Prebiotic Chemistry: Geochemical Context and Reaction Screening
Abstract
:1. Introduction
2. Historical Factors

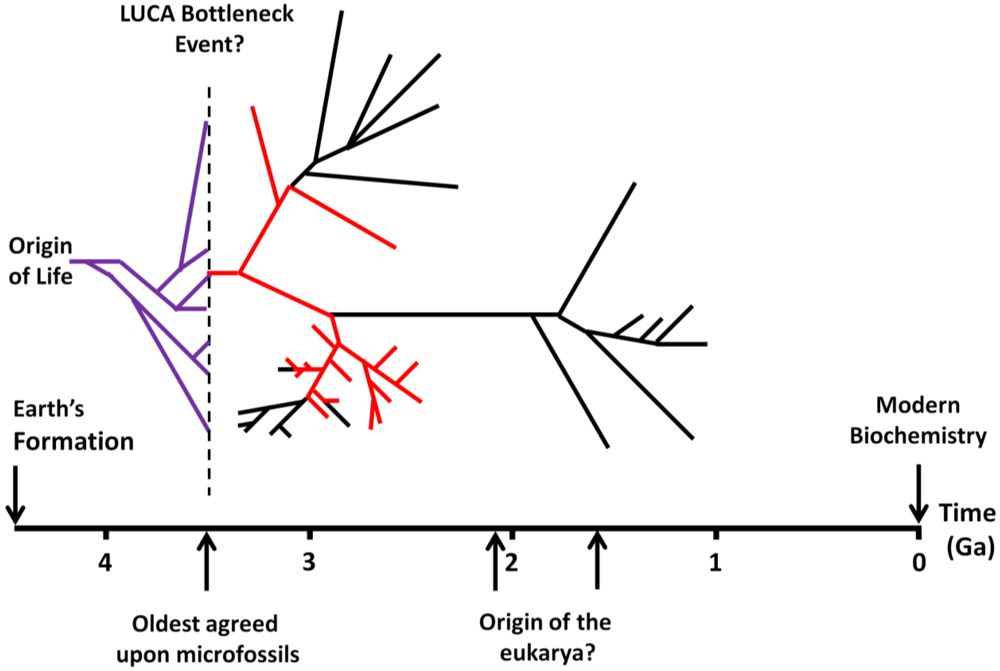
3. The Tempo and Timing of the Origin of Life
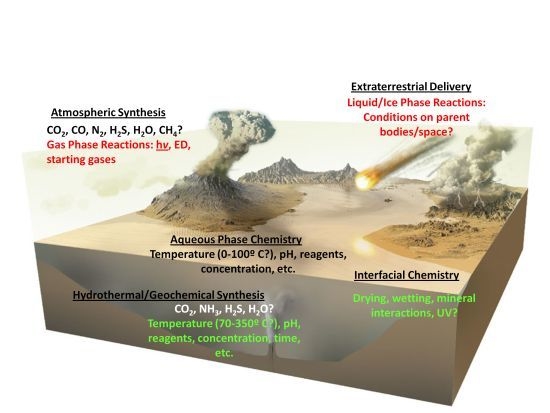
4. Historical Contingency and Immeasurable Variables
- The composition of the early atmosphere. While it was initially estimated that the early atmosphere was highly reducing, others have argued that it was not, and an atmosphere essentially similar to the present day one, albeit without abundant molecular oxygen was prevalent. This would affect the flux of solar radiation at various wavelengths reaching surface environments [62], the braking rates of impactors [35], as well as the flux and type of organics reaching the surface.
- The composition and properties of global and local primitive water bodies (e.g., pH, salinity, temperature), which would affect aqueous chemistry in many subtle ways (as discussed below).
- Prevailing temperatures on the prebiotic Earth’s surface, which has fundamental consequences for organic chemical processes (also discussed below).
