The Emerging Role of Protein Phosphatase in Regeneration
Abstract
:1. Introduction
2. Classification of Protein Phosphatases
| Superfamily | Family | Subfamily | Reference |
|---|---|---|---|
| PTPs | Class I | RPTPs | [16,17] |
| NRPTPs | |||
| DUSPs | |||
| Class II | LMW-PTP | [17] | |
| Class III | CDC25 | [17,19] | |
| Class IV | Eya | [17,20] | |
| Class V | Sts | [14] | |
| PSTPs | PPPs | PP1 | [22,24] |
| PP2A | |||
| PP2B | |||
| PP4 | |||
| PP5 | |||
| PP6 | |||
| PP7 | |||
| PPMs | PP2C | [18,22,25] | |
| PDP | |||
| DxDxTs | FCP/SCP | [18,22] | |
| HAD |
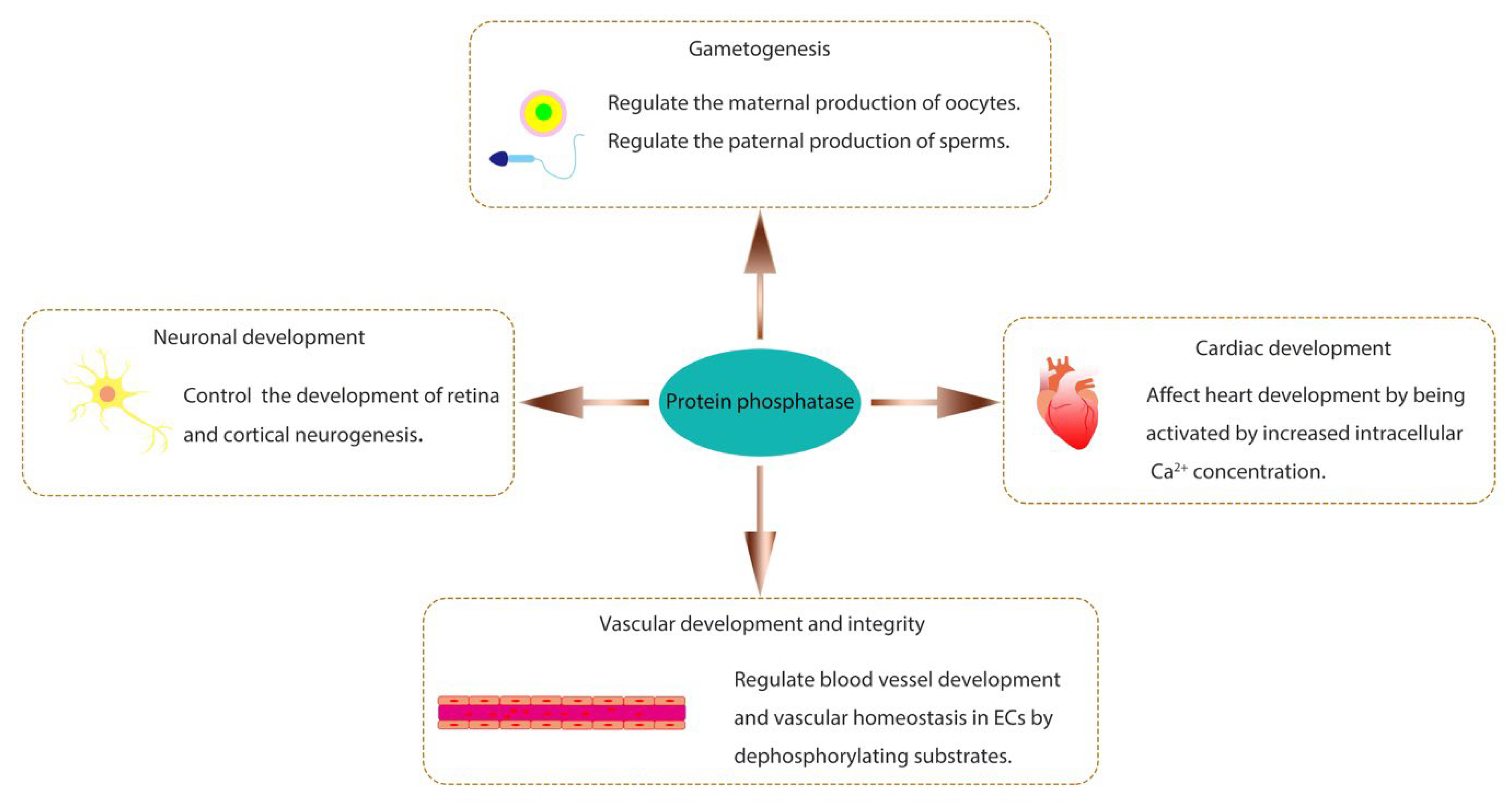
3. Various Developmental Functions of Protein Phosphatase
4. Roles of Protein Phosphatases in Regeneration
4.1. Liver Regeneration
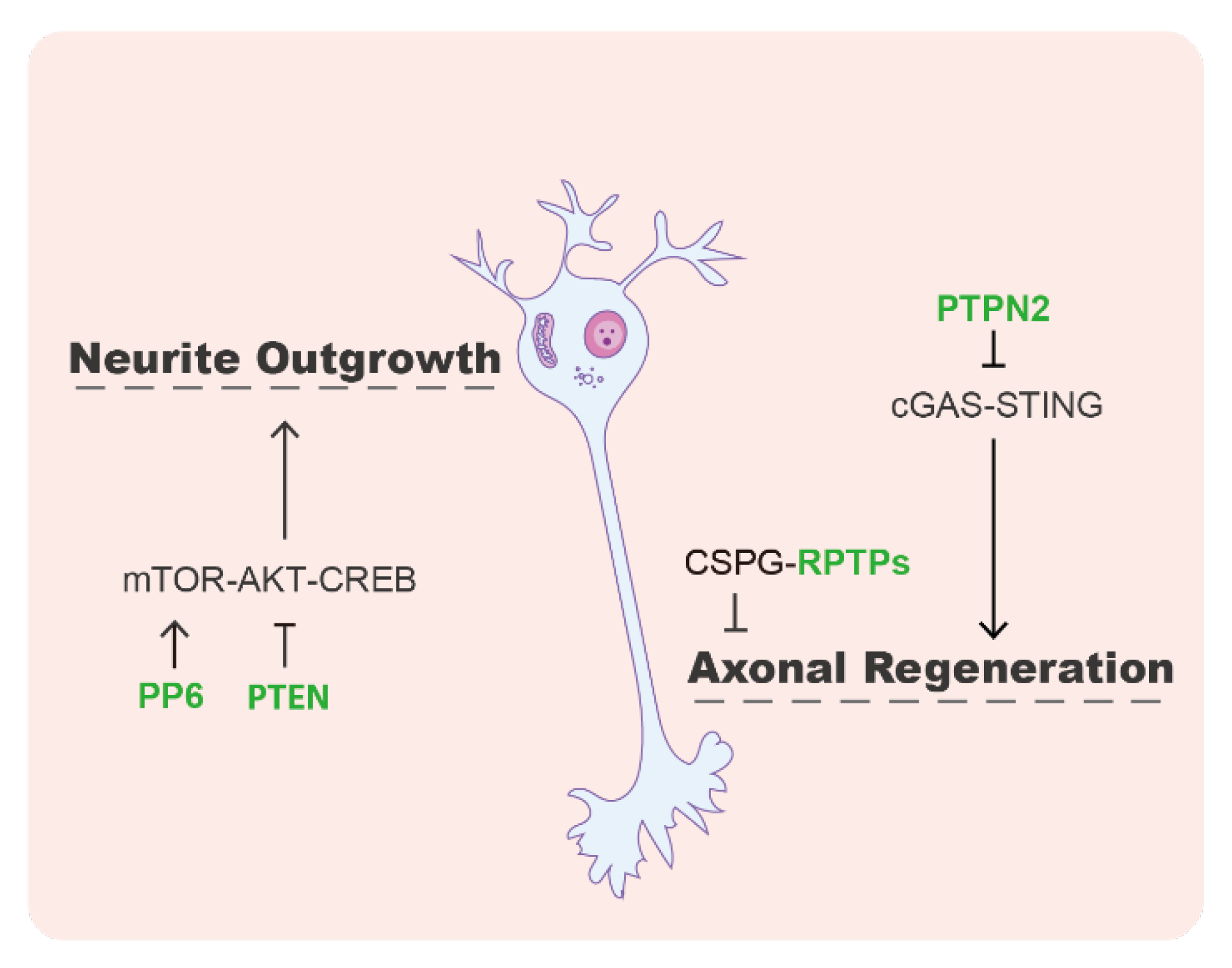
4.2. Nerve Regeneration
4.3. Heart Regeneration
4.4. The Regeneration of Other Organs or Cells
5. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, V.; Ram, M.; Kumar, R.; Prasad, R.; Roy, B.K.; Singh, K.K. Phosphorylation: Implications in Cancer. Protein J. 2017, 36, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P. The regulation of protein function by multisite phosphorylation—A 25 year update. Trends Biochem. Sci. 2000, 25, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, S.J.; James, D.E.; Mann, M. Protein Phosphorylation: A Major Switch Mechanism for Metabolic Regulation. Trends Endocrinol. Metab. 2015, 26, 676–687. [Google Scholar] [CrossRef] [PubMed]
- Olsen, J.V.; Blagoev, B.; Gnad, F.; Macek, B.; Kumar, C.; Mortensen, P.; Mann, M. Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell 2006, 127, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Virshup, D.M.; Shenolikar, S. From promiscuity to precision: Protein phosphatases get a makeover. Mol. Cell 2009, 33, 537–545. [Google Scholar] [CrossRef]
- Manning, G.; Whyte, D.B.; Martinez, R.; Hunter, T.; Sudarsanam, S. The protein kinase complement of the human genome. Science 2002, 298, 1912–1934. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, L.; Chi, C.; Lan, W.; Su, Y. The emerging roles of phosphatases in Hedgehog pathway. Cell Commun. Signal. 2017, 15, 35. [Google Scholar] [CrossRef]
- Cao, Z.; Meng, Y.; Gong, F.; Xu, Z.; Liu, F.; Fang, M.; Zou, L.; Liao, X.; Wang, X.; Luo, L.; et al. Calcineurin controls proximodistal blastema polarity in zebrafish fin regeneration. Proc. Natl. Acad. Sci. USA 2021, 118, e2009539118. [Google Scholar] [CrossRef]
- Brockes, J.P.; Kumar, A. Comparative aspects of animal regeneration. Annu. Rev. Cell Dev. Biol. 2008, 24, 525–549. [Google Scholar] [CrossRef]
- Poss, K.D. Advances in understanding tissue regenerative capacity and mechanisms in animals. Nat. Rev. Genet. 2010, 11, 710–722. [Google Scholar] [CrossRef]
- Shi, Y. Serine/threonine phosphatases: Mechanism through structure. Cell 2009, 139, 468–484. [Google Scholar] [CrossRef]
