Bioremediation of Landfill Leachate with Fungi: Autochthonous vs. Allochthonous Strains
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Autochthonous Biomasses
2.3. Allochthonous Biomasses
2.4. Biodegradation Screening with Free Biomasses
2.5. Biodegradation Experimental with Immobilized Biomasses
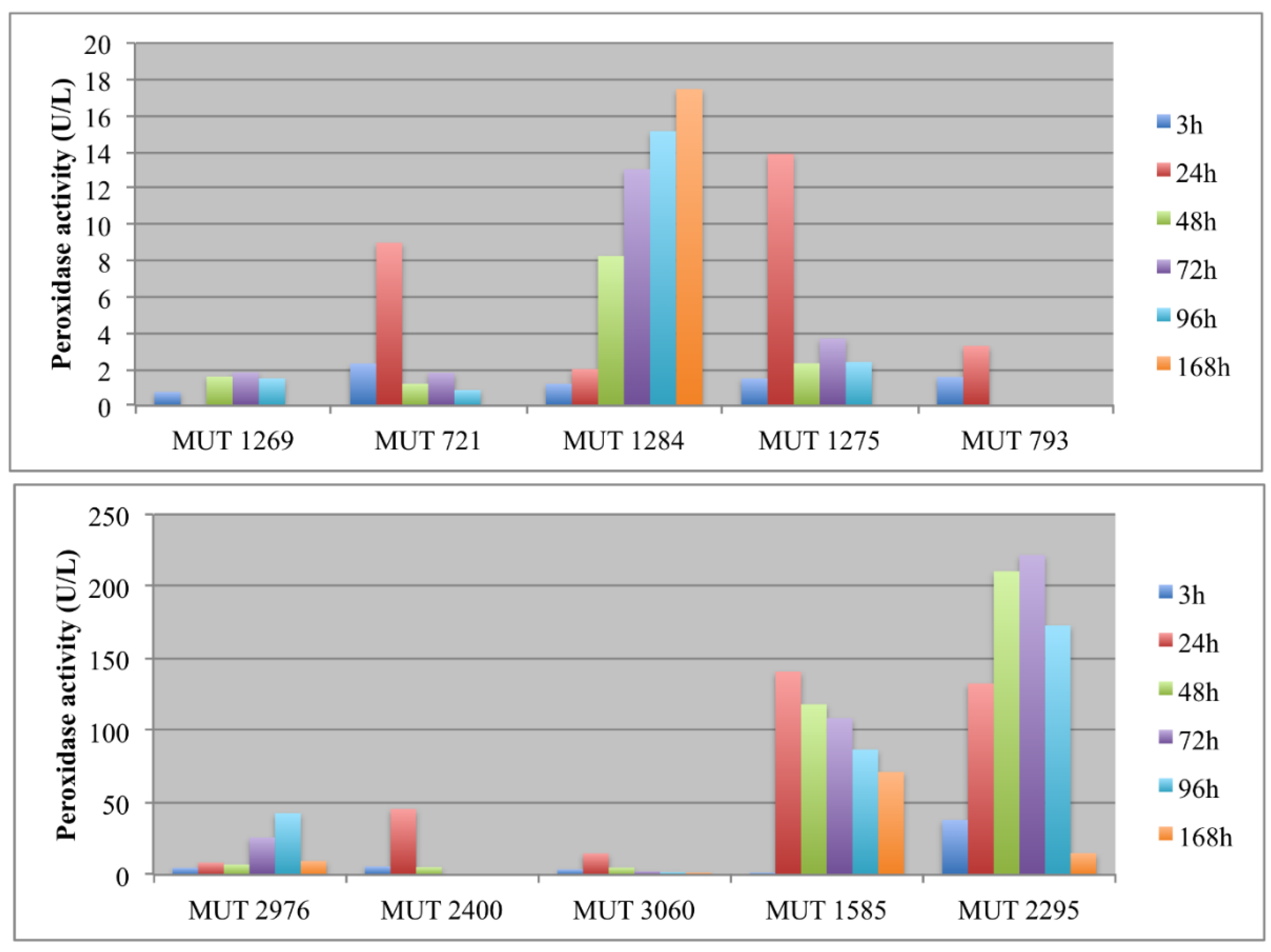
2.6. Enzymatic Activity Assay
2.7. Ecotoxicity Tests
2.8. Statistical Analyses
3. Results and Discussion
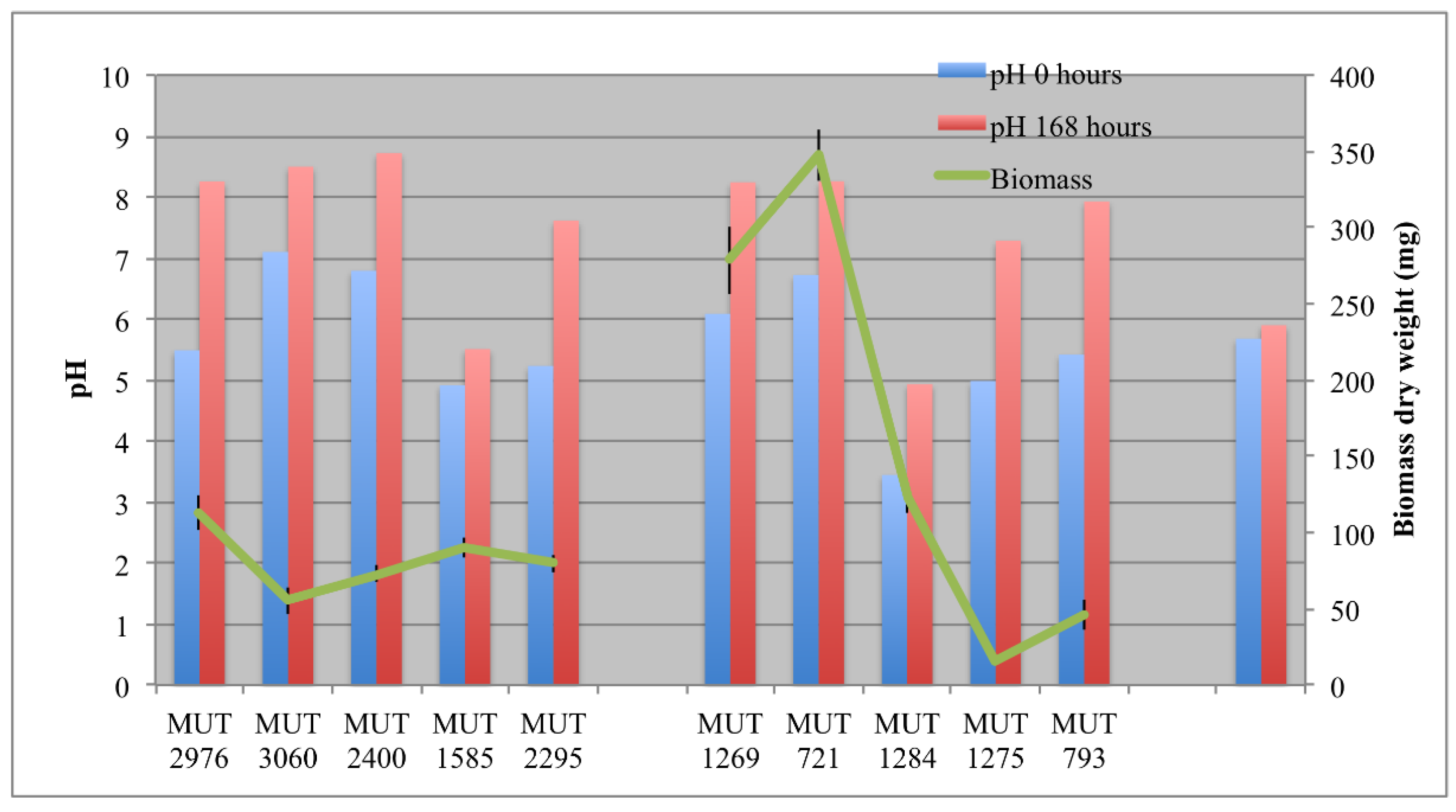
3.1. Biodegradation Screening with Free Biomasses
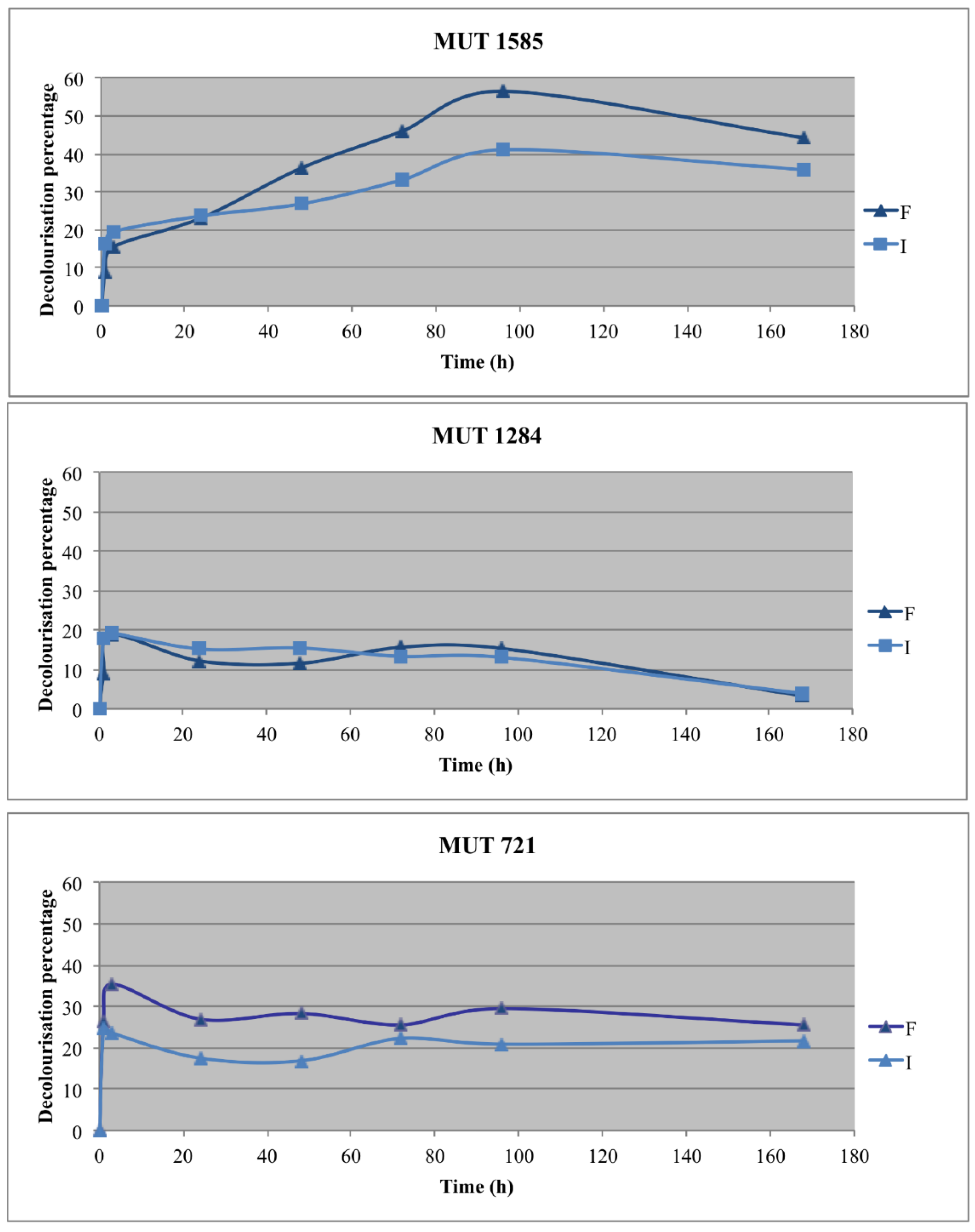
3.2. Biodegradation Experiment with Immobilized Biomasses
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McDonald, J.E.; Allison, H.E.; McCarthy, A.J. Composition of the Landfill Microbial Community as Determined by Application of Domain- and Group-Specific 16S and 18S rRNA-Targeted Oligonucleotide Probes. Appl. Environ. Microbiol. 2010, 76, 1301–1306. [Google Scholar] [CrossRef] [PubMed]
- Matejczyk, M.; Płaza, G.A.; Nałęcz-Jawecki, G.; Ulfig, K.; Markowska-Szczupak, A. Estimation of the environmental risk posed by landfills using chemical, microbiological and ecotoxicological testing of leachates. Chemosphere 2011, 82, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Tigini, V.; Prigione, V.; Varese, G.C. Mycological and ecotoxicological characterisation of landfill leachate before and after traditional treatments. Sci. Total Environ. 