infants born to hiv-infected mothers in africa: A pooled analysis. Lancet 2004, 364, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Petti, C.A.; Polage, C.R.; Quinn, T.C.; Ronald, A.R.; Sande, M.A. Laboratory medicine in africa: A barrier to effective health care. Clin. Infect. Dis. 2006, 42, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Berkelman, R.; Cassell, G.; Specter, S.; Hamburg, M.; Klugman, K. The “achilles heel” of global efforts to combat infectious diseases. Clin. Infect. Dis. 2006, 42, 1503–1504. [Google Scholar] [CrossRef] [PubMed]
- Peeling, R.; McNerney, R. Increasing Access to Diagnostics Through Technology Transfer and Local Production; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- Kock, R.; Croft, S.; Dixon, M.; Fletcher, C.; Good, L.; Guzman, J.; Heymann, D.; Liyanage, R.; Mckeever, D.; McNerney, R.; et al. Prioritising the Need for New Diagnostics, Medicine, Vaccines and Management Practices of Zoonoses which Have Significant Impact in the Developing World; DFID Zoonoses Report 6; Department for International Development: London, UK, 2012. [Google Scholar]
- McNerney, R.; Sollis, K.; Peeling, R. Improving access to new diagnostics through harmonised regulation: Priorities for action. Afr. J. Lab. Med. 2014, 3. [Google Scholar] [CrossRef]
- Dowdy, D.W.; Steingart, K.R.; Pai, M. Serological testing versus other strategies for diagnosis of active tuberculosis in india: A cost-effectiveness analysis. PLoS Med. 2011, 8, e1001074. [Google Scholar] [CrossRef] [PubMed]
- Peeling, R.W.; McNerney, R. Emerging technologies in point-of-care molecular diagnostics for resource-limited settings. Expert Rev. Mol. Diagn. 2014, 14, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Bissonnette, L.; Bergeron, M.G. Infectious disease management through point-of-care personalized medicine molecular diagnostic technologies. J. Personalized Med. 2012, 2, 50–70. [Google Scholar] [CrossRef]
- Target Product Profiles. Available online: http://www.idc-dx.org/resources?tid_2[]=47&keys= (accessed on 18 March 2015).
- World Health Organisation. High-priority Target Product Profiles for New Tuberculosis Diagnostics: Report of a Consensus Meeting; World Health Organisation: Geneva, Switzerland, 2014. [Google Scholar]
- McNerney, R.; Daley, P. Towards a point-of-care test for active tuberculosis: Obstacles and opportunities. Nat. Rev. Microbiol. 2011, 9, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Albertini, A.; Lee, E.; Coulibaly, S.O.; Sleshi, M.; Faye, B.; Mationg, M.L.; Ouedraogo, K.; Tsadik, A.G.; Feleke, S.M.; Diallo, I.; et al. Malaria rapid diagnostic test transport and storage conditions in burkina faso, senegal, ethiopia and the philippines. Malar. J. 2012, 11, 406. [Google Scholar] [CrossRef] [PubMed]
- Kroidl, I.; Clowes, P.; Mwakyelu, J.; Maboko, L.; Kiangi, A.; Rachow, A.; Reither, K.; Jung, J.; Nsojo, A.; Saathoff, E.; et al. Reasons for false-positive lipoarabinomannan elisa results in a tanzanian population. Scand. J. Infect. Dis. 2014, 46, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Steingart, K.R.; Sohn, H.; Schiller, I.; Kloda, L.A.; Boehme, C.C.; Pai, M.; Dendukuri, N. Xpert® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Diagn. Test Accuracy Rev. 2013. [Google Scholar] [CrossRef]
- Raizada, N.; Sachdeva, K.S.; Sreenivas, A.; Vadera, B.; Gupta, R.S.; Parmar, M.; Kulsange, S.; Babre, A.; Thakur, R.; Gray, C.; et al. Feasibility of decentralised deployment of Xpert MTB/RIF test at lower level of health system in india. PLoS ONE 2014, 9, e89301. [Google Scholar] [CrossRef] [PubMed]
- Creswell, J.; Codlin, A.J.; Andre, E.; Micek, M.A.; Bedru, A.; Carter, E.J.; Yadav, R.P.; Mosneaga, A.; Rai, B.; Banu, S.; et al. Results from early programmatic implementation of Xpert MTB/RIF testing in nine countries. BMC Infect. Dis. 2014, 14, 2. [Google Scholar] [CrossRef] [PubMed]
- Abdurrahman, S.T.; Emenyonu, N.; Obasanya, O.J.; Lawson, L.; Dacombe, R.; Muhammad, M.; Oladimeji, O.; Cuevas, L.E. The hidden costs of installing Xpert machines in a tuberculosis high-burden country: Experiences from nigeria. Pan Afr. Med. J. 2014, 18, 277. [Google Scholar] [CrossRef] [PubMed]
- Keeler, E.; Perkins, M.D.; Small, P.; Hanson, C.; Reed, S.; Cunningham, J.; Aledort, J.E.; Hillborne, L.; Rafael, M.E.; Girosi, F.; et al. Reducing the global burden of tuberculosis: The contribution of improved diagnostics. Nature 2006, 444 (Suppl. 1), 49–57. [Google Scholar] [CrossRef] [PubMed]
- Altman, D.G. Diagnostic tests: 2 predictive values. BMJ 1994, 309, 102. [Google Scholar] [CrossRef] [PubMed]
- Van Dyck, E.; Buve, A.; Weiss, H.A.; Glynn, J.R.; Brown, D.W.; de Deken, B.; Parry, J.; Hayes, R.J. Performance of commercially available enzyme immunoassays for detection of antibodies against herpes simplex virus type 2 in african populations. J. Clin. Microbiol. 2004, 42, 2961–2965. [Google Scholar] [CrossRef] [PubMed]