- Hunter, T. Protein kinases and phosphatases: The yin and yang of protein phosphorylation and signaling. Cell 1995, 80, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Olloquequi, J.; Cano, A.; Sanchez-Lopez, E.; Carrasco, M.; Verdaguer, E.; Fortuna, A.; Folch, J.; Bullo, M.; Auladell, C.; Camins, A.; et al. Protein tyrosine phosphatase 1B (PTP1B) as a potential therapeutic target for neurological disorders. Biomed Pharmacother. 2022, 155, 113709. [Google Scholar] [CrossRef] [PubMed]
- Sadatomi, D.; Tanimura, S.; Ozaki, K.; Takeda, K. Atypical protein phosphatases: Emerging players in cellular signaling. Int. J. Mol. Sci. 2013, 14, 4596–4612. [Google Scholar] [CrossRef]
- Ardito, F.; Giuliani, M.; Perrone, D.; Troiano, G.; Lo Muzio, L. The crucial role of protein phosphorylation in cell signaling and its use as targeted therapy (Review). Int. J. Mol. Med. 2017, 40, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.; Sasin, J.; Bottini, N.; Friedberg, I.; Friedberg, I.; Osterman, A.; Godzik, A.; Hunter, T.; Dixon, J.; Mustelin, T. Protein tyrosine phosphatases in the human genome. Cell 2004, 117, 699–711. [Google Scholar] [CrossRef]
- Caselli, A.; Paoli, P.; Santi, A.; Mugnaioni, C.; Toti, A.; Camici, G.; Cirri, P. Low molecular weight protein tyrosine phosphatase: Multifaceted functions of an evolutionarily conserved enzyme. Biochim. Biophys. Acta 2016, 1864, 1339–1355. [Google Scholar] [CrossRef]
- Moorhead, G.B.; De Wever, V.; Templeton, G.; Kerk, D. Evolution of protein phosphatases in plants and animals. Biochem. J. 2009, 417, 401–409. [Google Scholar] [CrossRef]
- Brenner, A.K.; Reikvam, H.; Lavecchia, A.; Bruserud, O. Therapeutic targeting the cell division cycle 25 (CDC25) phosphatases in human acute myeloid leukemia—The possibility to target several kinases through inhibition of the various CDC25 isoforms. Molecules 2014, 19, 18414–18447. [Google Scholar] [CrossRef]
- Tadjuidje, E.; Wang, T.S.; Pandey, R.N.; Sumanas, S.; Lang, R.A.; Hegde, R.S. The EYA tyrosine phosphatase activity is pro-angiogenic and is inhibited by benzbromarone. PLoS ONE 2012, 7, e34806. [Google Scholar] [CrossRef]
- San Luis, B.; Sondgeroth, B.; Nassar, N.; Carpino, N. Sts-2 is a phosphatase that negatively regulates zeta-associated protein (ZAP)-70 and T cell receptor signaling pathways. J. Biol. Chem. 2011, 286, 15943–15954. [Google Scholar] [CrossRef] [PubMed]
- Brautigan, D.L. Protein Ser/Thr phosphatases--the ugly ducklings of cell signalling. FEBS J. 2013, 280, 324–345. [Google Scholar] [CrossRef] [PubMed]
- Brauer, B.L.; Wiredu, K.; Mitchell, S.; Moorhead, G.B.; Gerber, S.A.; Kettenbach, A.N. Affinity-based profiling of endogenous phosphoprotein phosphatases by mass spectrometry. Nat. Protoc. 2021, 16, 4919–4943. [Google Scholar] [CrossRef] [PubMed]
- Brautigan, D.L.; Shenolikar, S. Protein Serine/Threonine Phosphatases: Keys to Unlocking Regulators and Substrates. Annu. Rev. Biochem. 2018, 87, 921–964. [Google Scholar] [CrossRef]
- Stanford, S.M.; Bottini, N. Targeting protein phosphatases in cancer immunotherapy and autoimmune disorders. Nat. Rev. Drug Discov. 2023, 22, 273–294. [Google Scholar] [CrossRef]
