Effects of Low-Temperature Plasma-Sterilization on Mars Analog Soil Samples Mixed with Deinococcus radiodurans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Incubation Medium
2.2. Growing and Handling of Cells
2.3. Plasma Sterilization Procedure
2.4. Colony Forming Unit (CFU) Counts
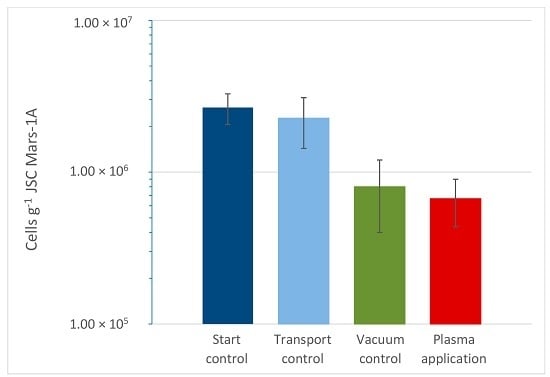
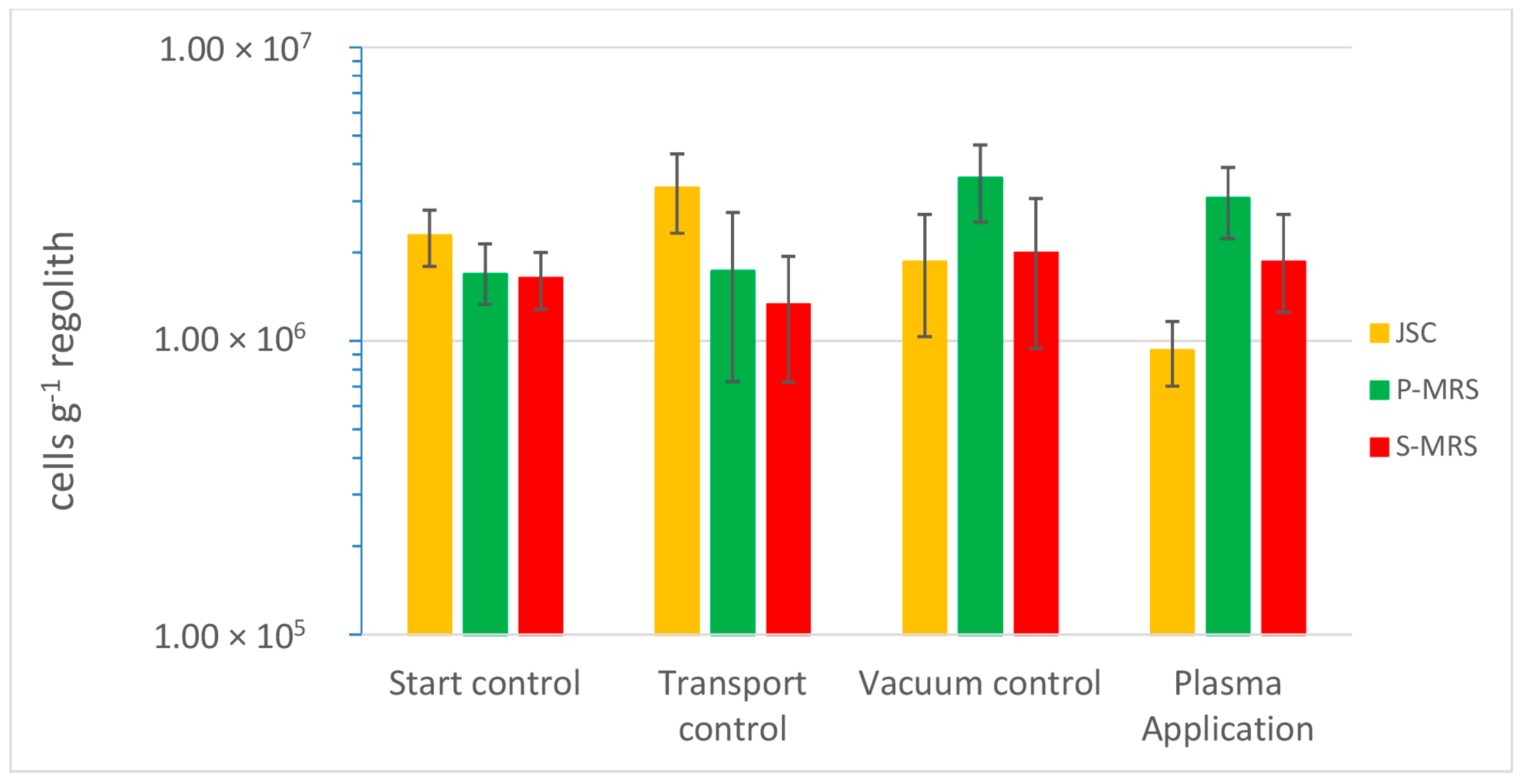
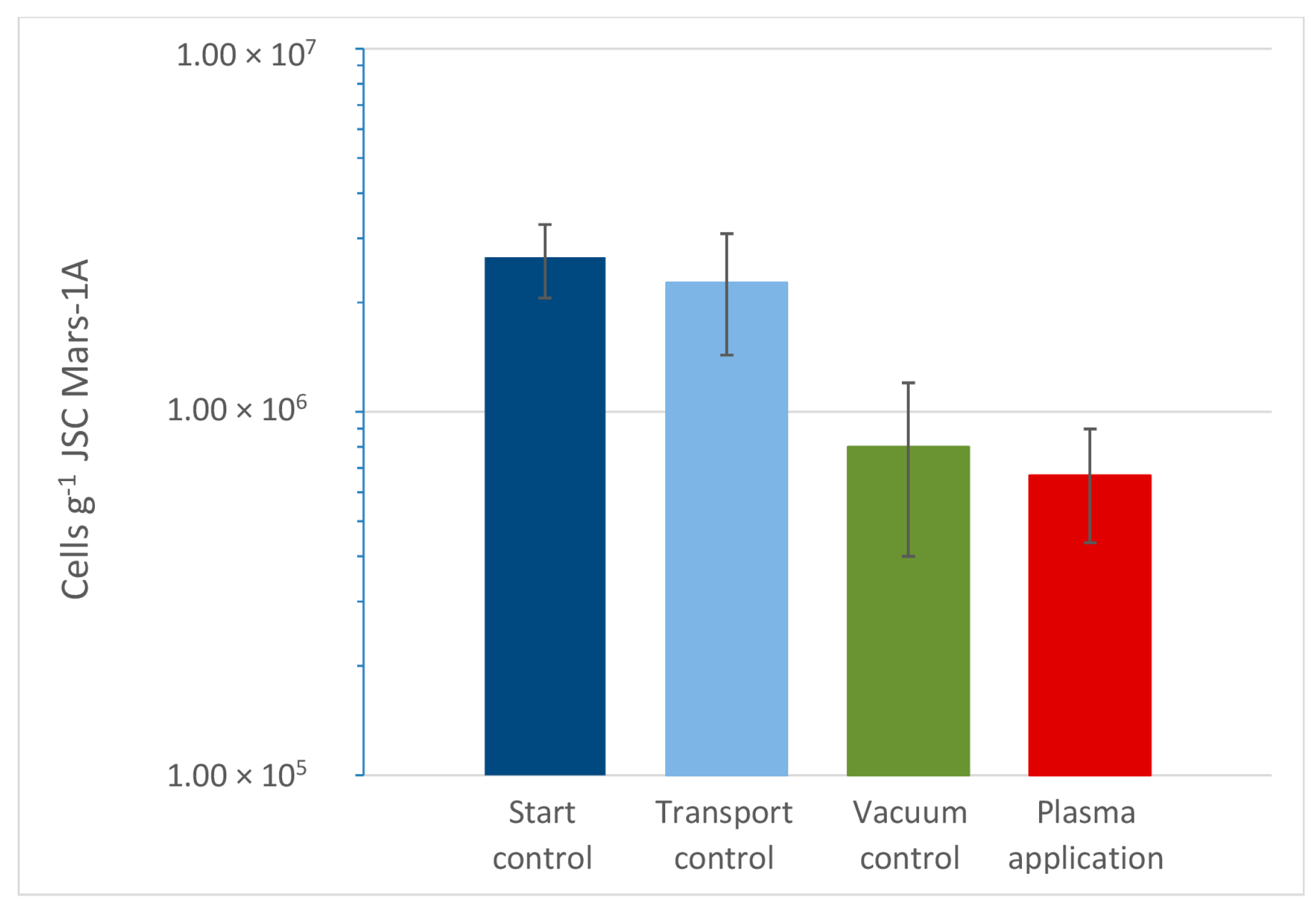
3. Results
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CFU | Colony forming units |
| TGY (medium) | Trypticase—Glucose—Yeast extract cultivation medium |
| PBS | Phosphate-buffered saline |
| JSC Mars-1A | Johnson Space Center Mars regolith analog |
| P-MRS | Phylosilicatic Mars regolith analog |
| S-MRS | Sulfatic Mars regolith analog |
| DICP | Double inductively plasma system |
References
- Stapelmann, K.; Fiebrandt, M.; Raguse, M.; Awakowicz, P.; Reitz, G.; Moeller, R. Utilization of Low-Pressure Plasma to Inactivate Bacterial Spores on Stainless Steel Screws. Astrobiology 2013, 13, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Bauermeister, A.; Moeller, R.; Reitz, G.; Sommer, S.; Rettberg, P. Effect