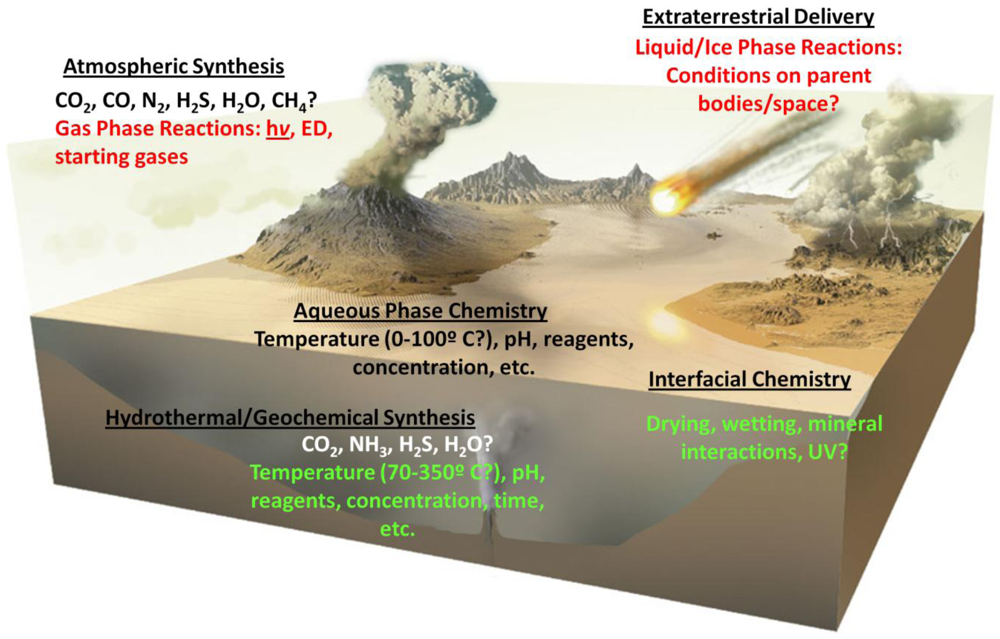
5. Organic Chemistry as the Critical, Ahistorical Factor
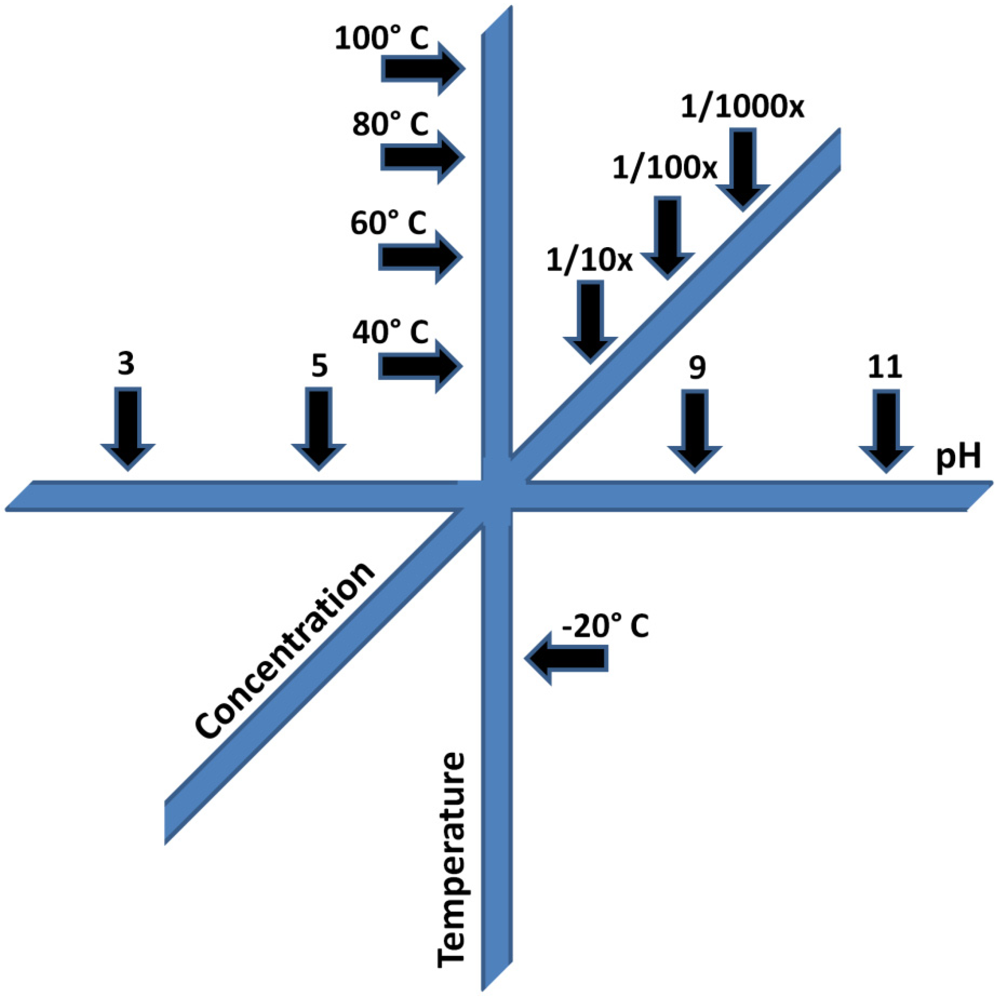
- pH. A change in the pH value of the reaction environment by single pH unit can easily change reaction rates by a factor of 10. At some pH values reactions may be so inhibited, no observable reaction occurs at all. The implications for prebiotic chemistry are large, and numerous examples of dramatic pH-dependent effects exist in the literature. For example the keto-enol equilibria of the nitrogenous bases in nucleic acids, e.g., [66]; vesicle formation and stability [67], primary synthetic reactions such as the formose reaction and cyanide polymerization [68,69], and the stability of various organic compounds potentially important for the origin of life, including, but not limited to sugars [55], nucleobases [54] and small molecules such as HCN and formamide (HCONH2) [70].