- Kornbluth, S.; Fissore, R. Vertebrate Reproduction. Cold Spring Harb. Perspect. Biol. 2015, 7, a006064. [Google Scholar] [CrossRef]
- Lei, W.L.; Qian, W.P.; Sun, Q.Y. Critical Functions of PP2A-Like Protein Phosphotases in Regulating Meiotic Progression. Front. Cell Dev. Biol. 2021, 9, 638559. [Google Scholar] [CrossRef]
- Lemonnier, T.; Daldello, E.M.; Poulhe, R.; Le, T.; Miot, M.; Lignieres, L.; Jessus, C.; Dupre, A. The M-phase regulatory phosphatase PP2A-B55delta opposes protein kinase A on Arpp19 to initiate meiotic division. Nat. Commun. 2021, 12, 1837. [Google Scholar] [CrossRef]
- Lei, W.L.; Han, F.; Hu, M.W.; Liang, Q.X.; Meng, T.G.; Zhou, Q.; Ouyang, Y.C.; Hou, Y.; Schatten, H.; Wang, Z.B.; et al. Protein phosphatase 6 is a key factor regulating spermatogenesis. Cell Death Differ. 2020, 27, 1952–1964. [Google Scholar] [CrossRef]
- Hu, M.W.; Wang, Z.B.; Teng, Y.; Jiang, Z.Z.; Ma, X.S.; Hou, N.; Cheng, X.; Schatten, H.; Xu, X.; Yang, X.; et al. Loss of protein phosphatase 6 in oocytes causes failure of meiosis II exit and impaired female fertility. J. Cell Sci. 2015, 128, 3769–3780. [Google Scholar] [CrossRef]
- Han, F.; Dong, M.Z.; Lei, W.L.; Xu, Z.L.; Gao, F.; Schatten, H.; Wang, Z.B.; Sun, X.F.; Sun, Q.Y. Oligoasthenoteratospermia and sperm tail bending in PPP4C-deficient mice. Mol. Hum. Reprod. 2021, 27, gaaa083. [Google Scholar] [CrossRef]
- Mass, E.; Wachten, D.; Aschenbrenner, A.C.; Voelzmann, A.; Hoch, M. Murine Creld1 controls cardiac development through activation of calcineurin/NFATc1 signaling. Dev. Cell 2014, 28, 711–726. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, T.; Hasegawa, K.; Wada, H.; Kakita, T.; Kaburagi, S.; Yanazume, T.; Sasayama, S. Calcineurin-GATA4 pathway is involved in beta-adrenergic agonist-responsive endothelin-1 transcription in cardiac myocytes. J. Biol. Chem. 2001, 276, 34983–34989. [Google Scholar] [CrossRef]
- Crabtree, G.; Olson, E. NFAT signaling: Choreographing the social lives of cells. Cell 2002, 109, S67–S79. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, Z.; Gan, X.; Zhai, G.; Gao, J.; Xiong, C.; Qiu, X.; Wang, X.; Yin, Z.; Zheng, F. Deletion of Pr130 interrupts cardiac development in zebrafish. Int. J. Mol. Sci. 2016, 17, 1746. [Google Scholar] [CrossRef]
- Song, G.; Han, M.; Li, Z.; Gan, X.; Chen, X.; Yang, J.; Dong, S.; Yan, M.; Wan, J.; Wang, Y.; et al. Deletion of Pr72 causes cardiac developmental defects in zebrafish. PLoS ONE 2018, 13, e0206883. [Google Scholar] [CrossRef]
- Wyatt, L.; Wadham, C.; Crocker, L.A.; Lardelli, M.; Khew-Goodall, Y. The protein tyrosine phosphatase Pez regulates TGFbeta, epithelial-mesenchymal transition, and organ development. J. Cell Biol. 2007, 178, 1223–1235. [Google Scholar] [CrossRef] [PubMed]
- Drexler, H.C.A.; Vockel, M.; Polaschegg, C.; Frye, M.; Peters, K.; Vestweber, D. Vascular Endothelial Receptor Tyrosine Phosphatase: Identification of Novel Substrates Related to Junctions and a Ternary Complex with EPHB4 and TIE2. Mol. Cell. Proteom. 2019, 18, 2058–2077. [Google Scholar] [CrossRef] [PubMed]