2014, 278, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Alimba, C.G.; Gandhi, D.; Sivanesan, S.; Bhanarkar, M.D.; Naoghare, P.K.; Bakare, A.A.; Krishnamurthi, K. Chemical characterization of simulated landfill soil leachates from Nigeria and India and their cytotoxicity and DNA damage inductions on three human cell lines. Chemosphere 2016, 164, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.J.L.; Tyrrel, S.F.; Smith, R.; Farrow, S. Bioassays for the Evaluation of Landfill Leachate Toxicity. J. Toxicol. Environ. Health B 2009, 12, 83–105. [Google Scholar] [CrossRef] [PubMed]
- Vedrenne, M.; Vasquez-Medrano, R.; Prato-Garcia, D.; Frontana-Uribe, B.A.; Ibanez, J.G. Characterization and detoxification of a mature landfill leachate using a combined coagulation-flocculation/photo Fenton treatment. J. Hazard. Mater. 2012, 205, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Renou, S.; Givaudan, J.G.; Poulain, S.; Dirassouyan, F.; Moulin, P. Landfill leachate treatment: Review and opportunity. J. Hazard. Mater. 2008, 150, 468–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurniawan, T.A.; Lo, W.; Chan, G.; Sillanpaa, M.E. Biological processes for treatment of landfill leachate. J. Environ. Monit. 2010, 12, 2032–2047. [Google Scholar] [CrossRef] [PubMed]
- Schiopu, A.-M.; Gavrilescu, M. Options for the treatment and management of municipal landfill leachate: Common and specific issues. Clean-Soil Air Water 2010, 38, 1101–1110. [Google Scholar] [CrossRef]
- Razarinah, W.A.R.W.; Zalina, M.N.; Abdullah, N. Utilization of the White-rot Fungus, Trametes menziesii for Landfill Leachate Treatment. Sains Malays. 2015, 44, 309–316. [Google Scholar] [CrossRef]