- Concentration. Reaction rates, with few exceptions, are often proportional to reactant concentration, since the probability that two molecules will come together and react decreases as the concentration of reactants is lowered. While some multi-reactant processes may proceed in extremely dilute solution, e.g., [71], others are markedly slowed or do not proceed at all upon dilution, e.g., [68,72]. Concentration is also pathway-dependant; compounds must find their way by plausible processes to the environment in which they become concentrated. For example, a considerable body of work has studied the possible role of HCONH2 as a precursor to prebiotic organics. To date, reactions have not been shown to work in dilute aqueous solution, and appear to largely require temperatures above the boiling point of water [73]. Concentration also includes two limits, a precipitation limit, beyond which compounds are insoluble, and a dilution limit, beyond which reaction is undetectable. In practice, the analytical limit of detection may be reached, but this can be overcome by various techniques. These are relatively trivial problems compared with the problem of not knowing where reactions become unproductive.
- Temperature. Most reaction rates scale as a function of temperature, according to the well-known Arrhenius equation. A typical organic transformation may increase or decrease in rate by a factor of 2 to 3 for each 10 °C change in temperature. For complex multi-component or multi-pathway reactions, these ratios may be extremely important in determining the course of reactions and their outcomes. In cases where multiple chemical steps occur simultaneously, and there are multiple reactants which are sensitive to temperature effects, the most sensitive component deserves the most careful consideration. While it may be possible to speed reactions by heating them, there may be activation barriers which produce unexpected effects at both higher and lower temperatures. As an example, recent experiments demonstrating increasing efficiency of RNA replicases have resorted to conducting reactions in ice eutectics, owing to the decreased rates of organic degradation at low temperatures [74]. Such experiments also take advantage of the concentrating effects of ice eutectics and the stabilization of weak interactions, such as those mediated by hydrogen bonding, at low temperatures.
- Time. Lazcano and Miller observed that all known prebiotic reactions are fast [75]. Orgel responded by pointing out that our knowledge of prebiotic chemistry may be more constrained by the lengths of post-doctoral fellowships than by any requirements of the chemistry involved [76]. This consideration relates intrinsically to variables of concentration, pH and temperature discussed above. In some cases, it may be almost impossible to produce a laboratory reconstruction of a process for temporal reasons. Extrapolation is perfectly reasonable in such cases, with some degree of caution. To an extent, time is exchanged for temperature in prebiotic simulations. As mentioned above, it is unknown what the average or range of surface temperatures was on the primitive Earth. Micro-environments must also be considered, for example, surface temperatures on the modern Earth range from ~−90 °C to ~+60 °C.
- Pressure. Environments where pressure becomes a significant variable already make some assumptions about other relevant variables, for example temperature. The pH of ambient conditions and the pKa of reactants may change dramatically under extreme pressure conditions, and some remarkable pressure-dependant effects have been observed in prebiotic studies of peptide oligomerization, as might occur in deep marine sediments [77]. It is interesting to speculate how difficulties of working with this parameter may skew our knowledge of abiotic organic chemistry.
- Ionic Strength. Depending on the reaction in question, this may have a relatively minor influence on chemistry, or be a significant factor. Some predictions can be made, but this is certainly worth exploring, for example in the way inorganic solute concentration may limit the ability of processes such as eutectic freezing to concentrate reactive organic solutes [78,79].
- Influence of Inorganic Reactants. Ammonia, sulfide, various transition metals, etc. can significantly affect the course of reactions. Ammonia and sulfide, depending on pH, can be significantly reactive with important prebiotic reactants [80]. It is probably not reasonable, and possibly not productive to explore them all experimentally, and it may irrelevant to do so in some cases, however some merit at least cursory consideration where they might be expected to be relevant. For example, it is well-documented that transition metals can have significant effects on the reactivity of various compounds including amino acids and peptides [52,81]. Simple oxoanions, such as borate and silicate, have also been found to have profound effects on more complex chemistries such as sugar forming reactions [82,83,84].
- Reactant Continuity. Each reactant in any proposed scheme or reaction must fit viably with the above-mentioned considerations. For example, while glyceraldehyde may be a perfectly reasonable reactant, its derivation from a formose like process would inevitably lead to the presence of other carbohydrates and carbohydrate-like compounds [85,86]. These would, in the absence of geochemically viable purification schemes, carry over into subsequent reaction steps. As discussed below, many prebiotic processes produce extraordinarily complex mixtures, and the mere presence of a compound cannot guarantee its ability to react with a second reagent. Even novel syntheses of glyceraldehyde from other processes also result in the presence of numerous other compounds [87].
- Reagent Purity. In almost all known examples of prebiotic synthesis starting from primary reactants such as atmospheric gases, complex mixtures result, in which competing side reactions would be difficult to avoid. For example, in many typical primary syntheses, the yield of glycine is often quite high relative to other measured organic species, though its overall yield is rather low. Glycine represents ~0.01 wt% of the organic content of the Murchison meteorite [88] and is present alongside some thousands or millions of other organic molecule types [37]. The same is true of the yield of glycine from electric discharge experiments, where despite being one of the most abundant single products, it only accounts for ~2% of the input carbon [89], or 0.6% of the input carbon from an aqueous HCN oligomerization reaction [90], in both cases again being accompanied by hundreds or thousands of other organic species (Figure 4).