- Vestweber, D. Vascular Endothelial Protein Tyrosine Phosphatase Regulates Endothelial Function. Physiology 2021, 36, 84–93. [Google Scholar] [CrossRef]
- Winderlich, M.; Keller, L.; Cagna, G.; Broermann, A.; Kamenyeva, O.; Kiefer, F.; Deutsch, U.; Nottebaum, A.F.; Vestweber, D. VE-PTP controls blood vessel development by balancing Tie-2 activity. J. Cell Biol. 2009, 185, 657–671. [Google Scholar] [CrossRef]
- Baumer, S.; Keller, L.; Holtmann, A.; Funke, R.; August, B.; Gamp, A.; Wolburg, H.; Wolburg-Buchholz, K.; Deutsch, U.; Vestweber, D. Vascular endothelial cell-specific phosphotyrosine phosphatase (VE-PTP) activity is required for blood vessel development. Blood 2006, 107, 4754–4762. [Google Scholar] [CrossRef]
- Martin, M.; Geudens, I.; Bruyr, J.; Potente, M.; Bleuart, A.; Lebrun, M.; Simonis, N.; Deroanne, C.; Twizere, J.C.; Soubeyran, P.; et al. PP2A regulatory subunit Balpha controls endothelial contractility and vessel lumen integrity via regulation of HDAC7. EMBO J. 2013, 32, 2491–2503. [Google Scholar] [CrossRef]
- Jiang, X.; Hu, J.; Wu, Z.; Cafarello, S.T.; Di Matteo, M.; Shen, Y.; Dong, X.; Adler, H.; Mazzone, M.; Ruiz de Almodovar, C.; et al. Protein Phosphatase 2A Mediates YAP Activation in Endothelial Cells Upon VEGF Stimulation and Matrix Stiffness. Front. Cell Dev. Biol. 2021, 9, 675562. [Google Scholar] [CrossRef] [PubMed]
- Breau, M.A.; Trembleau, A. Chemical and mechanical control of axon fasciculation and defasciculation. Semin. Cell Dev. Biol. 2023, 140, 72–81. [Google Scholar] [CrossRef]
- Bradke, F.; Dotti, C.G. Establishment of neuronal polarity: Lessons from cultured hippocampal neurons. Curr. Opin. Neurobiol. 2000, 10, 574–581. [Google Scholar] [CrossRef]
- Stoker, A.W. RPTPs in axons, synapses and neurology. Semin. Cell Dev. Biol. 2015, 37, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Tomita, H.; Cornejo, F.; Aranda-Pino, B.; Woodard, C.L.; Rioseco, C.C.; Neel, B.G.; Alvarez, A.R.; Kaplan, D.R.; Miller, F.D.; Cancino, G.I. The Protein Tyrosine Phosphatase Receptor Delta Regulates Developmental Neurogenesis. Cell Rep. 2020, 30, 215–228.e5. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.H.; Yeh, Y.J.; Wang, J.Y.; Liu, Y.W.; Chen, Y.L.; Cheng, H.W.; Cheng, C.M.; Chuang, Y.J.; Yuh, C.H.; Chen, Y.R. NEAP/DUSP26 suppresses receptor tyrosine kinases and regulates neuronal development in zebrafish. Sci. Rep. 2017, 7, 5241. [Google Scholar] [CrossRef]
- Knight, M.N.; Hankenson, K.D. Mesenchymal Stem Cells in Bone Regeneration. Adv. Wound Care 2013, 2, 306–316. [Google Scholar] [CrossRef]
- Lin, X.; Duan, X.; Liang, Y.Y.; Su, Y.; Wrighton, K.H.; Long, J.; Hu, M.; Davis, C.M.; Wang, J.; Brunicardi, F.C.; et al. PPM1A functions as a Smad phosphatase to terminate TGFbeta signaling. Cell 2006, 125, 915–928. [Google Scholar] [CrossRef]