- Primo, O.; Rivero, M.J.; Ortiz, I. Photo-Fenton process as an efficient alternative to the treatment of landfill leachates. J. Hazard. Mater. 2012, 153, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Ruby, G.-O.V.; Marquez-Benavidez, L.; Juan Manuel, S.-Y.J. Evaluation of the phytotoxicity of Landfill leachate on Phaseolus vulgaris L and survival of microorganisms of public health importance. J. Selva Andin. Res. Soc. 2015, 6, 51–63. [Google Scholar]
- Remmas, N.; Melidis, P.; Zerva, I.; Kristoffersen, J.B.; Nikolaki, S.; Tsiamis, G.; Ntougias, S. Dominance of candidate Saccharibacteria in a membrane bioreactor treating medium age landfill leachate: Effects of organic load on microbial communities, hydrolytic potential and extracellular polymeric substances. Bioresour. Technol. 2017, 238, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Selbmann, L.; Egidi, E.; Isola, D.; Onofri, S.; Zucconi, L.; de Hoog, G.S.; Chinaglia, S.; Testa, L.; Tosi, S.; Balestrazzi, A.; et al. Biodiversity, evolution and adaptation of fungi in extreme environments. Plant Biosyst. 2013, 147, 237–246. [Google Scholar] [CrossRef] [Green Version]
- Ardeshir, R.A.; Rastgar, S.; Peyravi, M.; Jahanshahi, M.; Rad, A.S. A new route of bioaugmentation by allochthonous and autochthonous through biofilm bacteria for soluble chemical oxygen demand removal of old leachate. Environ. Technol. 2017, 38, 2447–2455. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, P.; Malik, A. Fungal dye decolourization: Recent advances and future potential. Environ. Int. 2009, 35, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Wesemberg, D.; Kyriakides, I.; Agathos, S.N. White-rot fungi and their enzymes for the treatment of industrial dye effluents. Biotechnol. Adv. 2003, 22, 161–187. [Google Scholar] [CrossRef]