- Light. The flux of solar radiation to the primitive Earths’ surface is somewhat poorly constrained, though the sun’s total luminosity was likely considerably lower [62]. The lack of an ozone layer likely permitted much lower and more energetic wavelength radiation to reach the Earth’s surface, however trivial environmental considerations, such as protection by various dissolved inorganic species, or reactions proceeding in interstitial pore water, could have rendered such considerations essentially moot [92]. Nevertheless, the potentially significant effects of both UV and visible light on prebiotic chemistry remain seriously understudied, underscored by the recent results of Sutherland and colleagues, among other older studies [87,93,94,95].
- Minerals. There are a great number of possible minerals which might have contributed some form of catalysis or concentration of various species [96]. For example, iron oxide minerals have been shown to be catalysts for the both the degradation and synthesis of peptides, as well as the degradation of amino acids [51,52]. It would be unreasonable to attempt to test every possible mineral from the start, but it might be instructive to examine the effects of minerals on reactions once those reactions have been shown to work in their absence. It might be a useful exercise to consider which minerals would be likely to be common in any given environment.
- Cycling. Relatively few studies have taken into account the effects of thermal cycling. Among those that have been reported, one showed the formation of peptides in cycling hydrothermal solutions [97], while another showed the formation of peptides in cycling tidal environments [98]. In both cases is was later suggested that the ultimate product yield is very close to the expected thermodynamic outcome in the absence of cycling [72], as may be expected for a simple reversible process, but the phenomenon likely deserves further consideration.
6. The Way Forward?
Acknowledgments
References
- Cleaves, H.J., II. Prebiotic chemistry: What we know, what we don’t. Evol. Educ. Outreach 2012, 5, 342–360. [Google Scholar] [CrossRef]
- Wächtershäuser, G. Before enzymes and templates: Theory of surface metabolism. Microbiol. Rev. 1988, 52, 452–484. [Google Scholar]
- Hanczyc, M.M.; Fujikawa, S.M.; Szostak, J.W. Experimental models of primitive cellular compartments: Encapsulation, growth, and division. Science 2003, 302, 618–622. [Google Scholar] [CrossRef]
- Russell, M.J.; Daia, D.E.; Hall, A.J. The emergence of life from FeS bubbles at alkaline hot springs in an acid ocean. In Thermophiles: The Keys to the Molecular Evolution and the Origin of Life? Wiegel, J., Adams, M.W.W., Eds.; Taylor and Francis: London, UK, 1998; pp. 77–126. [Google Scholar]
- Fox, S.W.; Vegotsky, A.; Harada, K.; Hoagland, P.D. Spontaneous generation of anabolic pathways, protein, and nucleic acid. Ann. N. Y. Acad. Sci. 1957, 69, 328–337. [Google Scholar] [CrossRef]
- Cleaves, H.J., 2nd; Chalmers, J.H. Extremophiles may be irrelevant to the origin of life. Astrobiology 2004, 4, 1–9. [Google Scholar] [CrossRef]
- Shapiro, R. Origins: A Skeptic’s Guide to the Creation of Life on Earth; Bantam Books: New York, NY, USA, 1987. [Google Scholar]
- Schwartz, A.W.; van der Veen, M.; Bisseling, T.; Chittenden, G.J. Prebiotic nucleotide synthesis-demonstration of a geologically plausible pathway. Orig. Life 1975, 6, 163–168. [Google Scholar] [CrossRef]
- Pross, A. What is Life? How Chemistry Becomes Biology; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Eschenmoser, A. The search for the chemistry of life’s origin. Tetrahedron 2007, 63, 12821–12844. [Google Scholar] [CrossRef]
- Shapiro, R. The improbability of prebiotic nucleic acid synthesis. Orig. Life Evol. B 1984, 14, 565–570. [Google Scholar] [CrossRef]
- McCollom, T.M. Miller-Urey and beyond: What have we learned about prebiotic organic synthesis reactions in the past 60 years? Ann. Rev. Earth Planet. Sci. 2003, 41. [Google Scholar] [CrossRef]
- Kirschvink, J.L.; Weiss, B.P. Mars, panspermia, and the origin of life: Where did it all begin. Palaeontol. Electron. 2002, 4, 8–15. [Google Scholar]