- Kokabu, S.; Nojima, J.; Kanomata, K.; Ohte, S.; Yoda, T.; Fukuda, T.; Katagiri, T. Protein phosphatase magnesium-dependent 1A-mediated inhibition of BMP signaling is independent of Smad dephosphorylation. J. Bone Miner. Res. 2010, 25, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Kim, D.Y.; Lee, D.S.; Kim, J.W.; Koh, J.T.; Kim, E.J.; Jang, W.G. Peroxiredoxin II negatively regulates BMP2-induced osteoblast differentiation and bone formation via PP2A Calpha-mediated Smad1/5/9 dephosphorylation. Exp. Mol. Med. 2019, 51, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xiao, M.; Sun, B.; Zhang, Z.; Shen, T.; Duan, X.; Yu, P.B.; Feng, X.H.; Lin, X. C-terminal domain (CTD) small phosphatase-like 2 modulates the canonical bone morphogenetic protein (BMP) signaling and mesenchymal differentiation via Smad dephosphorylation. J. Biol. Chem. 2014, 289, 26441–26450. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, X.; Du, Y.; Hu, M.; Tian, Y.; Li, Z.; Lv, L.; Zhang, X.; Liu, Y.; Zhou, Y.; et al. DUSP5 promotes osteogenic differentiation through SCP1/2-dependent phosphorylation of SMAD1. Stem. Cells 2021, 39, 1395–1409. [Google Scholar] [CrossRef]
- Back, S.H.; Adapala, N.S.; Barbe, M.F.; Carpino, N.C.; Tsygankov, A.Y.; Sanjay, A. TULA-2, a novel histidine phosphatase, regulates bone remodeling by modulating osteoclast function. Cell. Mol. Life Sci. 2013, 70, 1269–1284. [Google Scholar] [CrossRef]
- Ponder, K.P. Analysis of liver development, regeneration, and carcinogenesis by genetic marking studies. FASEB J. 1996, 10, 673–682. [Google Scholar] [CrossRef]
- Pahlavan, P.S.; Feldmann, R.E., Jr.; Zavos, C.; Kountouras, J. Prometheus’ challenge: Molecular, cellular and systemic aspects of liver regeneration. J. Surg. Res. 2006, 134, 238–251. [Google Scholar] [CrossRef]
- Michalopoulos, G.K. Principles of liver regeneration and growth homeostasis. Compr. Physiol. 2013, 3, 485–513. [Google Scholar] [CrossRef]
- Chen, P.J.; Cai, S.P.; Huang, C.; Meng, X.M.; Li, J. Protein tyrosine phosphatase 1B (PTP1B): A key regulator and therapeutic target in liver diseases. Toxicology 2015, 337, 10–20. [Google Scholar] [CrossRef]
- Revuelta-Cervantes, J.; Mayoral, R.; Miranda, S.; González-Rodríguez, A.; Fernández, M.; Martín-Sanz, P.; Valverde, A.M. Protein Tyrosine Phosphatase 1B (PTP1B) deficiency accelerates hepatic regeneration in mice. Am. J. Pathol. 2011, 178, 1591–1604. [Google Scholar] [CrossRef]
- Samino, S.; Revuelta-Cervantes, J.; Vinaixa, M.; Rodríguez, M.; Valverde, A.M.; Correig, X. A (1)H NMR metabolic profiling to the assessment of protein tyrosine phosphatase 1B role in liver regeneration after partial hepatectomy. Biochimie 2013, 95, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Janssens, V.; Goris, J. Protein phosphatase 2A: A highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem. J. 2001, 353, 417–439. [Google Scholar] [CrossRef]
