Prenatal Diagnosis of Chromosome Abnormalities: A 13-Year Institution Experience
Abstract
:1. Introduction
2. Objectives
3. Methods
4. Results
4.1. Impact of Increased Utilization of Aneuploidy Screening on Genetic Invasive Tests
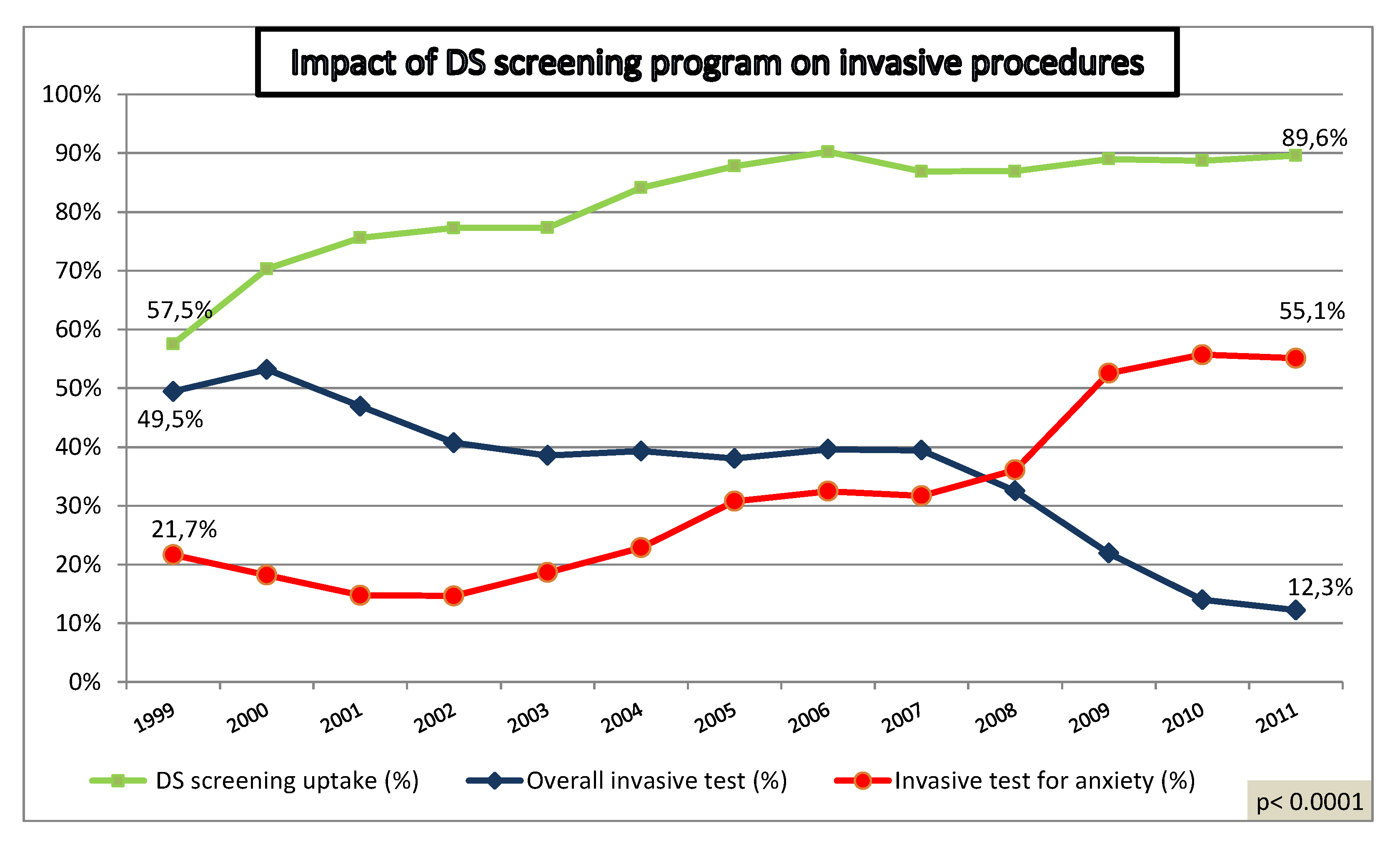
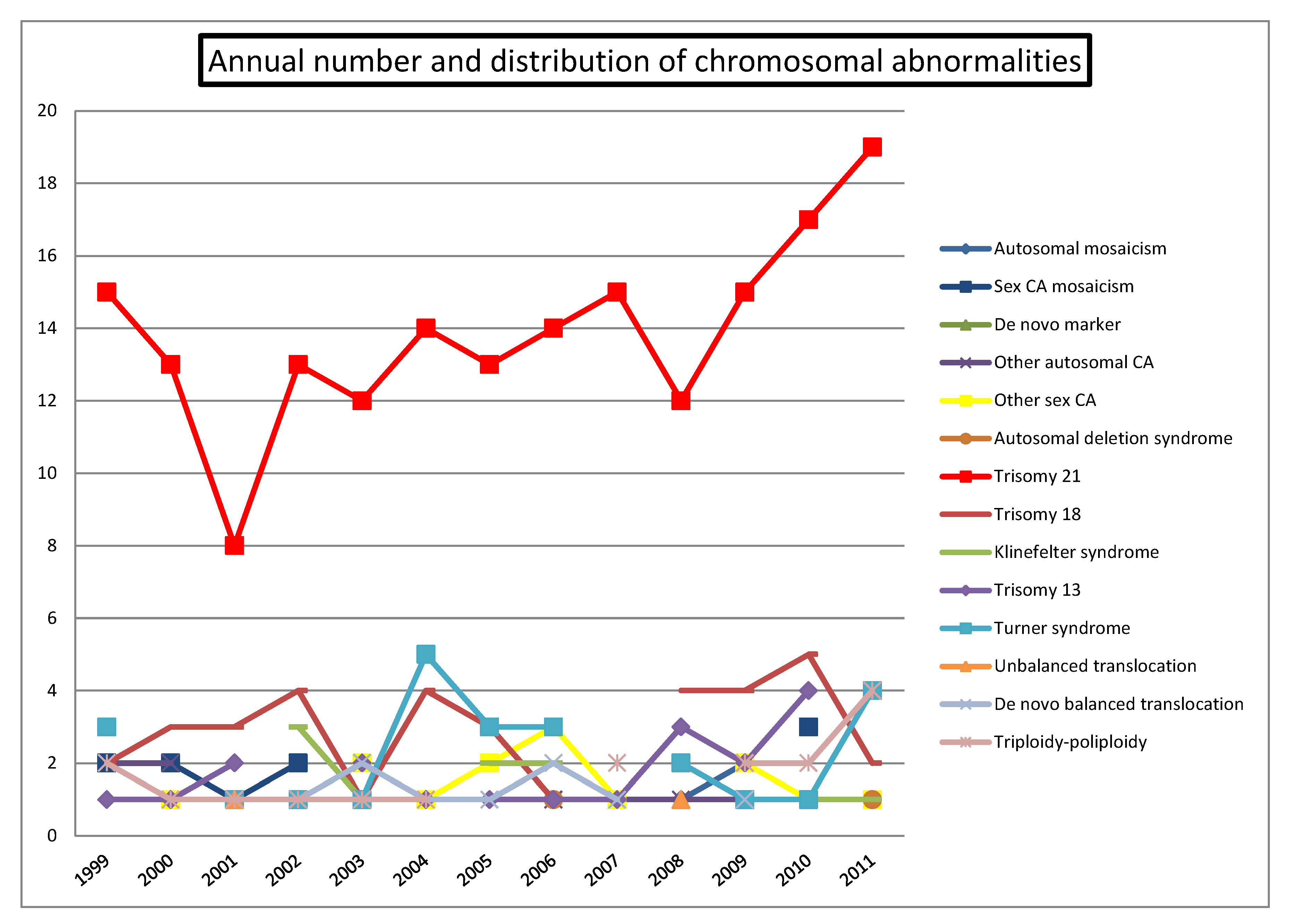
4.2. Chromosomal Abnormalities
| Chromosomal abnormality | Medical indication | Non-medical indication (anxiety) | Overall |
|---|---|---|---|
| N overall | 7,877 | 3,129 | 11,006 |
| Autosomal mosaicism | 6 | 1 | 7 |
| Sex CA mosaicism | 10 | 1 | 11 |
| De novo marker | 4 | 1 | 5 |
| Other autosomal CA | 12 | 12 | |
| Other sex CA | 11 | 5 | 16 |
| Autosomal deletion syndrome | 4 | 4 | |
| Trisomy 21 | 178 | 2 | 180 |
| Trisomy 18 | 36 | 36 | |
| Klinefelter syndrome (47, XXY) | 10 | 2 | 12 |
| Trisomy 13 | 19 | 19 | |
| Turner syndrome (45, X) | 25 | 25 | |
| Unbalanced translocation | 2 | 2 | |
| De novo balanced translocation | 8 | 1 | 9 |
| Triploidy-Poliploidy | 17 | 17 | |
| Overall | 342 | 13 | 355 |
| Prevalence relevant CA | 4.34% | 0.42% | 3.23% |
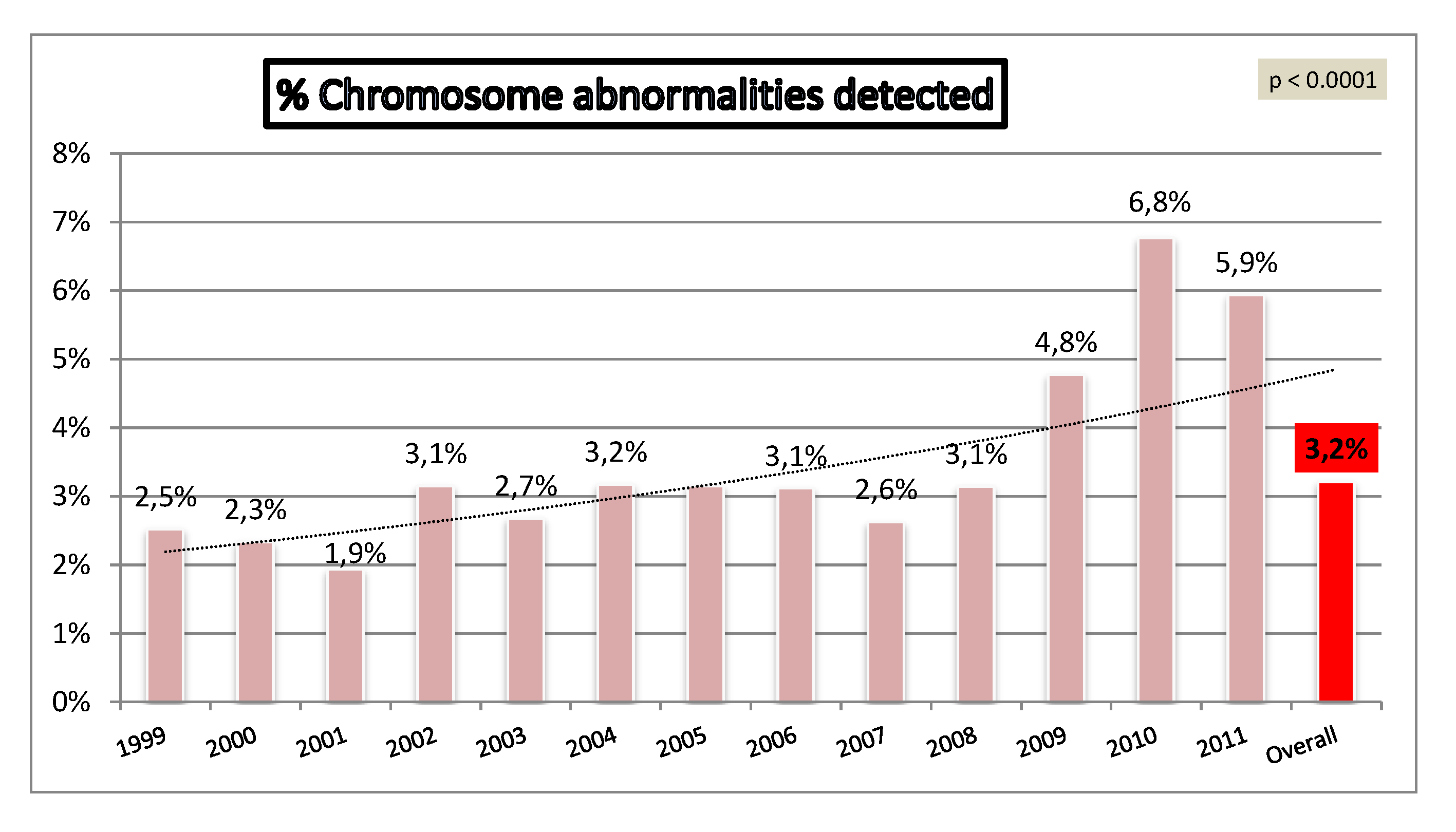
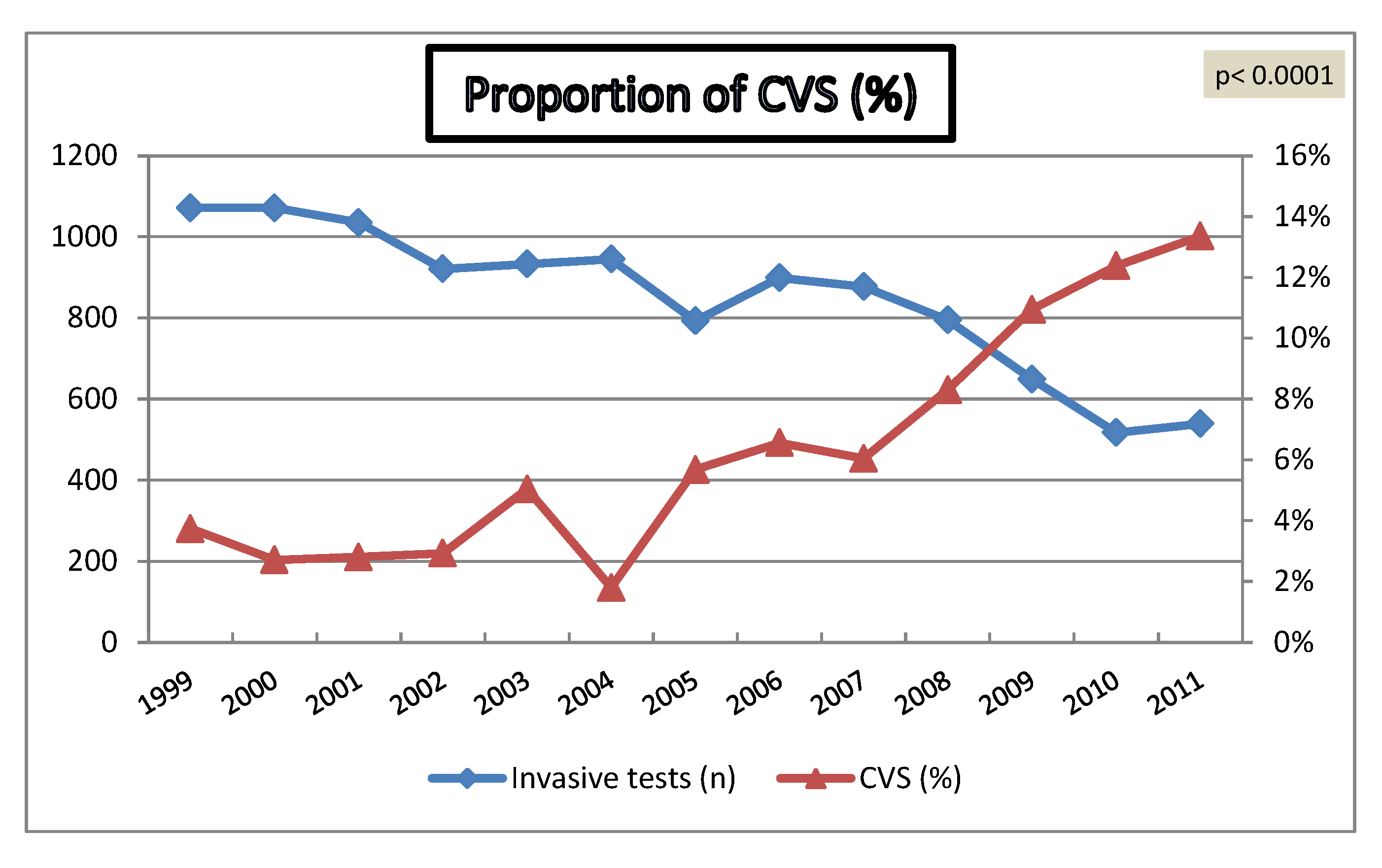
4.3. Indications for Referral

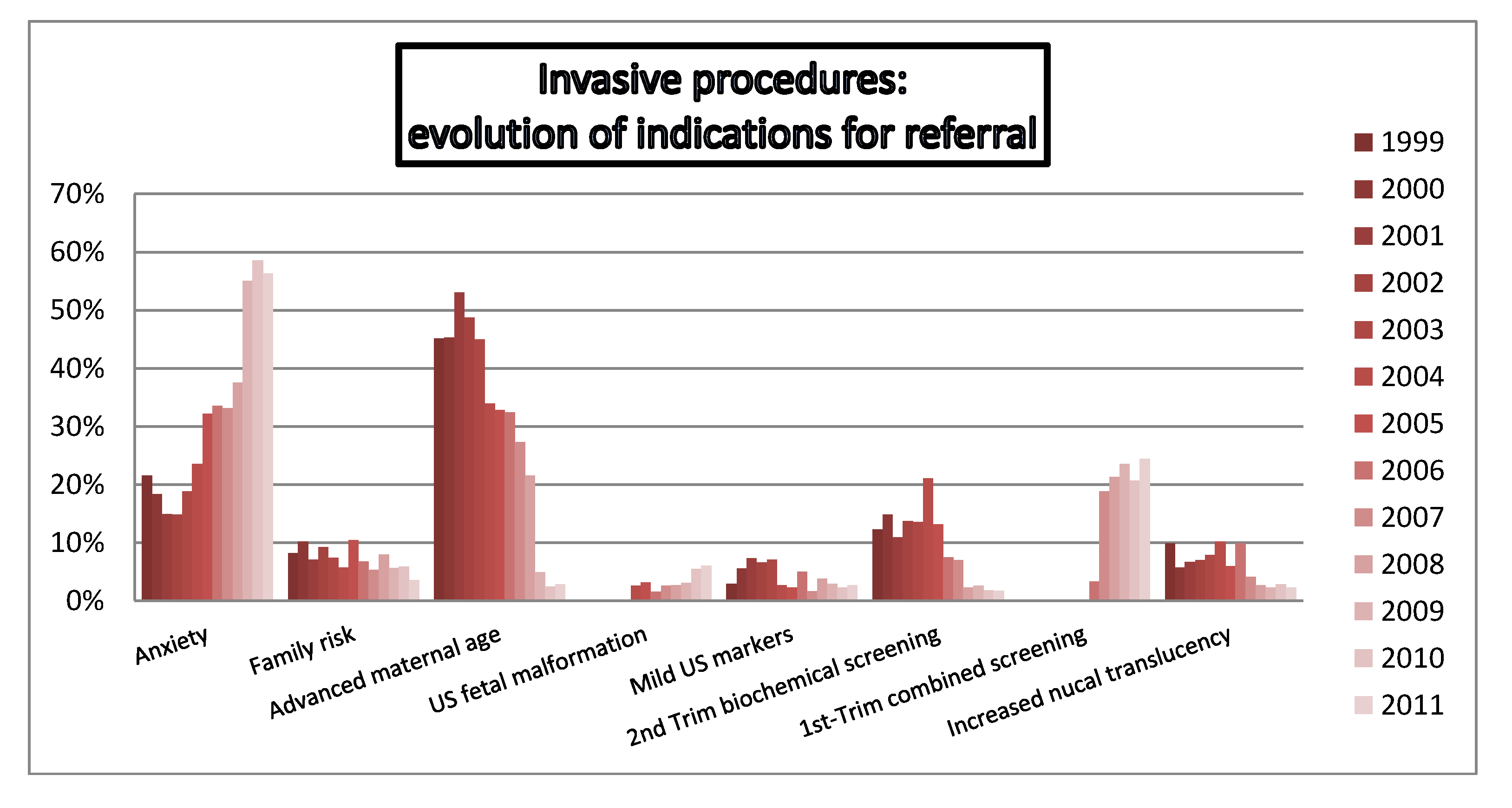