- Herrero, M.; Stuckey, D.C. Bioaugmentation and its application in wastewater treatment: A review. Chemosphere 2015, 140, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Tigini, V.; Varese, G.C. Biosorption with autochthonous and allochthonous fungal biomasses for bioremediation and detoxification of landfill leachate. Environ. Earth Sci. 2018, 77, 342. [Google Scholar] [CrossRef]
- Anastasi, A.; Spina, F.; Romagnolo, A.; Tigini, V.; Prigione, V.; Varese, G.C. Integrated fungal biomass and activated sludge treatment for textile wastewater bioremediation. Bioresour. Technol. 2012, 123, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Anastasi, A.; Spina, F.; Prigione, V.; Tigini, V.; Varese, G.C. Textile wastewater treatment: Scale-up of a bioprocess using the fungus Bjerkandera adusta. J. Biotechnol. 2010, 150, 52. [Google Scholar] [CrossRef]
- Niku-Paavola, M.L.; Karhunen, E.; Salola, P.; Raunio, V. Ligninolytic enzymes of the white-rot fungus Phlebia radiate. J. Biochem. 1988, 254, 877–884. [Google Scholar] [CrossRef]
- Vyas, B.R.M.; Volc, J.; Sasek, V. Effects of temperature on the production of manganese peroxidase and lignin peroxidase by Phanerochaete-Chrysosporium. Folia Microbiol. 1994, 39, 19–22. [Google Scholar] [CrossRef]
- Leifa, F.; Ashok Pandey, A.; Soccol, C.R. Production of Flammulina velutipes on Coffee Husk and Coffee Spent-ground. Braz. Arch. Biol. Technol. 2001, 44, 2. [Google Scholar] [CrossRef]