- Hoover, R.B. Comets, Carbonaceous Meteorites, and the Origin of the Biosphere. In Biosphere Origin and Evolution; Springer: New York, NY, USA, 2008; pp. 55–68. [Google Scholar]
- Russell, M.J.; Hall, A.J.; Boyce, A.J.; Fallick, A.E. 100th anniversary special paper: On hydrothermal convection systems and the emergence of life. Econ. Geol. 2005, 100, 419–438. [Google Scholar]
- Wächtershäuser, G. Groundworks for an evolutionary biochemistry—The iron sulfur world. Prog. Biophys. Mol. Biol. 1992, 58, 85–201. [Google Scholar] [CrossRef]
- Corliss, J.; Baross, J.; Hoffman, S. An hypothesis concerning the relationship between submarine hot springs and the origin of life on earth. Oceanol. Acta 1981, 4, 59–69. [Google Scholar]
- Fox, S.W. Thermal synthesis of amino acids and the origin of life. Geochim. Cosmochim Acta 1995, 59, 1213–1214. [Google Scholar] [CrossRef]
- Nelson, K.E.; Robertson, M.P.; Levy, M.; Miller, S.L. Concentration by evaporation and the prebiotic synthesis of cytosine. Orig. Life Evol. B 2001, 31, 221–229. [Google Scholar] [CrossRef]
- Gesteland, R.F.; Cech, T.; Atkins, J.F. The RNA World: The Nature of Modern RNA Suggests a Prebiotic RNA World, 3rd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, New York, NY, USA, 2006; p. 768. [Google Scholar]
- Joyce, G.F.; Schwartz, A.W.; Miller, S.L.; Orgel, L.E. The case for an ancestral genetic system involving simple analogues of the nucleotides. Proc. Natl. Acad. Sci. USA 1987, 84, 4398–4402. [Google Scholar] [CrossRef]
- Luisi, P.L.; Varela, F.J. Self-replicating micelles—A chemical version of a minimal autopoietic system. Orig. Life Evol B 1989, 19, 633–643. [Google Scholar] [CrossRef]
- Luisi, P.L.; Walde, P.; Oberholzer, T. Lipid vesicles as possible intermediates in the origin of life. Curr. Opin. Colloid Interface 1999, 4, 33–39. [Google Scholar] [CrossRef]
- Kauffman, S. Question 1: Origin of life and the living state. Orig. Life Evol. B 2007, 37, 315–322. [Google Scholar] [CrossRef]
- Cody, G.D.; Boctor, N.Z.; Hazen, R.M.; Brandes, J.A.; Morowitz, H.J.; Yoder, H.S., Jr. Geochemical roots of autotrophic carbon fixation: Hydrothermal experiments in the system citric acid, H2O-(±FeS)-(±NiS). Geochim. Cosmochim. Acta 2001, 65, 3557–3576. [Google Scholar] [CrossRef]
- Chen, I.A.; Roberts, R.W.; Szostak, J.W. The emergence of competition between model protocells. Science 2004, 305, 1474–1476. [Google Scholar] [CrossRef]
- Szostak, J.W.; Bartel, D.P.; Luisi, P.L. Synthesizing life. Nature 2001, 409, 387–390. [Google Scholar] [CrossRef]
- Ehrenfreund, P.; Rasmussen, S.; Cleaves, J.; Chen, L. Experimentally tracing the key steps in the origin of life: The aromatic world. Astrobiology 2006, 6, 490–520. [Google Scholar] [CrossRef]
- Segre, D.; Ben-Eli, D.; Lancet, D. Compositional genomes: Prebiotic information transfer in mutually catalytic noncovalent assemblies. Proc. Natl. Acad. Sci. USA 2000, 97, 4112–4117. [Google Scholar] [CrossRef]
- Fox, S.W.; Jungck, J.R.; Nakashima, T. From proteinoid microsphere to contemporary cell: Formation of internucleotide and peptide bonds by proteinoid particles. Orig. Life 1974, 5, 227–237. [Google Scholar] [CrossRef]
- DeClue, M.S.; Monnard, P.-A.; Bailey, J.A.; Maurer, S.E.; Collis, G.E.; Ziock, H.-J.; Rasmussen, S.; Boncella, J.M. Nucleobase mediated, photocatalytic vesicle formation from an ester precursor. J. Am. Chem. Soc. 2008, 131, 931–933. [Google Scholar]
- Mulkidjanian, A.Y.; Bychkov, A.Y.; Dibrova, D.V.; Galperin, M.Y.; Koonin, E.V. Origin of first cells at terrestrial, anoxic geothermal fields. Proc. Natl. Acad. Sci. USA 2012, 109, E821–E830. [Google Scholar]
- Ball, P. Astrobiology: Water, water, everywhere? Nature 2004, 427, 19–20. [Google Scholar]
- Mojzsis, S.J.; Harrison, T.M.; Pidgeon, R.T. Oxygen-isotope evidence from ancient zircons for liquid water at the Earth’s surface 4,300 myr ago. Nature 2001, 409, 178–181. [Google Scholar] [CrossRef]
- Chyba, C.; Sagan, C. Endogenous production, exogenous delivery and impact-shock synthesis of organic molecules: An inventory for the origins of life. Nature 1992, 355, 125–132. [Google Scholar] [CrossRef]