4.4. Efficiency of Genetic Invasive Testing According to Indication of Referral
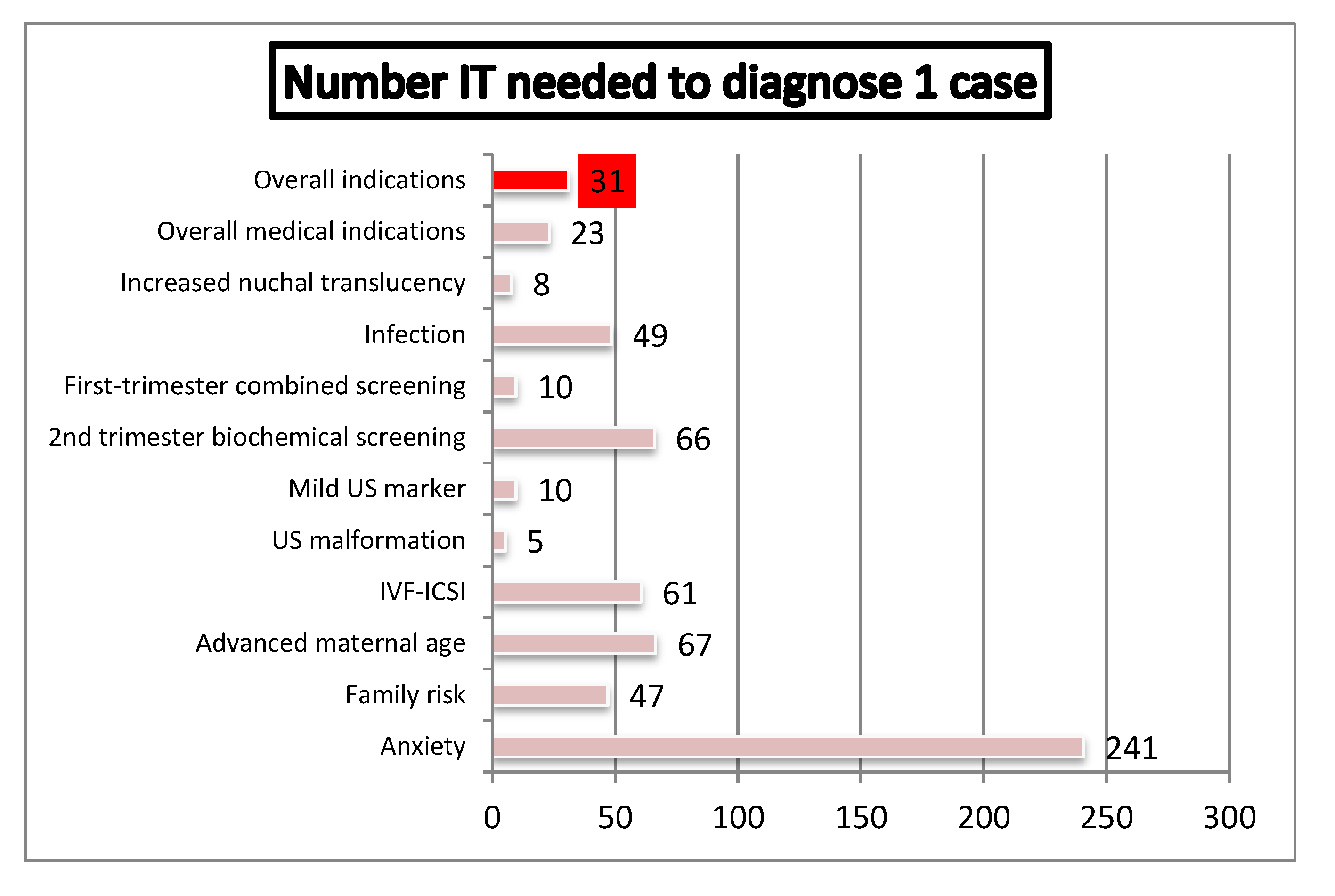

4.5. Residual Risk of Chromosomal Anomalies in Low Risk Pregnancies
5. Discussion
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Sherman, L.; Allen, E.G.; Bean, L.H.; Freeman, S.B. Epidemiology of Down syndrome. Ment Retard Dev. Disabil. Res. Rev. 2007, 13, 221–227. [Google Scholar] [CrossRef]
- Ogivile, C.M. Prenatal diagnosis for chromosome abnormalities: past, present and future. Pathol. Biol. 2003, 51, 156–160. [Google Scholar] [CrossRef]
- Lewis, M.; Faed, M.J.; Howie, P.W. Screening for Down’s syndrome based on individual risk. BMJ 1991, 7, 551–553. [Google Scholar]
- Spencer, K.; Spencer, C.E.; Power, M.; Dawson, C.; Nicolaides, K.H. Screening for chromosomal abnormalities in the first trimester using ultrasound and maternal serum biochemistry in a one-stop clinic: A review of three years prospective experience. BJOG 2003, 110, 281–286. [Google Scholar] [CrossRef]
- Borrell, A.; Casals, E.; Fortuny, A.; Farre, M.T.; Gonce, A.; Sanchez, A.; Soler, A.; Cararach, V.; Vanrell, J.A. First-trimester screening for trisomy 21 combining biochemistry and ultrasound at individually optimal gestational ages. An interventional study. Prenatal Diag. 2004, 24, 541–545. [Google Scholar] [CrossRef]
