Portrait of a Geothermal Spring, Hunter’s Hot Springs, Oregon
Abstract
:1. Introduction
2. The Synechococcus/Chloroflexus Zone



3. The Geitlerinema/Chloroflexus Zone
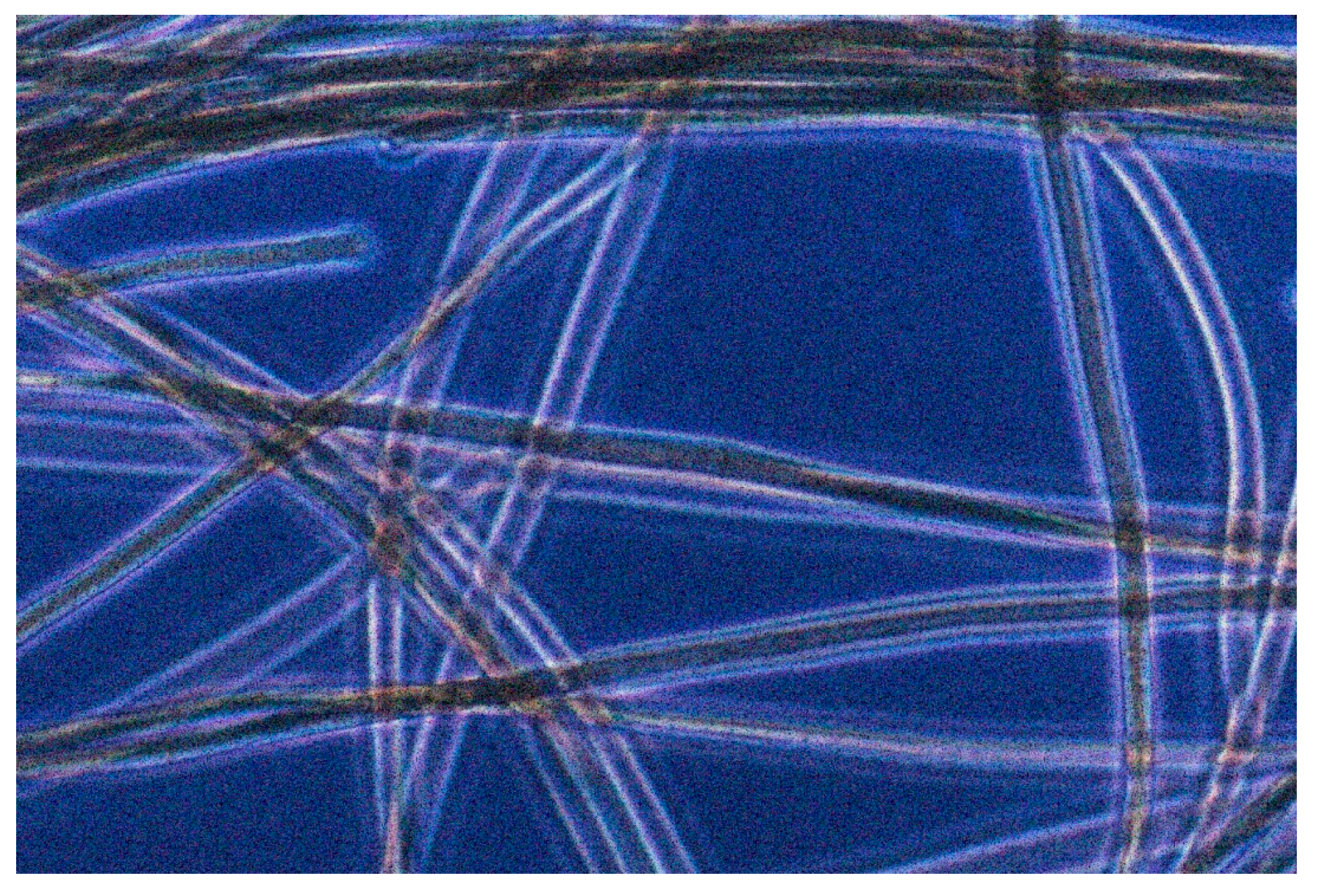
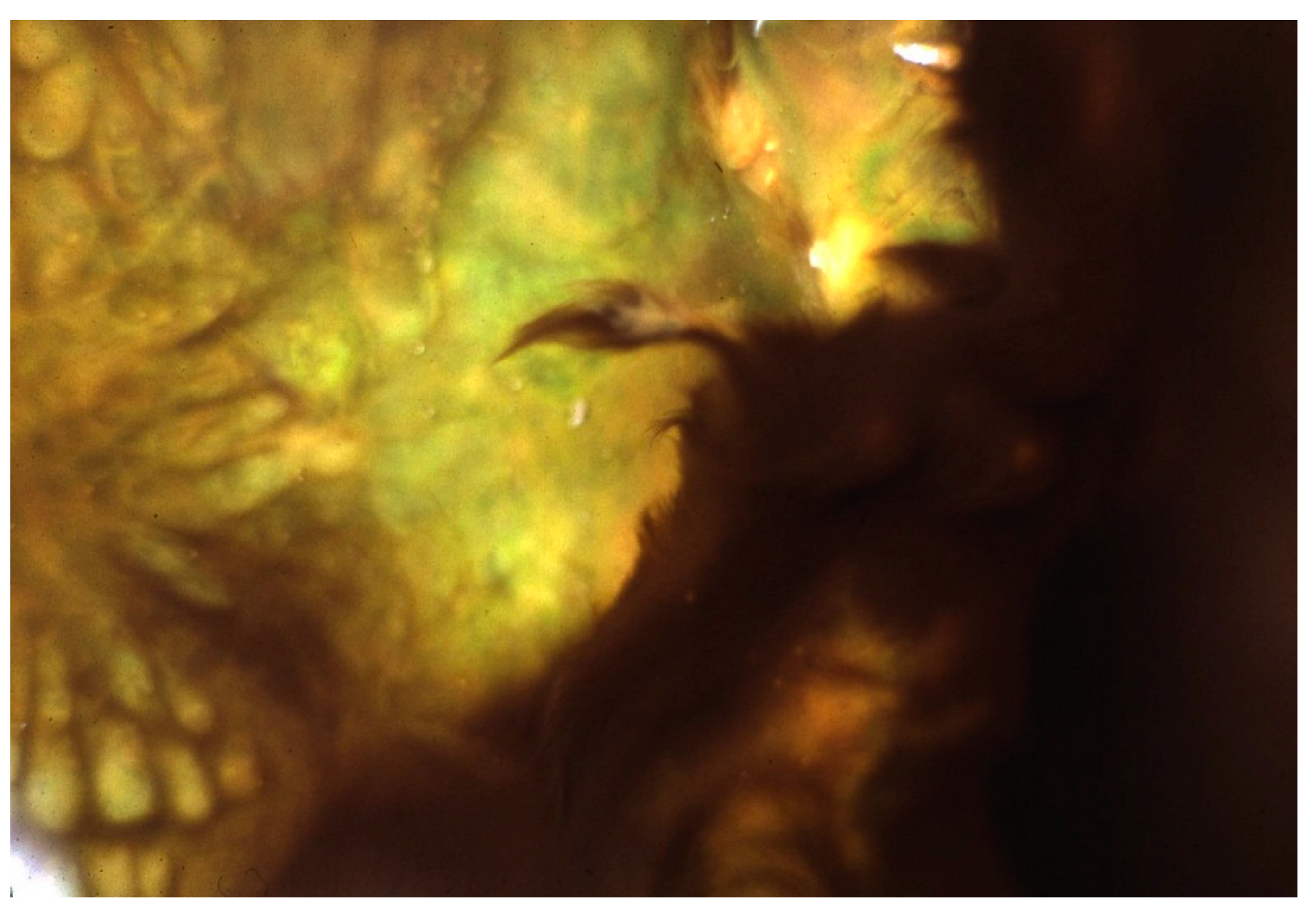


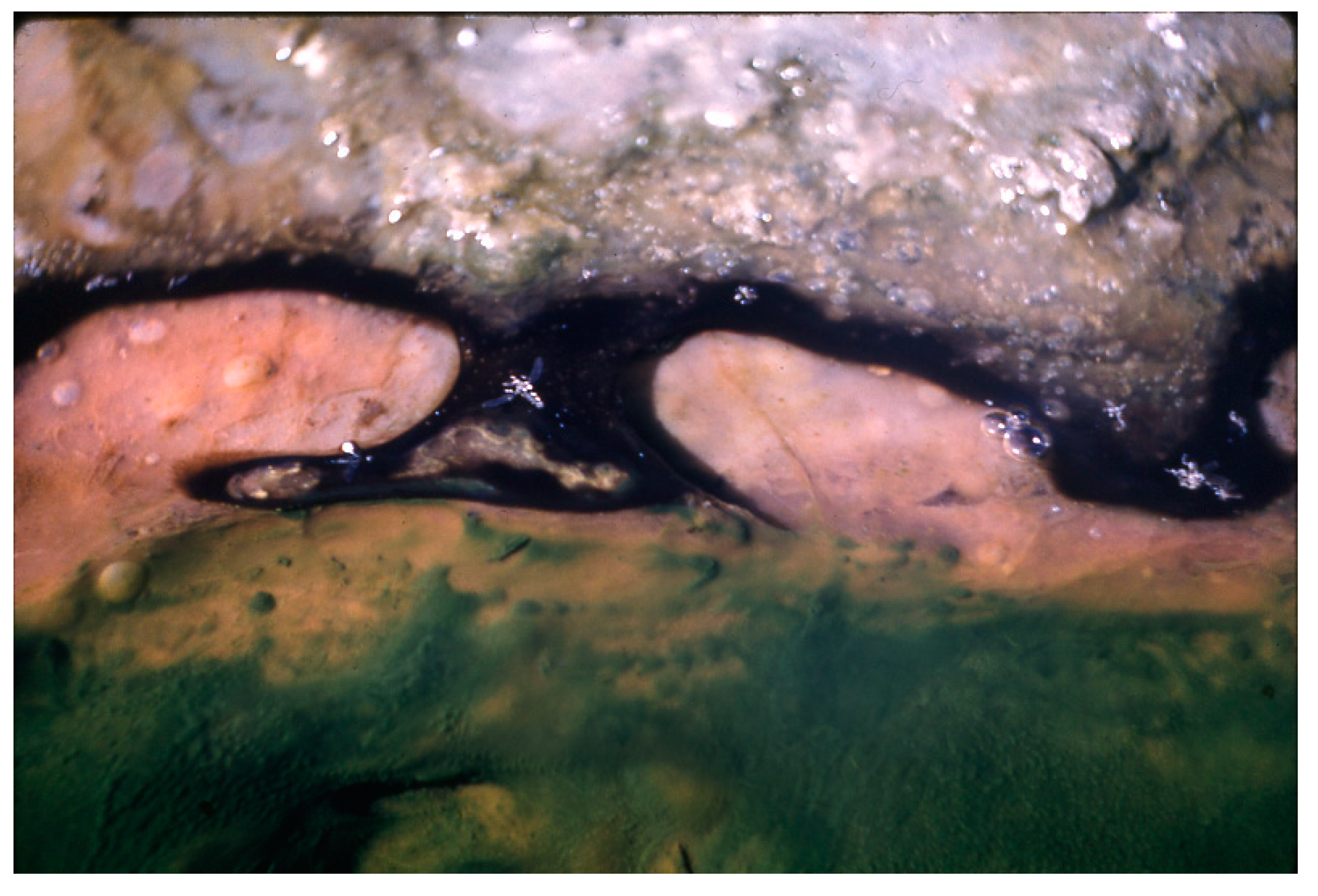
4. The Ostracod/Pleurocapsa/Calothrix Zone
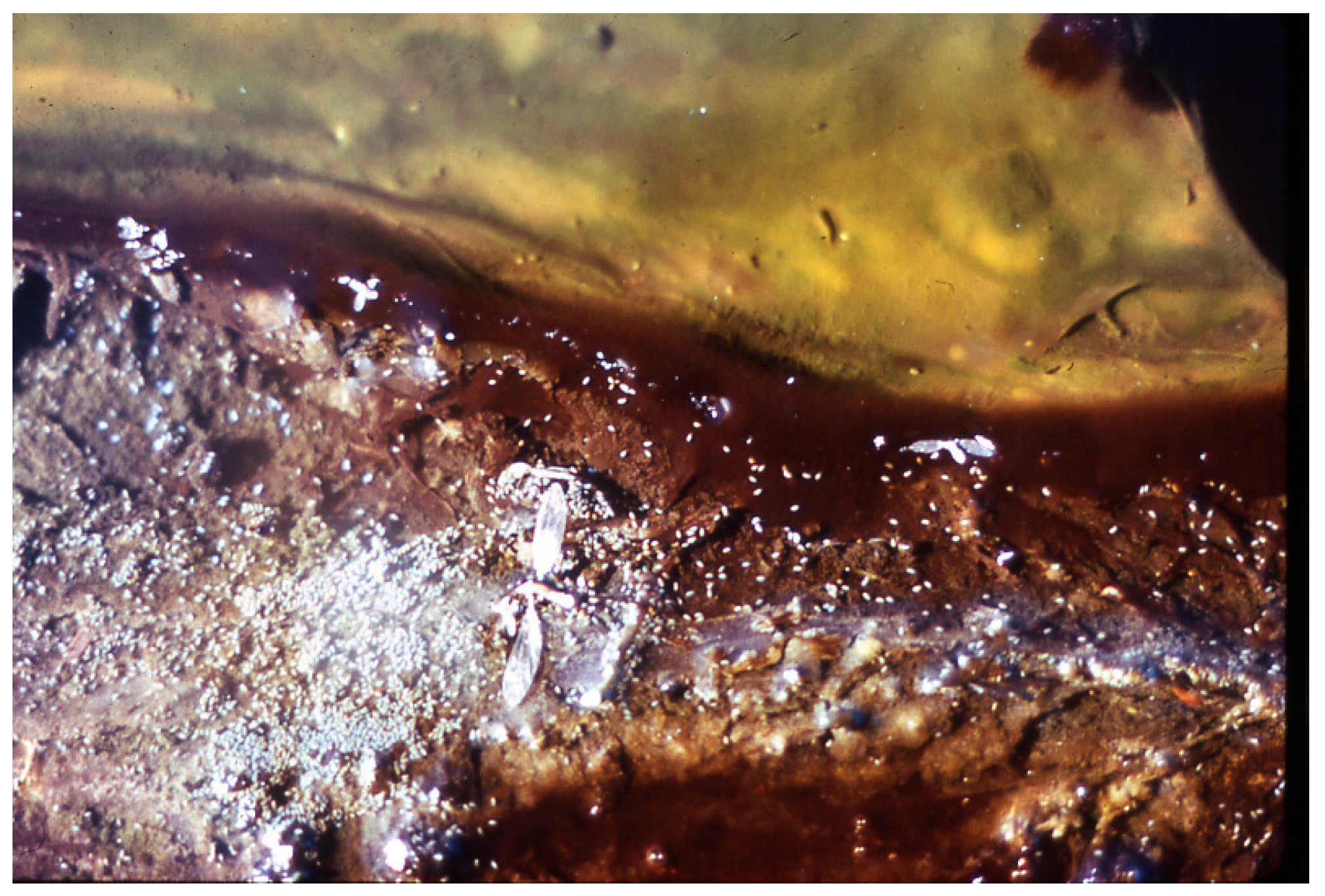
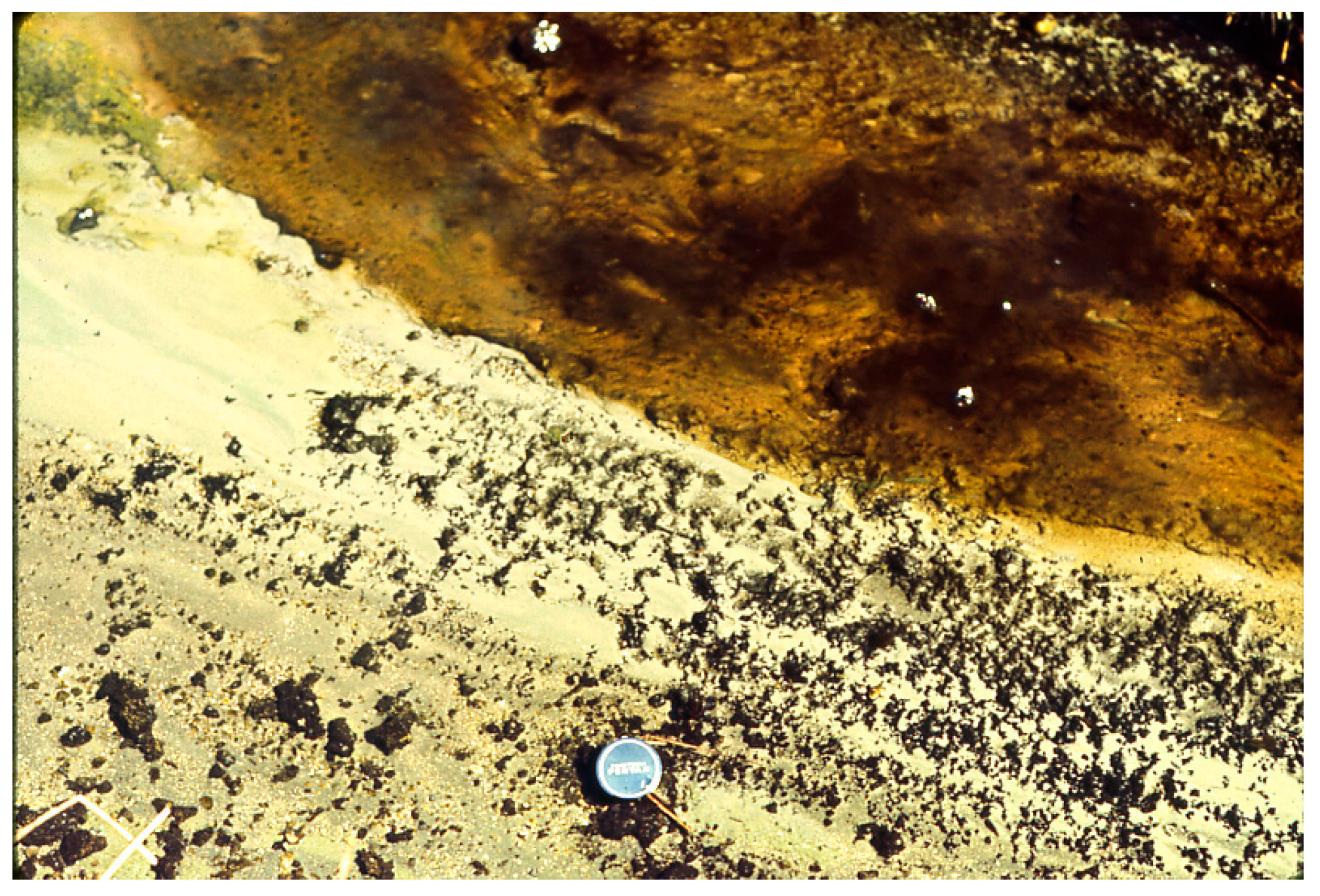


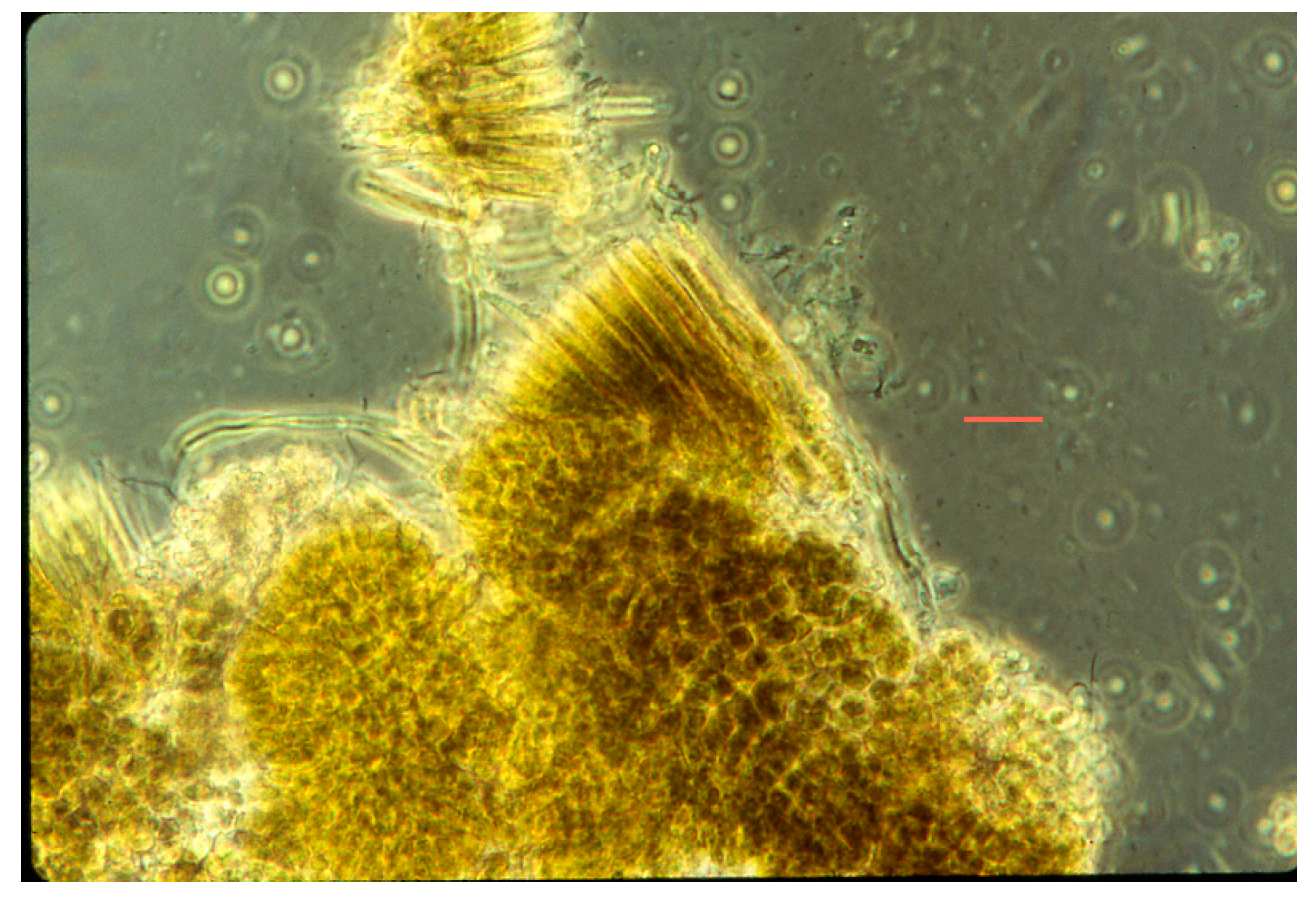

5. Sulfate Reducing Zone: Thermochromatium/Beggiatoa/Geitlerinema


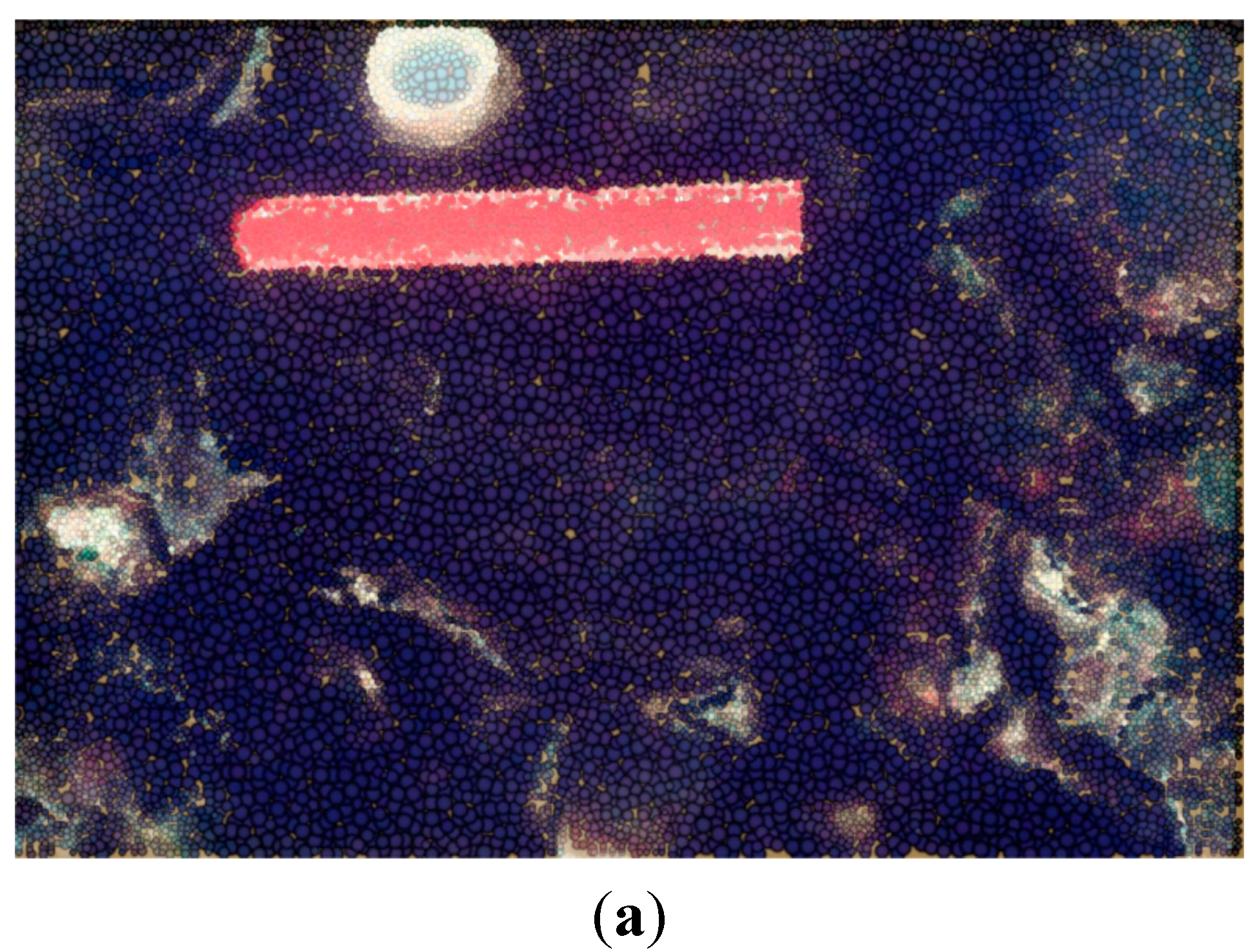

6. Discussion

Acknowledgments
Conflicts of Interest
References
- Wingard, C.E.; Schiller, J.R.; Miller, S.R.; Castenholz, R.W. An Inventory of the Photosynthetic Microorganisms of Hot Springs of Borax Lake, Oregon and Selected Hot Springs of the Northern Great Basin, USA; The Nature Conservancy: Arlington, VA, USA, 1996; p. 40. [Google Scholar]