- Lai, S.S.; Zhao, D.D.; Cao, P.; Lu, K.; Luo, O.Y.; Chen, W.B.; Liu, J.; Jiang, E.Z.; Yu, Z.H.; Lee, G.; et al. PP2Acalpha positively regulates the termination of liver regeneration in mice through the AKT/GSK3beta/Cyclin D1 pathway. J. Hepatol. 2016, 64, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Zhang, J.; Fu, X.; Xie, W.; Qiu, Y. PP2Acalpha inhibits PFKFB2-induced glycolysis to promote termination of liver regeneration. Biochem. Biophys. Res. Commun. 2020, 526, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Fu, V.; Plouffe, S.W.; Guan, K.L. The Hippo pathway in organ development, homeostasis, and regeneration. Curr. Opin. Cell Biol. 2017, 49, 99–107. [Google Scholar] [CrossRef]
- Avruch, J.; Zhou, D.; Fitamant, J.; Bardeesy, N.; Mou, F.; Barrufet, L.R. Protein kinases of the Hippo pathway: Regulation and substrates. Semin. Cell Dev. Biol. 2012, 23, 770–784. [Google Scholar] [CrossRef]
- Lu, L.; Li, Y.; Kim, S.M.; Bossuyt, W.; Liu, P.; Qiu, Q.; Wang, Y.; Halder, G.; Finegold, M.J.; Lee, J.S.; et al. Hippo signaling is a potent in vivo growth and tumor suppressor pathway in the mammalian liver. Proc. Natl. Acad. Sci. USA 2010, 107, 1437–1442. [Google Scholar] [CrossRef]
- Song, H.; Mak, K.K.; Topol, L.; Yun, K.; Hu, J.; Garrett, L.; Chen, Y.; Park, O.; Chang, J.; Simpson, R.M.; et al. Mammalian Mst1 and Mst2 kinases play essential roles in organ size control and tumor suppression. Proc. Natl. Acad. Sci. USA 2010, 107, 1431–1436. [Google Scholar] [CrossRef]
- Liu, C.Y.; Lv, X.; Li, T.; Xu, Y.; Zhou, X.; Zhao, S.; Xiong, Y.; Lei, Q.Y.; Guan, K.L. PP1 cooperates with ASPP2 to dephosphorylate and activate TAZ. J. Biol. Chem. 2011, 286, 5558–5566. [Google Scholar] [CrossRef]
- Lv, X.B.; Liu, C.Y.; Wang, Z.; Sun, Y.P.; Xiong, Y.; Lei, Q.Y.; Guan, K.L. PARD3 induces TAZ activation and cell growth by promoting LATS1 and PP1 interaction. EMBO Rep. 2015, 16, 975–985. [Google Scholar] [CrossRef]
- Schlegelmilch, K.; Mohseni, M.; Kirak, O.; Pruszak, J.; Rodriguez, J.R.; Zhou, D.; Kreger, B.T.; Vasioukhin, V.; Avruch, J.; Brummelkamp, T.R.; et al. Yap1 acts downstream of α-catenin to control epidermal proliferation. Cell 2011, 144, 782–795. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Huang, J.; Wang, X.; Yuan, J.; Li, X.; Feng, L.; Park, J.I.; Chen, J. PTPN14 is required for the density-dependent control of YAP1. Genes Dev. 2012, 26, 1959–1971. [Google Scholar] [CrossRef] [PubMed]
- Mello, S.S.; Valente, L.J.; Raj, N.; Seoane, J.A.; Flowers, B.M.; McClendon, J.; Bieging-Rolett, K.T.; Lee, J.; Ivanochko, D.; Kozak, M.M.; et al. A p53 Super-tumor Suppressor Reveals a Tumor Suppressive p53-Ptpn14-Yap Axis in Pancreatic Cancer. Cancer Cell 2017, 32, 460–473.e6. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Wu, Q.; Wang, M.; Irani, S.; Li, X.; Zhang, Q.; Meng, F.; Liu, S.; Zhang, F.; Wu, L.; et al. The protein phosphatase PPM1A dephosphorylates and activates YAP to govern mammalian intestinal and liver regeneration. PLoS Biol. 2021, 19, e3001122. [Google Scholar] [CrossRef]
- Steward, M.M.; Sridhar, A.; Meyer, J.S. Neural regeneration. Curr. Top. Microbiol. Immunol. 2013, 367, 163–191. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; de Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation 2017, 135, e146–e603. [Google Scholar] [CrossRef]