- Comas Gabriel, C.; Echevarria Tellería, M.; Muñoz Prades, A.; Rodríguez García, I.; Carrera Martínez, M.; Serra Zantop, B. Invasive prenatal diagnostic practice: A review of a ten years experience at Dexeus Institut. Prenatal Diag. 2011, 22, 117–127. [Google Scholar] [CrossRef]
- Ekelund, C.K.; Jørgensen, F.S.; Petersen, O.B.; Sundberg, K.; Tabor, A. Impact of a new national screening policy for Down's syndrome in Denmark: population based cohort study. BMJ 2008, 27. [Google Scholar]
- Vestergaard, C.H.; Lidegaard, Ø.; Tabor, A. Invasive prenatal diagnostic practice in Denmark 1996 to 2006. Acta Obstet. Gynecol. Scand. 2009, 88, 362–365. [Google Scholar] [CrossRef]
- Lichtenbelt, K.D.; Alizadeh, B.Z.; Scheffer, P.G.; Stoutenbeek, P.; Schielen, P.C.; Page-Christiaens, L.C.; Schuring-Blom, G.H. Trends in the utilization of invasive prenatal diagnosis in The Netherlands during 2000–2009. Prenatal Diag. 2011, 31, 765–772. [Google Scholar] [CrossRef]
- Morris, J.K.; Waters, J.J.; de Souza, E. The population impact of screening for Down syndrome: Audit of 19326 invasive diagnostic tests in England and Wales in 2008. Prenatal Diag. 2012, 32, 596–601. [Google Scholar] [CrossRef]