- Bardi, A.; Yuan, Q.; Siracusa, G.; Chicca, I.; Islam, M.; Spennati, F.; Tigini, V.; Di Gregorio, S.; Levin, D.B.; Petroni, G.; et al. Effect of cellulose as co-substrate on old landfill leachate treatment using white-rot fungi. Bioresour. Technol. 2017, 241, 1067–1076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leitão, A.L. Potential of Penicillium Species in the Bioremediation Field. Int. J. Environ. Res. Public Health 2009, 6, 1393–1417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinto, A.P.; Serrano, C.; Pires, T.; Mestrinho, E.; Dias, L.; Martins Teixeira, T.; Caldeira, A.T. Degradation of terbuthylazine, difenoconazole and pendimethalin pesticides by selected fungi cultures. Sci. Total Environ. 2012, 435–436, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Singh, P.; Liu, Y.; Pan, S.G.; Wang, G.Y. Diversity and biochemical features of culturable fungi from the coastal waters of Southern China. AMB Expr. 2014, 4, 60. [Google Scholar] [CrossRef] [PubMed]
- Lapierre, S.G.; Phelippeau, M.; Hakimi, C.; Didier, Q.; Reynaud-Gaubert, M.; Dubus, J.-C.; Drancourt, M. Cystic fibrosis respiratory tract salt concentration: An Exploratory Cohort Study. Medicine 2017, 96, 8423. [Google Scholar] [CrossRef] [PubMed]
- Ketola, T.; Hiltunen, T. Rapid evolutionary adaptation to elevated salt concentrations in pathogenic freshwater bacteria Serratia marcescens. Ecol. Evol. 2014, 4, 3901–3908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tigini, V.; Bevione, F.; Prigione, V.; Poli, A.; Ranieri, L.; Spennati, F.; Munz, G.; Varese, G.C. Tannery mixed liquors from an ecotoxicological and mycological point of view: Risks vs potential biodegradation application. Sci. Total Environ. 