- Dheda, K.; van Zyl Smit, R.; Badri, M.; Pai, M. T-cell interferon-gamma release assays for the rapid immunodiagnosis of tuberculosis: Clinical utility in high-burden vs. Low-burden settings. Curr. Opin. Pulm. Med. 2009, 15, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Elliott, A.M.; Luo, N.; Tembo, G.; Halwiindi, B.; Steenbergen, G.; Machiels, L.; Pobee, J.; Nunn, P.; Hayes, R.J.; McAdam, K.P. Impact of hiv on tuberculosis in zambia: A cross sectional study. BMJ 1990, 301, 412–415. [Google Scholar] [CrossRef] [PubMed]
- Lawn, S.D.; Kerkhoff, A.D.; Vogt, M.; Wood, R. Diagnostic accuracy of a low-cost, urine antigen, point-of-care screening assay for HIV-associated pulmonary tuberculosis before antiretroviral therapy: A descriptive study. Lancet Infect. Dis. 2012, 12, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Everett, D.B.; Baisely, K.J.; McNerney, R.; Hambleton, I.; Chirwa, T.; Ross, D.A.; Changalucha, J.; Watson-Jones, D.; Helmby, H.; Dunne, D.W.; et al. Association of schistosomiasis with false-positive HIV test results in an african adolescent population. J. Clin. Microbiol. 2010, 48, 1570–1577. [Google Scholar] [CrossRef] [PubMed]
- Gasasira, A.F.; Dorsey, G.; Kamya, M.R.; Havlir, D.; Kiggundu, M.; Rosenthal, P.J.; Charlebois, E.D. False-positive results of enzyme immunoassays for human immunodeficiency virus in patients with uncomplicated malaria. J. Clin. Microbiol. 2006, 44, 3021–3024. [Google Scholar] [CrossRef] [PubMed]
- Nathanson, C.M.; Cuevas, L.E.; Cunningham, J.; Perkins, M.D.; Peeling, R.W.; Guillerm, M.; Moussy, F.; Ramsay, A. The TDR Tuberculosis Specimen Bank: A resource for diagnostic test developers. Int. J. Tuberc. Lung Dis. 2010, 14, 1461–1467. [Google Scholar] [PubMed]
- Bates, M.; Mudenda, V.; Shibemba, A.; Kaluwaji, J.; Tembo, J.; Kabwe, M.; Chimoga, C.; Chilukutu, L.; Chilufya, M.; Kapata, N.; et al. Burden of tuberculosis at post mortem in inpatients at a tertiary referral centre in sub-saharan africa: A prospective descriptive autopsy study. Lancet Infect. Dis. 2015, 15, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Rana, F.S.; Hawken, M.P.; Mwachari, C.; Bhatt, S.M.; Abdullah, F.; Ng'ang'a, L.W.; Power, C.; Githui, W.A.; Porter, J.D.; Lucas, S.B. Autopsy study of HIV-1-positive and HIV-1-negative adult medical patients in Nairobi, Kenya. J. Acquir. Immune Defic. Syndr. 2000, 24, 23–29. [Google Scholar] [CrossRef] [PubMed]
- US Department of Health and Human Services; Public Health Service; Centers for Disease Control and Prevention; National Institutes of Health. Biosafety in Microbiological and Biomedical Laboratories, 5th ed.; CDC: Atlanta, GA, USA, 2009. [Google Scholar]
- Rugera, S.P.; McNerney, R.; Poon, A.K.; Akimana, G.; Mariki, R.F.; Kajumbula, H.; Kamau, E.; Mpawenimana, S.; Said, S.Y.; Toroitich, A.; et al. Regulation of medical diagnostics and medical devices in the east african community partner states. BMC Health Serv. Res. 2014, 14, 524. [Google Scholar] [CrossRef] [PubMed]
- Special Programme for Research & Training in Tropical Diseases (TDR). Regulation of vitro Diagnostics: A Global Perspective 2002; Reproduced as Annex to Diagnostics for Tuberculosis: Global Demand and Market Potential; World health organization: Geneva, Switzerland, 2006. [Google Scholar]
- Study Protocols. Available online: http://www.idc-dx.org/resources?tid_2[]=48&keys= (accessed on 20 March 2015).
- Evaluation Study Sites. Available online: http://www.idc-dx.org/resources?tid_2[]=50&keys= (accessed on 20 March 2015).
- World Health Organisation. Prequalification Program for in vitro Diagnostic Devices. Available online: http://www.who.int/diagnostics_laboratory/evaluations/en/ (accessed on 20 March 2015).
- Pai, M.; Minion, J.; Steingart, K.; Ramsay, A. New and improved tuberculosis diagnostics: Evidence, policy, practice, and impact. Curr. Opin. Pulm. Med. 2010, 16, 271–284. [Google Scholar] [PubMed]
- Schunemann, A.H.J.; Oxman, A.D.; Brozek, J.; Glasziou, P.; Jaeschke, R.; Vist, G.E.; Williams, J.W.; Kunz, R.; Craig, J.; Montori, V.M.; et al. Grade: Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. Bri. Med. J. 2008, 336, 1106–1110. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
McNerney, R. Diagnostics for Developing Countries. Diagnostics 2015, 5, 200-209. https://doi.org/10.3390/diagnostics5020200
McNerney R. Diagnostics for Developing Countries. Diagnostics. 2015; 5(2):200-209. https://doi.org/10.3390/diagnostics5020200
Chicago/Turabian StyleMcNerney, Ruth. 2015. "Diagnostics for Developing Countries" Diagnostics 5, no. 2: 200-209. https://doi.org/10.3390/diagnostics5020200