- Chien, P.N.; Ryu, S.E. Protein tyrosine phosphatase sigma in proteoglycan-mediated neural regeneration regulation. Mol. Neurobiol. 2013, 47, 220–227. [Google Scholar] [CrossRef]
- Aricescu, A.R.; McKinnell, I.W.; Halfter, W.; Stoker, A.W. Heparan sulfate proteoglycans are ligands for receptor protein tyrosine phosphatase sigma. Mol. Cell. Biol. 2002, 22, 1881–1892. [Google Scholar] [CrossRef]
- Shen, Y.; Tenney, A.P.; Busch, S.A.; Horn, K.P.; Cuascut, F.X.; Liu, K.; He, Z.; Silver, J.; Flanagan, J.G. PTPsigma is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science 2009, 326, 592–596. [Google Scholar] [CrossRef]
- Tran, A.P.; Sundar, S.; Yu, M.; Lang, B.T.; Silver, J. Modulation of Receptor Protein Tyrosine Phosphatase Sigma Increases Chondroitin Sulfate Proteoglycan Degradation through Cathepsin B Secretion to Enhance Axon Outgrowth. J. Neurosci. Off. J. Soc. Neurosci. 2018, 38, 5399–5414. [Google Scholar] [CrossRef]
- Luo, F.; Wang, J.; Zhang, Z.; You, Z.; Bedolla, A.; Okwubido-Williams, F.; Huang, L.F.; Silver, J.; Luo, Y. Inhibition of CSPG receptor PTPsigma promotes migration of newly born neuroblasts, axonal sprouting, and recovery from stroke. Cell Rep. 2022, 40, 111137. [Google Scholar] [CrossRef] [PubMed]
- Fisher, D.; Xing, B.; Dill, J.; Li, H.; Hoang, H.H.; Zhao, Z.; Yang, X.L.; Bachoo, R.; Cannon, S.; Longo, F.M.; et al. Leukocyte common antigen-related phosphatase is a functional receptor for chondroitin sulfate proteoglycan axon growth inhibitors. J. Neurosci. 2011, 31, 14051–14066. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, N.; Fujiwara, N.; Hayakawa, K.; Ohama, T.; Sato, K. Protein phosphatase 6 promotes neurite outgrowth by promoting mTORC2 activity in N2a cells. J. Biochem. 2021, 170, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Park, K.K.; Liu, K.; Hu, Y.; Smith, P.D.; Wang, C.; Cai, B.; Xu, B.; Connolly, L.; Kramvis, I.; Sahin, M.; et al. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science 2008, 322, 963–966. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, C.; Wang, X.; Miao, J.; Chen, W.; Zhou, Y.; Xu, Y.; An, Y.; Cheng, A.; Ye, W.; et al. Driving axon regeneration by orchestrating neuronal and non-neuronal innate immune responses via the IFNγ-cGAS-STING axis. Neuron 2022, 111, 236–255. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Rosa, J.M.; Burns, C.E.; Burns, C.G. Zebrafish heart regeneration: 15 years of discoveries. Regeneration 2017, 4, 105–123. [Google Scholar] [CrossRef]
- Zhao, L.; Gao, F.; Gao, S.; Liang, Y.; Long, H.; Lv, Z.; Su, Y.; Ye, N.; Zhang, L.; Zhao, C.; et al. Biodiversity-based development and evolution: The emerging research systems in model and non-model organisms. Sci. China Life Sci. 2021, 64, 1236–1280. [Google Scholar] [CrossRef]
- Bergmann, O.; Bhardwaj, R.D.; Bernard, S.; Zdunek, S.; Barnabé-Heider, F.; Walsh, S.; Zupicich, J.; Alkass, K.; Buchholz, B.A.; Druid, H.; et al. Evidence for cardiomyocyte renewal in humans. Science 2009, 324, 98–102. [Google Scholar] [CrossRef]