- Mademont-Soler, I.; Morales, C.; Clusellas, N.; Soler, A.; Sánchez, A. Prenatal cytogenetic diagnosis in Spain: Analysis and evaluation of the results obtained from amniotic fluid samples during the last decade. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 157, 156–160. [Google Scholar] [CrossRef]
- Han, S.H.; An, J.W.; Jeong, G.Y.; Yoon, H.R.; Lee, A.; Yang, Y.H.; Lee, K.P.; Lee, K.R. Clinical and cytogenetic findings on 31,615 mid-trimester amniocenteses. Korean J. Lab. Med. 2008, 28, 378–385. [Google Scholar] [CrossRef]
- Hassold, T.; Abruzzo, M.; Adkins, K.; Griffin, D.; Merrill, M.; Millie, E.; Saker, D.; Shen, J.; Zaragoza, M. Human aneuploidy: incidence, origin, and etiology. Environ. Mol. Mutagen. 1996, 28, 167–175. [Google Scholar] [CrossRef]
- Verp, M.S.; Bombard, A.T.; Simpson, J.L.; Elias, S. Parental decision following prenatal diagnosis of fetal chromosome abnormality. Am. J. Med. Genet. 1988, 29, 613–622. [Google Scholar] [CrossRef]
- Ratcliffe, S. Long-term outcome in children of sex chromosome abnormalities. Arch. Dis. Child. 1999, 80, 192–195. [Google Scholar] [CrossRef]
- Linden, M.G.; Bender, B.G. Fifty-one prenatally diagnosed children and adolescents with sex chromosome abnormalities. Am. J. Med. Genet. 2002, 110, 11–18. [Google Scholar] [CrossRef]