- Mariner, R.H.; Rapp, J.B.; Willey, L.M.; Presser, T.S. The Chemical Composition and Estimated Minimum Thermal Reservoir Temperature of Selected Hot Springs in Oregon; US Geological Survey: Menlo Park, CA, USA, 1974; p. 27. [Google Scholar]
- Peary, J.; Castenholz, R.W. Temperature strains of a thermophilic blue-green alga. Nature 1964, 202, 720–721. [Google Scholar] [CrossRef]
- Ward, D.M.; Miller, S.R.; Castenholz, R.W. Cyanobacteria in geothermal habitats. In Ecology of Cynobacteria II; Whitton, B.A., Ed.; Springer: New York, NY, USA, 2012; pp. 39–63. [Google Scholar]
- Miller, S.R.; Castenholz, R.W. The evolution of thermotolerance in hot spring cyanobacteria of the genus Synechococcus. Appl. Environ. Microbiol. 2000, 66, 4222–4229. [Google Scholar] [PubMed]
- Miller, S.R. Evidence for the adaptive evolution of the carbon fixation gene rbcL during diversification in temperature tolerance of a clade of hot spring cyanobacteria. Mol. Ecol. 2003, 12, 1237–1246. [Google Scholar]
- Miller, S.R.; McGuirl, M.A.; Carvey, D. The evolution of RiBisCO stability at the thermal limit of photoautotrophy. Mol. Biol. Evol. 2013, 30, 752–760. [Google Scholar] [CrossRef]
- Meeks, J.C.; Castenholz, R.W. Growth and photosynthesis in an extreme thermophile, Synechococcus lividus (Cyanophyta). Arch. Mikrobiol. 1971, 78, 25–41. [Google Scholar] [CrossRef]
- Meeks, J.C.; Castenholz, R.W. Photosynthetic properties of the extreme thermophile, Synechococcus lividus. I. Effect of temperature on fluorescence and enhancement of CO2 assimilation. J. Thermal. Biol. 1978, 3, 11–18. [Google Scholar]
- Meeks, J.C.; Castenholz, R.W. Photosynthetic properties of the extreme thermophile, Synechococcus lividus. II. Stoichiometry between oxygen evolution and carbon dioxide assimilation. J. Thermal. Biol. 1978, 3, 19–24. [Google Scholar]