- Miller, S.L.; Schlesinger, G. Carbon and energy yields in prebiotic syntheses using atmospheres containing CH4, CO and CO2. Orig. Life 1984, 14, 83–90. [Google Scholar] [CrossRef]
- Schmitt-Kopplin, P.; Gabelica, Z.; Gougeon, R.D.; Fekete, A.; Kanawati, B.; Harir, M.; Gebefuegi, I.; Eckel, G.; Hertkorn, N. High molecular diversity of extraterrestrial organic matter in Murchison meteorite revealed 40 years after its fall. Proc. Natl. Acad. Sci. USA 2010, 107, 2763–2768. [Google Scholar] [CrossRef]
- Schwartz, A.W. Intractable mixtures and the origin of life. Chem. Biodivers. 2007, 4, 656–664. [Google Scholar] [CrossRef]
- Arrhenius, G.; Bada, J.L.; Joyce, G.F.; Lazcano, A.; Miller, S.; Orgel, L.E. Origin and ancestor: Separate environments. Science 1999, 283, 792–792. [Google Scholar]
- Line, M.A. The enigma of the origin of life and its timing. Microbiology 2002, 148, 21–27. [Google Scholar]
- Caetano-Anolles, G.; Sun, F.-J.; Wang, M.; Yafremava, L.S.; Harish, A.; Kim, H.S.; Knudsen, V.; Caetano-Anolles, D.; Mittenthal, J.E. Origins and evolution of modern biochemistry: Insights from genomes and molecular structure. Front. Biosci. 2008, 13, 5212–5240. [Google Scholar]
- Vermeij, G.J. Historical contingency and the purported uniqueness of evolutionary innovations. Proc. Natl. Acad. Sci. USA 2006, 103, 1804–1809. [Google Scholar] [CrossRef]
- Freeland, S.J.; Hurst, L.D. The genetic code is one in a million. J. Mol. Evol. 1998, 47, 238–248. [Google Scholar] [CrossRef]
- Feng, D.-F.; Cho, G.; Doolittle, R.F. Determining divergence times with a protein clock: Update and reevaluation. Proc. Nat. Acad. Sci. USA 1997, 94, 13028–13033. [Google Scholar] [CrossRef]
- Sheridan, P.P.; Freeman, K.H.; Brenchley, J.E. Estimated minimal divergence times of the major bacterial and archaeal phyla. Geomicrobiol. J. 2003, 20, 1–14. [Google Scholar] [CrossRef]
- Sleep, N.H. The Hadean-Archaean environment. Cold Spring Harb. Perspect. Biol. 2010, 2. [Google Scholar] [CrossRef]
- Ayala, F.J. Molecular clock mirages. Bioessays 1999, 21, 71–75. [Google Scholar] [CrossRef]
- Schidlowski, M. A 3,800-million-year isotopic record of life from carbon in sedimentary rocks. Nature 1988, 333, 313–318. [Google Scholar] [CrossRef]
- Schopf, J.W. Microfossils of the early archean apex chert: New evidence of the antiquity of life. Science 1993, 260, 640–646. [Google Scholar]
- Wacey, D.; Kilburn, M.R.; Saunders, M.; Cliff, J.; Brasier, M.D. Microfossils of sulphur-metabolizing cells in 3.4-billion-year-old rocks of western Australia. Nat. Geosci. 2011, 4, 698–702. [Google Scholar]
- McCollom, T.M. The influence of minerals on decomposition of the n-alkyl-α-amino acid norvaline under hydrothermal conditions. Geochim. Cosmochim. Acta 2013, 104, 330–357. [Google Scholar] [CrossRef]
- Marshall-Bowman, K.; Ohara, S.; Sverjensky, D.A.; Hazen, R.M.; Cleaves, H.J. Catalytic peptide hydrolysis by mineral surface: Implications for prebiotic chemistry. Geochim. Cosmochim. Acta 2010, 74, 5852–5861. [Google Scholar] [CrossRef]
- Vallentyne, J.R. Biogeochemistry of organic matter—II thermal reaction kinetics and transformation products of amino compounds. Geochim. Cosmochim. Acta 1964, 28, 157–188. [Google Scholar] [CrossRef]
- Levy, M.; Miller, S.L. The stability of the RNA bases: Implications for the origin of life. Proc. Natl. Acad. Sci. USA 1998, 95, 7933–7938. [Google Scholar] [CrossRef]
- Larralde, R.; Robertson, M.P.; Miller, S.L. Rates of decomposition of ribose and other sugars: Implications for chemical evolution. Proc. Natl. Acad. Sci. USA 1995, 92, 8158–8160. [Google Scholar] [CrossRef]
- Knoll, A.H.; Javaux, E.J.; Hewitt, D.; Cohen, P. Eukaryotic organisms in proterozoic oceans. Philos. Trans. Roy. Soc. B 2006, 361, 1023–1038. [Google Scholar] [CrossRef]
- Stetter, K.O. Hyperthermophilic procaryotes. FEMS Microbiol. Rev. 1996, 18, 149–158. [Google Scholar] [CrossRef]
- Phylogenetic Tree. Available online: http://www.gla.ac.uk/projects/originoflife/html/2001/figures/fig2.htm/ (accessed on 14 March 2013).