of Relative Humidity on Deinococcus radiodurans’ Resistance to Prolonged Desiccation, Heat, Ionizing, Germicidal, and Environmentally Relevant UV Radiation. Microb. Ecol. 2011, 61, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Cox, M.M.; Battista, J.R. Deinococcus radiodurans—The consummate survivor. Nat. Rev. Microbiol. 2005, 3, 882–892. [Google Scholar] [CrossRef] [PubMed]
- Schirmack, J.; Alawi, M.; Wagner, D. Influence of Martian regolith analogs on the activity and growth of methanogenic archaea, with special regard to long-term desiccation. Front. Microbiol. 2015, 6, 210. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.V.; Ruff, S.W.; Douglas, R.G.; Ming, W.; Arvidson, R.E.; Clark, B.C.; Golden, C.D.; Siebach, K.; Klingelhöfer, G.; Schröder, C.; et al. Identification of Carbonate-Rich Outcrops on Mars by the Spirit Rover. Science 2010, 329, 421. [Google Scholar] [CrossRef] [PubMed]
- Halfmann, H.; Bibinov, N.; Wunderlich, J.; Awakowicz, P. A double inductively coupled plasma for sterilization of medical devices. J. Phys. D Appl. Phys. 2007, 40, 4145–4154. [Google Scholar] [CrossRef]