2018, 627, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Rougeron, A.; Giraud, S.; Alastruey-Izquierdo, A.; Cano-Lira, J.; Rainer, J.; Mouhajir, A.; Le Gal, S.; Nevez, G.; Meyer, W.; Bouchara, J.P. Ecology of Scedosporium Species: Present Knowledge and Future Research. Mycopathologia 2018, 183, 185–200. [Google Scholar] [CrossRef] [PubMed]
- Anastasi, A.; Spina, F.; Prigione, V.; Tigini, V.; Giansanti, P.; Varese, G.C. Scale-up of a bioprocess for textile wastewater treatment using Bjerkandera adusta. Bioresour. Technol. 2010, 101, 3067–3075. [Google Scholar] [CrossRef] [PubMed]
- Svobodová, K.; Novotný, Č. Bioreactors based on immobilized fungi: Bioremediation under non-sterile conditions. Appl. Microbiol. Biotechnol. 2018, 102, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Hai, F.I.; Yamamoto, K.; Nakajima, F.; Fukushi, K.; Nghiem, L.D.; Price, W.E.; Jin, B. Degradation of azo dye acid orange 7 in a membrane bioreactor by pellets and attached growth of Coriolus versicolour. Bioresour. Technol. 2013, 141, 29–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.; Hai, F.I.; Nghiem, L.D.; Nguyen, L.N.; Roddick, F.; Price, W.E. Removal of bisphenol A and diclofenac by a novel fungal membrane bioreactor operated under nonsterile conditions. Int. Biodeterior. Biodegrad. 2013, 85, 483–490. [Google Scholar] [CrossRef]
- Spennati, F. Use of Fungi and Bacteria for the Removal of Recalcitrant Compounds from Tannery Wastewater. Ph.D. Thesis, University of Florence, Firenze, Italy, Autonomous University of Barcelona, Barcelona, Spain, 2018. Available online: https://flore.unifi.it/retrieve/handle/2158/1126916/334393/Spennati_PhD_Thesis.pdf (accessed on 2 July 2018).