- Tera, F.; Papanastassiou, D.; Wasserburg, G. Isotopic evidence for a terminal lunar cataclysm. Earth Planet. Sci. Lett. 1974, 22, 1–21. [Google Scholar] [CrossRef]
- Maher, K.A.; Stevenson, D.J. Impact frustration of the origin of life. Nature 1988, 331, 612–614. [Google Scholar] [CrossRef]
- Spudis, P.D.; Wilhelms, D.E.; Robinson, M.S. The sculptured hills of the Taurus highlands: Implications for the relative age of Serenitatis, basin chronologies and the cratering history of the Moon. J. Geophys. Res. Planets 2011, 116, E00H03. [Google Scholar] [CrossRef]
- Cnossen, I.; Sanz-Forcada, J.; Favata, F.; Witasse, O.; Zegers, T.; Arnold, N.F. Habitat of early life: Solar X-ray and UV radiation at Earth’s surface 4–3.5 billion years ago. J. Geophys. Res. Planets 2007, 112. [Google Scholar] [CrossRef]
- Costanzo, G.; Saladino, R.; Crestini, C.; Ciciriello, F.; di Mauro, E. Formamide as the main building block in the origin of nucleic acids. BMC Evol. Biol. 2007, 7, S1. [Google Scholar]
- Lazcano, A. The origins of life: Have too many cooks spoiled the prebiotic soup? Nat. Hist. 2006, 115, 36–41. [Google Scholar]
- Hoenigsberg, H.F. From geochemistry and biochemistry to prebiotic evolution.We necessarily enter into Gánti’s fluid automata. Genet. Mol. Res. 2007, 6, 358–373. [Google Scholar]
- Dreyfus, M.; Dodin, G.; Bensaude, O.; Dubois, J. Tautomerism of purines. I. N(7)H ⬄ N(9)H equilibrium in adenine. J. Am. Chem. Soc. 1975, 97, 2369–2376. [Google Scholar]
- Monnard, P.-A.; Deamer, D.W. Preparation of vesicles from nonphospholipid amphiphiles. Method. Enzymol. 2003, 372, 133–151. [Google Scholar] [CrossRef]
- Sanchez, R.A.; Ferris, J.P.; Orgel, L.E. Studies in prebiotic synthesis. II. Synthesis of purine precursors and amino acids from aqueous hydrogen cyanide. J. Mol. Biol. 1967, 30, 223–253. [Google Scholar]
- Shigemasa, Y.; Fujitani, T.; Sakazawa, C.; Matsuura, T. Formose reactions. III. Evaluation of various factors affecting the formose reaction. Bull. Chem. Soc. Jpn. 1977, 50, 1527–1531. [Google Scholar] [CrossRef]
- Miyakawa, S.; Cleaves, H.J.; Miller, S.L. The cold origin of life: A Implications based on the hydrolytic stabilities of hydrogen cyanide and formamide. Orig. Life Evol. B 2002, 32, 195–208. [Google Scholar] [CrossRef]
- Nelson, K.E.; Levy, M.; Miller, S.L. Peptide nucleic acids rather than RNA may have been the first genetic molecule. Proc. Natl. Acad. Sci. USA 2000, 97, 3868–3871. [Google Scholar] [CrossRef]
- Cleaves, H.J.; Aubrey, A.D.; Bada, J.L. An evaluation of the critical parameters for abiotic peptide synthesis in submarine hydrothermal systems. Orig. Life Evol. B 2009, 39, 109–126. [Google Scholar] [CrossRef]
- Saladino, R.; Crestini, C.; Pino, S.; Costanzo, G.; di Mauro, E. Formamide and the origin of life. Phys. Life Rev. 2012, 9, 84–104. [Google Scholar] [CrossRef] [Green Version]
- Wochner, A.; Attwater, J.; Coulson, A.; Holliger, P. Ribozyme-catalyzed transcription of an active ribozyme. Science 2011, 332, 209–212. [Google Scholar] [CrossRef]
- Lazcano, A.; Miller, S.L. How long did it take for life to begin and evolve to cyanobacteria? J. Mol. Evol. 1994, 39, 546–554. [Google Scholar] [CrossRef]
- Orgel, L.E. The origin of life—How long did it take? Orig. Life Evol. B 1998, 28, 91–96. [Google Scholar] [CrossRef]
- Ohara, S.; Kakegawa, T.; Nakazawa, H. Pressure effects on the abiotic polymerization of glycine. Orig. Life Evol. B 2007, 37, 215–223. [Google Scholar] [CrossRef]
- Cleaves, H.J., 2nd; Nelson, K.E.; Miller, S.L. The prebiotic synthesis of pyrimidines in frozen solution. Naturwissenschaften 2006, 93, 228–231. [Google Scholar] [CrossRef]
- Miller, S.L.; Orgel, L.E. The Origins of Life on the Earth; Prentice-Hall: Englewood Cliffs, NJ, USA, 1974; p. 229. [Google Scholar]
- Cleaves, H.J. The prebiotic geochemistry of formaldehyde. Precambrian Res. 2008, 164, 111–118. [Google Scholar] [CrossRef]
- Rode, B.M.; Son, H.L.; Suwannachot, Y. The combination of salt induced peptide formation reaction and clay catalysis: A way to higher peptides under primitive Earth conditions. Orig. Life Evol. B 1999, 29, 273–286. [Google Scholar] [CrossRef]