- Raguse, M.; Fiebrandt, M.; Denis, B.; Stapelmann, K.; Eichenberger, P.; Driks, A.; Eaton, P.; Awakowicz, P.; Moeller, R. Understanding of the importance of the spore coat structure and pigmentation in the Bacillus subtilis spore resistance to low pressure plasma sterilization. J. Phys. D Appl. Phys. 2016. under review. [Google Scholar]
- Halfmann, H.; Denis, B.; Bibinov, N.; Wunderlich, J.; Awakowicz, P. Utilization of Identification of the most efficient VUV/UV radiation for plasma based inactivation of Bacillus atrophaeus spores. J. Phys. D Appl. Phys. 2007, 40, 5907–5911. [Google Scholar] [CrossRef]
- Horneck, G.; Rettberg, P.; Reitz, G.; Wehner, J.; Eschweiler, U.; Strauch, K.; Panitz, C.; Starke, V.; Baumstrak-Khan, C. Protection of Bacterial Spares in Space, A Contribution to the Discussion on Panspermia. Orig. Life Evol. Biosph. 2001, 31, 527–547. [Google Scholar] [CrossRef] [PubMed]
- Raguse, M.; Fiebrandt, M.; Stapelmann, K.; Madela, K.; Laue, M.; Lackmann, J.-W.; Thwaite, J.E.; Setlow, P.; Awakowicz, P.; Moeller, R. Improvement of biological indicators by uniformLy distributing Bacillus subtilis spores in monolayers to evaluate enhanced spore decontamination technologies. Appl. Environ. Microbiol. 2016, 82, 2031–2038. [Google Scholar] [CrossRef] [PubMed]