- Christian, S.M.; Koehn, D.; Pillay, R.; MacDougall, A.; Wilson, R.D. Parental decisions following prenatal diagnosis of sex chromosome aneuploidy: A trend over time. Prenatal Diag. 2000, 20, 37–40. [Google Scholar] [CrossRef]
- Hamamy, H.A.; Dahoun, S. Parental decisions following the prenatal diagnosis of sex chromosome abnormalities. Eur. J. Obstet. Gynecol. Reprod. Biol. 2004, 116, 58–62. [Google Scholar] [CrossRef]
- Shaffer, B.L.; Caughey, A.B.; Norton, M.E. Variation in the decision to terminate pregnancy in the setting of fetal aneuploidy. Prenatal Diag. 2006, 26, 667–671. [Google Scholar] [CrossRef]
- Lee, C.N.; Lin, S.Y.; Lin, C.H.; Shih, J.C.; Lin, T.H.; Su, Y.N. Clinical utility of array comparative genomic hybridisation for prenatal diagnosis: A cohort study of 3171 pregnancies. BJOG 2012, 119, 1282. [Google Scholar] [CrossRef]
- Querejeta, M.E.; Nieva, B.; Navajas, J.; Cigudosa, J.C.; Suela, J. Diagnóstico prenatal y array-CGH II: gestaciones de bajo riesgo. Prenatal Diag. 2012, 23, 49–55. [Google Scholar] [CrossRef]