- Prieur, B.E. Étude de l’activité prébiotique potentielle de l’acide borique. C. R. Acad. Sci. Ser. 2001, 4, 667–670. [Google Scholar]
- Ricardo, A.; Carrigan, M.A.; Olcott, A.N.; Benner, S.A. Borate minerals stabilize ribose. Science 2004, 303, 196. [Google Scholar] [CrossRef]
- Lambert, J.B.; Gurusamy-Thangavelu, S.A.; Ma, K. The silicate-mediated formose reaction: Bottom-up synthesis of sugar silicates. Science 2010, 327, 984–986. [Google Scholar] [CrossRef]
- Schwartz, A.W.; Degraaf, R.M. The prebiotic synthesis of carbohydrates—A reassessment. J. Mol. Evol. 1993, 36, 101–106. [Google Scholar] [CrossRef]
- Decker, P.; Schweer, H.; Pohlamnn, R. Bioids: X. Identification of formose sugars, presumable prebiotic metabolites, using capillary gas chromatography/gas chromatography—Mass spectrometry of n-butoxime trifluoroacetates on OV-225. J. Chrom. A 1982, 244, 281–291. [Google Scholar]
- Ritson, D.; Sutherland, J.D. Prebiotic synthesis of simple sugars by photoredox systems chemistry. Nat. Chem. 2012, 4, 895–899. [Google Scholar] [CrossRef]
- Glavin, D.P.; Callahan, M.P.; Dworkin, J.P.; Elsila, J.E. The effects of parent body processes on amino acids in carbonaceous chondrites. Meteorit. Planet. Sci. 2010, 45, 1948–1972. [Google Scholar] [CrossRef]
- Miller, S.L. The mechanism of synthesis of amino acids by electric discharges. Biochim. Biophys. Acta 1957, 23, 480–489. [Google Scholar] [CrossRef]
- Ferris, J.P.; Joshi, P.C.; Edelson, E.H.; Lawless, J.G. Hcn: A plausible source of purines, pyrimidines and amino acids on the primitive Earth. J. Mol. Evol. 1978, 11, 293–311. [Google Scholar] [CrossRef]
- Yusenko, K.; Fox, S.; Guni, P.; Strasdeit, H. Model studies on the formation and reactions of solid glycine complexes at the coasts of a primordial salty ocean. Z. Anorg. Allg. Chem. 2008, 634, 2347–2354. [Google Scholar] [CrossRef]
- Cleaves, H.J.; Miller, S.L. Oceanic protection of prebiotic organic compounds from UV radiation. Proc. Nat. Acad. Sci. USA 1998, 95, 7260–7263. [Google Scholar] [CrossRef]
- Powner, M.W.; Anastasi, C.; Crowe, M.A.; Parkes, A.L.; Raftery, J.; Sutherland, J.D. On the prebiotic synthesis of ribonucleotides: Photoanomerisation of cytosine nucleosides and nucleotides revisited. Chembiochem 2007, 8, 1170–1179. [Google Scholar] [CrossRef]
- Sanchez, R.A.; Orgel, L.E. Studies in prebiotic synthesis. V. Synthesis and photoanomerization of pyrimidine nucleosides. J. Mol. Biol. 1970, 47, 531–543. [Google Scholar] [CrossRef]
- Zhang, X.V.; Martin, S.T. Driving parts of Krebs cycle in reverse through mineral photochemistry. J. Am. Chem. Soc. 2006, 128, 16032–16033. [Google Scholar] [CrossRef]
- Cleaves, H.J.; Michalkova Scott, A.; Hill, F.C.; Leszczynski, J.; Sahai, N.; Hazen, R. Mineral-organic interfacial processes: Potential roles in the origins of life. Chem. Soc. Rev. 2012, 41, 5502–5525. [Google Scholar] [CrossRef]
- Imai, E.; Honda, H.; Hatori, K.; Brack, A.; Matsuno, K. Elongation of oligopeptides in a simulated submarine hydrothermal system. Science 1999, 283, 831–833. [Google Scholar] [CrossRef]
- Lahav, N.; White, D.; Chang, S. Peptide formation in the prebiotic era: Thermal condensation of glycine in fluctuating clay environments. Science 1978, 201, 67–69. [Google Scholar]
- Draganić, I.G.; Draganić, Z.D.; Adloff, J.P. Radiation and Radioactivity on Earth and Beyond; CRC Press: London, UK, 1990. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cleaves, H.J., II. Prebiotic Chemistry: Geochemical Context and Reaction Screening. Life 2013, 3, 331-345. https://doi.org/10.3390/life3020331
Cleaves HJ II. Prebiotic Chemistry: Geochemical Context and Reaction Screening. Life. 2013; 3(2):331-345. https://doi.org/10.3390/life3020331
Chicago/Turabian StyleCleaves, Henderson James, II. 2013. "Prebiotic Chemistry: Geochemical Context and Reaction Screening" Life 3, no. 2: 331-345. https://doi.org/10.3390/life3020331
APA StyleCleaves, H. J., II. (2013). Prebiotic Chemistry: Geochemical Context and Reaction Screening. Life, 3(2), 331-345. https://doi.org/10.3390/life3020331