- Upadhyay, R.C.; Singh, M. Cultivation of Edible Mushrooms. In The Mycota—Industrial Applications, 2nd ed.; Esser, K., Ed.; Springer: Berlin, Germany, 2010; Volume 10, p. 94. ISBN 978-3-642-11457-1. [Google Scholar]
- De Melo, M.R.; Paccola-Meirelles, L.D.; de Jesus Faria, T.; Kazue Ishikawa, N. Influence of Flammulina velutipes mycelia culture conditions on antimicrobial metabolite production. Mycoscience 2009, 50, 78–81. [Google Scholar] [CrossRef]
- Cho, N.S.; Kim, D.H.; Cho, H.Y.; Ohga, S.; Leonowicz, A. Effect of various compounds on the activity of laccases from Basidiomycetes and their oxidative and demethoxylating activities. J. Faculty Agric. Kyushu Univ. 2006, 51, 211–218. [Google Scholar]
- Ntougias, S.; Baldrian, P.; Ehaliotis, C.; Nerud, F.; Merhautová, V.; Zervakis, G.I. Olive mill wastewater biodegradation potential of white-rot fungi—Mode of action of fungal culture extracts and effects of ligninolytic enzymes. Bioresour. Technol. 2015, 189, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Kalčíková, G.; Babič, J.; Pavko, A.; Žěgajnar Gotvajn, A. Fungal and enzymatic treatment of mature municipal landfill leachate. Waste Manag. 2014, 34, 798–803. [Google Scholar] [CrossRef] [PubMed]
- Saetang, J.; Babel, S. Biodegradation of organics in landfill leachate by immobilized white rot fungi, Trametes versicolor BCC 8725. Environ. Technol. 2012, 33, 2575–2584. [Google Scholar] [CrossRef] [PubMed]
- Razarinah, W.A.R.W.; Zalina, M.N.; Abdullah, N. Treatment of landfill leachate by immobilized Ganoderma australe and crude enzyme. ScienceAsia 2014, 40, 335–339. [Google Scholar] [CrossRef]
- Aleksandrova, G.P.; Petrov, A.N.; Medvedeva, S.A.; Babkin, V.A. Screening of lignin-degrading fungi for biotechnological purposes. Appl. Biochem. Microbiol. 1998, 34, 245–250. [Google Scholar]
- Kazartsev, I.A.; Soloviev, V.A. Application of mathematical models to describe the wood decomposition process caused by lignin-degrading fungi. Sydowia 2012, 64, 221–232. [Google Scholar]
- Xie, C.; Gong, W.; Yan, L.; Zhu, Z.; Hu, Z.; Peng, Y. Biodegradation of ramie stalk by Flammulina velutipes: Mushroom production and substrate utilization. Amb Express 2017, 7, 171. [Google Scholar] [CrossRef] [PubMed]
- Saetang, J.; Babel, S. Effect of leachate loading rate and incubation period on the treatment efficiency by T. versicolor immobilized on foam cubes. Int. J. Environ. Sci. Technol. 2009, 6, 457–466. [Google Scholar] [CrossRef] [Green Version]
- Nayanashree, G.; Thippeswamy, B. Natural rubber degradation by laccase and manganese peroxidase enzymes of Penicillium chrysogenum. Int. J. Environ. Sci. Technol. 2015, 12, 2665–2672. [Google Scholar] [CrossRef]
- Guisado, G.; LÓpez, M.J.; Vargas-García, M.C.; Suárez-Estrella, F.; Moreno, J. Pseudallescheria angusta, a ligninolytic microorganism for wood fibres biomodification. BioResources 2012, 7, 464–474. [Google Scholar]
- Tigini, V.; Prigione, V.; Di Toro, S.; Fava, F.; Varese, G.C. Isolation and characterisation of polychlorinated biphenyl (PCB) degrading fungi from a historically contaminated soil. Microb. Cell Factories 2009, 8, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loffredo, E.; Castellana, G. Comparative evaluation of the efficiency of low-cost adsorbents and ligninolytic fungi to remove a combination of xenoestrogens and pesticides from a landfill leachate and abate its phytotoxicity. J. Environ. Sci. Health A 2015, 50, 958–970. [Google Scholar]