- Armengol, L.; Nevado, J.; Serra-Juhé, C.; Plaja, A.; Mediano, C.; García-Santiago, F.A.; García-Aragonés, M.; Villa, O.; Mansilla, E.; Preciado, C.; Fernández, L.; Ángeles Mori, M.; García-Pérez, L.; Lapunzina, P.D.; Pérez-Jurado, L.A. Clinical utility of chromosomal microarray analysis in invasive prenatal diagnosis. Hum. Genet. 2012, 131, 513–523. [Google Scholar] [CrossRef]
- Fiorentino, F.; Caiazzo, F.; Napolitano, S.; Spizzichino, L.; Bono, S.; Sessa, M.T.; Nuccitelli, A.; Biricik, A.; Gordon, A.; Rizzo, G; Baldi, M. Introducing array comparative genomic hybridization into routine prenatal diagnosis practice: A prospective study on over 1000 consecutive clinical cases. Prenatal Diag. 2011, 31, 1270–1282. [Google Scholar] [CrossRef]
- Breman, A.; Pursley, A.N.; Hixson, P.; Bi, W.; Ward, P.; Bacino, C.A.; Shaw, C.H.; Lupski, J.R.; Beaudet, A.; Patel, A.; Cheung, S.W.; van den Veyver, I. Prenatal chromosomal microarray analysis in a diagnostic laboratory; experience with >1000 cases and review of the literature. Prenatal Diag. 2012, 32, 351–361. [Google Scholar] [CrossRef]
- Fiorentino, F.; Baldi, M. Re: Microarray application in prenatal diagnosis: A position statement from cytogenetics working group of the Italian Society of Human Genetics (SIGU), Novemebr 2011. Ultrasound Obstet. Gynecol. 2012, 39, 601–602. [Google Scholar] [CrossRef]