- Pierson, B.K.; Castenholz, R.W. A phototrophic gliding filamentous bacterium of hot springs, Chloroflexus aurantiacus, gen. and sp. nov. Arch. Microbiol. 1974, 100, 5–24. [Google Scholar]
- Ramsing, N.B.; Ferris, M.J.; Ward, D.M. Highly ordered vertical structure of Synechococcus populations within the one-millimeter-thick photic zone of a hot spring cyanobacterial mat. Appl. Environ. Microbiol. 2000, 66, 1038–1049. [Google Scholar] [CrossRef] [PubMed]
- Stenou, A.-S.; Bhaya, D.; Bateson, M.M.; Melendrez, M.C.; Ward, D.M.; Brecht, E.; Peters, J.W.; Kühl, M.; Grossman, A.R. In situ analysis of nitrogen fixation and metabolic switching in unicellular thermophilic cyanobacteria inhabiting hot spring microbial mats. Proc. Natl. Acad. Sci. USA 2006, 103, 2398–2403. [Google Scholar] [PubMed]
- Castenholz, R.W. Thermophilic blue-green algae and the thermal environment. Bact. Rev. 1969, 33, 476–504. [Google Scholar]
- Castenholz, R.W. The behavior of Oscillatoriaterebriformis in hot springs. J. Phycol. 1968, 4, 132–139. [Google Scholar]
- Castenholz, R.W. Aggregation in a thermophilic Oscillatoria. Nature 1967, 215, 1285–1286. [Google Scholar] [CrossRef] [PubMed]
- Castenholz, R.W. Movements. In The Biology of Blue-green Algae; Carr, N.G., Whitton, B.A., Eds.; Blackwell: Oxford, UK, 1973; pp. 320–339. [Google Scholar]
- Richardson, L.L.; Castenholz, R.W. Diel vertical movements of the cyanobacterium Oscillatoria terebriformis in a sulfide-rich hot spring microbial mat. Appl. Environ. Microbiol. 1987, 53, 2142–2150. [Google Scholar] [PubMed]
- Nelson, D.C.; Castenholz, R.W. Light responses of Beggiatoa. Arch. Microbiol. 1981, 131, 146–155. [Google Scholar]
- Wickstrom, C.E.; Castenholz, R.W. Dynamics of cyanobacteria-ostracod interactions in an Oregon hot spring. Ecology 1985, 66, 1024–1041. [Google Scholar] [CrossRef]