- Diaz, B.; Schulze-Makuch, D. Microbial Survival Rates of Escherichia coli and Deinococcus radiodurans under Low Temperature, Low Pressure, and UV-Irradiation Conditions, and Their Relevance to Possible Martian Life. Astrobiology 2006, 6, 332–347. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Wendner, R.; Cusatis, G. A Novel Material for In Situ Construction on Mars: Experiments and Numerical Simulations. Constr. Build. Mater. 2016, 120, 222–232. [Google Scholar] [CrossRef]
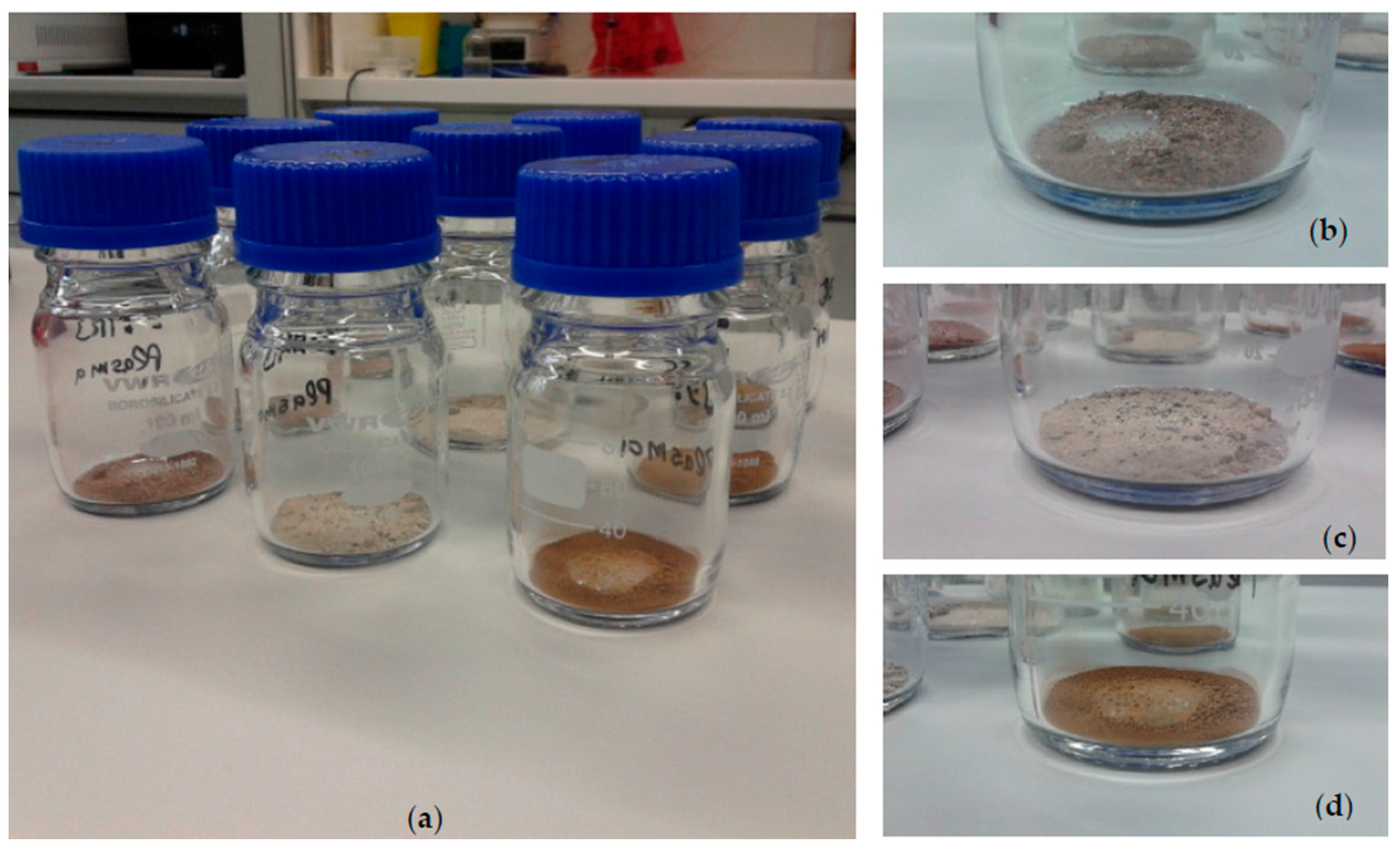
| Mineral Phase | JSC Mars-1A (wt %) | P-MRA (wt %) | S-MRA (wt %) |
|---|---|---|---|
| Plagioclase Feldspar | 64 | - | - |
| Olivine | 12 | - | - |
| Magnetite | 11 | - | - |
| Pyroxene and/or Glass | 9 | - | - |
| Fe2O3 | - | 5 | - |
| Montmorillonite | - | 45 | - |
| Chamosite | - | 20 | - |
| Kaolinite | - | 5 | - |
| Siderite | - | 5 | - |
| Hydromagnesite | - | 5 | - |
| Quartz | - | 10 | 3 |
| Gabbro | - | 3 | 31 |
| Dunite | - | 2 | 16 |
| Hematite | 5 | - | 17 |
| Goethite | - | - | 3 |