- Hillman, S.C.; Pretlove, S.; Coomarasamy, A.; McMullan, D.J.; Davison, E.V.; Maher, E.R.; Kilby, M.D. Additional information from array comparative genomic hybridization technology over conventional karyotyping in prenatal diagnosis: A systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2011, 37, 6–14. [Google Scholar] [CrossRef]
- Novelli, A.; Grati, F.R.; Ballarati, L.; Bernardini, L.; Bizzoco, D.; Camurri, L.; Casalone, R.; Cardarelli, L.; Cavalli, P.; Ciccone, R.; et al. Microarray application in prenatal diagnosis: A position statement from the cytogenetics working group of the Italian Society of Human Genetics (SIGU), November 2011. Ultrasound Obstet. Gynecol. 2012, 39, 384–388. [Google Scholar] [CrossRef]
- Chiu, R.W.K.; Akolekar, R.; Zheng, Y.W.L.; Leung, T.K.; Sun, H.; Chan, K.C.A.; Lun, F.M.F.; Go, A.T.J.I.; Lau, E.T.; To, W.W.K. Non-invasive prenatal assessment of trisomy 21 by multiplexed maternal plasma DNA sequencing: Large scale validation study. Brit. Med. J. 2011, 342, c7401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sparks, A.B.; Struble, C.A.; Wang, E.T.; Song, K.; Oliphant, A. Noninvasive prenatal detection and selective analysis of cell-free DNA obtained from maternal blood: Evaluation for trisomy 21 and trisomy 18. Am. J. Obstet. Gynecol. 2012, 206, 319e1–319e9. [Google Scholar] [CrossRef]