- Soni, D.; Naoghare, P.S.; Saravanadevi, S.; Pandey, R.A. Release, Transport and Toxicity of Engineered Nanoparticles. In Reviews of Environmental Contamination and Toxicology; Whitacre, D.M., Ed.; Springer: Cham, Switzerland, 2014; Volume 234, p. 23. [Google Scholar]
- Gadd, M.G. Biosorption: Critical review of scientific rationale, environmental importance and significance for pollution treatment. J. Chem. Technol. Biotechnol. 2009, 84, 13–28. [Google Scholar] [CrossRef]
- Gao, D.; Du, L.; Yang, J.; Wu, W.; Liang, H. A critical review of the application of white rot fungus to environmental pollution control. Crit. Rev. Biotechnol. 2010, 30, 70–77. [Google Scholar] [CrossRef] [PubMed]



| Parameter | Leachate | Effluent | Legal Threshold Limit |
|---|---|---|---|
| Cuprum (as Cu) | 0.60 mg L−1 | 0.15 mg L−1 | 0.1 mg L−1 |
| Zinc (as Zn) | 0.66 mg L−1 | 1.38 mg L−1 | 0.5 mg L−1 |
| Total hydrocarbons | <100 mg L−1 | 598.7 mg L−1 | 5 mg L−1 |
| Ammonium (as NH4) | 2266.0 mg L−1 | 408.0 mg L−1 | 15 mg L−1 |
| Total nitrogen (as N) | 2537.9 mg L−1 | 514.1 mg L−1 | 20 mg L−1 |
| Chlorides (as Cl−) | 3193 mg L−1 | 2550 mg L−1 | 1200 mg L−1 |
| Anionic surfactants (MBAS) | 23.07 mg L−1 | 37.9 mg L−1 | 2 mg L−1 |
| Non ionic surfactants (PPAS) | 23.48 mg L−1 | 17.2 mg L−1 | |
| Cationic surfactants | <10 mg L−1 | <10 mg L−1 | |
| COD (as O2) | 6166 mg L−1 | 2099 mg L−1 | 160 mg L−1 |
| BOD5 (as O2) | 4209 mg L−1 | 1410 mg L−1 | 40 mg L−1 |
| Suspended solids | 1064 mg L−1 | 604 mg L−1 | 80 mg L−1 |
| Colour | visible at 1:20 dilution | visible at 1:20 dilution | not visible at 1:20 dilution |
| R. subcapitata | L. sativum | ||
|---|---|---|---|
| Autochthonous fungi | MUT 1269 | −7% | −39% |
| MUT 721 | −100% | −40% | |
| MUT 793 | −53% | −4% | |
| MUT 1275 | 69% | −49% | |
| MUT 1284 | 211% | −13% | |
| Allochthonous fungi | MUT 2400 | 211% | −5% |
| MUT 1585 | 42% | −35% | |
| MUT 2295 | −72% | −44% | |
| MUT 2976 | −48% | −35% | |
| MUT 3060 | −33% | −16% | |
| Fungal Cultures | L. sativum 100% | R. subcapitata 6.25% |
|---|---|---|
| Free biomass MUT 1585 | 74.9% | 65.7% |
| Free biomass MUT 1284 | 82.3% | −60.9% |
| Free biomass MUT 721 | 24.3% | −0.9% |
| Immobilised biomass MUT 1585 | 74.8% | 29.1% |
| Immobilised biomass MUT 1284 | 11.0% | −2.9% |
| Immobilised biomass MUT 721 | 45.0% | −13.7% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spina, F.; Tigini, V.; Romagnolo, A.; Varese, G.C. Bioremediation of Landfill Leachate with Fungi: Autochthonous vs. Allochthonous Strains. Life 2018, 8, 27. https://doi.org/10.3390/life8030027
Spina F, Tigini V, Romagnolo A, Varese GC. Bioremediation of Landfill Leachate with Fungi: Autochthonous vs. Allochthonous Strains. Life. 2018; 8(3):27. https://doi.org/10.3390/life8030027
Chicago/Turabian StyleSpina, Federica, Valeria Tigini, Alice Romagnolo, and Giovanna Cristina Varese. 2018. "Bioremediation of Landfill Leachate with Fungi: Autochthonous vs. Allochthonous Strains" Life 8, no. 3: 27. https://doi.org/10.3390/life8030027