| Gypsum | - | - | 30 |
| Grain Size [µm] | P-MRS | S-MRS |
|---|---|---|
| 1000–550 | 13% | 30% |
| 550–300 | 11% | 26% |
| 300–150 | 12% | 16% |
| 150–50 | 50% | 14% |
| 50–0 | 14% | 14% |
| Grain Size [µm] | JSC Mars-1A |
|---|---|
| 1000–500 | 20% |
| 500–250 | 30% |
| 250–150 | 20% |
| 150–100 | 12% |
| 100–50 | 10% |
| 50–0 | 8% |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schirmack, J.; Fiebrandt, M.; Stapelmann, K.; Schulze-Makuch, D. Effects of Low-Temperature Plasma-Sterilization on Mars Analog Soil Samples Mixed with Deinococcus radiodurans. Life 2016, 6, 22. https://doi.org/10.3390/life6020022
Schirmack J, Fiebrandt M, Stapelmann K, Schulze-Makuch D. Effects of Low-Temperature Plasma-Sterilization on Mars Analog Soil Samples Mixed with Deinococcus radiodurans. Life. 2016; 6(2):22. https://doi.org/10.3390/life6020022
Chicago/Turabian StyleSchirmack, Janosch, Marcel Fiebrandt, Katharina Stapelmann, and Dirk Schulze-Makuch. 2016. "Effects of Low-Temperature Plasma-Sterilization on Mars Analog Soil Samples Mixed with Deinococcus radiodurans" Life 6, no. 2: 22. https://doi.org/10.3390/life6020022