- Norton, M.E.; Brar, H.; Weiss, J.; Karimi, A.; Laurent, L.C.; Caughey, A.B.; Rodriguez, M.H.; Williams, J.; Mitchell, M.E.; Adair, C.D.; et al. Non-Invasive Chromosomal Evaluation (NICE) Study: Results of a multicenter prospective cohort study for detection of fetal trisomy 21 and trisomy 18. Am. J. Obstet. Gynecol. 2012, 207, 137e1–137e8. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Comas, C.; Echevarria, M.; Rodríguez, M.Á.; Rodríguez, I.; Serra, B.; Cirigliano, V. Prenatal Diagnosis of Chromosome Abnormalities: A 13-Year Institution Experience. Diagnostics 2012, 2, 57-71. https://doi.org/10.3390/diagnostics2040057
Comas C, Echevarria M, Rodríguez MÁ, Rodríguez I, Serra B, Cirigliano V. Prenatal Diagnosis of Chromosome Abnormalities: A 13-Year Institution Experience. Diagnostics. 2012; 2(4):57-71. https://doi.org/10.3390/diagnostics2040057
Chicago/Turabian StyleComas, Carmen, Mónica Echevarria, María Ángeles Rodríguez, Ignacio Rodríguez, Bernat Serra, and Vincenzo Cirigliano. 2012. "Prenatal Diagnosis of Chromosome Abnormalities: A 13-Year Institution Experience" Diagnostics 2, no. 4: 57-71. https://doi.org/10.3390/diagnostics2040057