- Nelson, D.C.; Castenholz, R.W. Organic nutrition of Beggiatoasp. J. Bacteriol. 1981, 147, 236–247. [Google Scholar]
- Kulkoyluoglu, O.; Munch, C.; Rust, R.W. Thermopsis thermophile, n. gen., n sp. from hot springs in Nevada (Crustacea, Ostracoda). Hydrobiologia 2003, 499, 113–123. [Google Scholar]
- Wickstrom, C.E.; Castenholz, R.W. Thermophilic ostracod: Aquatic metazoan with the highest known temperature tolerance. Science 1973, 181, 1063–1064. [Google Scholar] [CrossRef] [PubMed]
- Castenholz, R.W.; Garcia-Pichel, F. Cyanobacterial responses to UV radiation. In Ecology of Cyanobacteria II; Whitton, B.A., Ed.; Springer: New York, NY, USA, 2012; pp. 481–499. [Google Scholar]
- Castenholz, R.W. Low temperature acclimation and survival in thermophilic Oscillatoria terebriformis. In Taxonomy and Biology of Blue-green Algae; Desikachary, T.V., Ed.; University of Madras: Madras, India, 1972; pp. 406–418. [Google Scholar]
- Wickstrom, C.E.; Castenholz, R.W. Association of Pleurocapsa and Calothrix (Cyanophyta) in thermal streams. J. Phycol. 1978, 14, 84–88. [Google Scholar]
- Castenholz, R.W. Ecology of Blue-green algae in hot springs. In The Biology of Blue-green Algae; Carr, N.G., Whitton, B.A., Eds.; Blackwell: Oxford, UK, 1973; pp. 379–414. [Google Scholar]
- Jue, C.-S. The effect of aerobic environments on Chromatium cf. tepidum, a thermophilic purple sulfur bacterium. Master’s Thesis, University of Oregon, Eugene, OR, USA, 1990; p. 79. [Google Scholar]
- Nelson, D.C.; Castenholz, R.W. The use of reduced sulfur compounds by Beggiatoa sp. J. Bacteriol. 1981, 147, 140–154. [Google Scholar]
- Halfen, L.N.; Castenholz, R.W. Gliding motility in the blue-green alga Oscillatoria princeps. J. Phycol. 1971, 7, 133–145. [Google Scholar]
- Richardson, L.L.; Castenholz, R.W. Enhanced survival of the cyanobacterium Oscillatoria terebriformis in darkness under anaerobic conditions. Appl. Environ. Microbiol. 1987, 53, 2151–2158. [Google Scholar] [PubMed]
- Richardson, L.L.; Castenholz, R.W. Chemokinetic motility responses of the cyanobacterium Oscillatoria terebriformis. Appl. Environ. Microbiol. 1989, 55, 261–263. [Google Scholar] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castenholz, R.W. Portrait of a Geothermal Spring, Hunter’s Hot Springs, Oregon. Life 2015, 5, 332-347. https://doi.org/10.3390/life5010332
Castenholz RW. Portrait of a Geothermal Spring, Hunter’s Hot Springs, Oregon. Life. 2015; 5(1):332-347. https://doi.org/10.3390/life5010332
Chicago/Turabian StyleCastenholz, Richard W. 2015. "Portrait of a Geothermal Spring, Hunter’s Hot Springs, Oregon" Life 5, no. 1: 332-347. https://doi.org/10.3390/life5010332