- Porrello, E.R.; Mahmoud, A.I.; Simpson, E.; Hill, J.A.; Richardson, J.A.; Olson, E.N.; Sadek, H.A. Transient regenerative potential of the neonatal mouse heart. Science 2011, 331, 1078–1080. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, E.; Zhao, M.; Chong, Z.; Fan, C.; Tang, Y.; Hunter, J.D.; Borovjagin, A.V.; Walcott, G.P.; Chen, J.Y.; et al. Regenerative Potential of Neonatal Porcine Hearts. Circulation 2018, 138, 2809–2816. [Google Scholar] [CrossRef]
- Poss, K.D.; Wilson, L.G.; Keating, M.T. Heart regeneration in zebrafish. Science 2002, 298, 2188–2190. [Google Scholar] [CrossRef] [PubMed]
- Helston, O.; Amaya, E. Reactive oxygen species during heart regeneration in zebrafish: Lessons for future clinical therapies. Wound Repair Regen. 2021, 29, 211–224. [Google Scholar] [CrossRef]
- Han, P.; Zhou, X.H.; Chang, N.; Xiao, C.L.; Yan, S.; Ren, H.; Yang, X.Z.; Zhang, M.L.; Wu, Q.; Tang, B.; et al. Hydrogen peroxide primes heart regeneration with a derepression mechanism. Cell Res. 2014, 24, 1091–1107. [Google Scholar] [CrossRef] [PubMed]
- Missinato, M.A.; Saydmohammed, M.; Zuppo, D.A.; Rao, K.S.; Opie, G.W.; Kühn, B.; Tsang, M. Dusp6 attenuates Ras/MAPK signaling to limit zebrafish heart regeneration. Development 2018, 145, dev157206. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, C.; Wu, X.; Hu, X.; Zhang, Y.; Wang, X.; Zheng, L.; Gao, P.; Du, J.; Zheng, W.; et al. Dusp6 deficiency attenuates neutrophil-mediated cardiac damage in the acute inflammatory phase of myocardial infarction. Nat. Commun. 2022, 13, 6672. [Google Scholar] [CrossRef] [PubMed]
- Maillet, M.; Purcell, N.H.; Sargent, M.A.; York, A.J.; Bueno, O.F.; Molkentin, J.D. DUSP6 (MKP3) null mice show enhanced ERK1/2 phosphorylation at baseline and increased myocyte proliferation in the heart affecting disease susceptibility. J. Biol. Chem. 2008, 283, 31246–31255. [Google Scholar] [CrossRef]
- Liang, T.; Gao, F.; Jiang, J.; Lu, Y.W.; Zhang, F.; Wang, Y.; Liu, N.; Fu, X.; Dong, X.; Pei, J.; et al. Loss of Phosphatase and Tensin Homolog Promotes Cardiomyocyte Proliferation and Cardiac Repair After Myocardial Infarction. Circulation 2020, 142, 2196–2199. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zheng, H.; Han, Y.; Chen, Y.; Li, B.; Chen, G.; Chen, X.; Huang, S.; He, X.; Wei, G.; et al. LncRNA Snhg1-driven self-reinforcing regulatory network promoted cardiac regeneration and repair after myocardial infarction. Theranostics 2021, 11, 9397–9414. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, Y.; He, J.; Wu, F.; Wu, D.; Shi, N.; Liu, W.; Li, Z.; Liu, W.; Zhou, H.; et al. Dual specificity phosphatase 1 attenuates inflammation-induced cardiomyopathy by improving mitophagy and mitochondrial metabolism. Mol. Metab. 2022, 64, 101567. [Google Scholar] [CrossRef]
- Wang, Y.; Han, D.; Zhou, T.; Chen, C.; Cao, H.; Zhang, J.Z.; Ma, N.; Liu, C.; Song, M.; Shi, J.; et al. DUSP26 induces aortic valve calcification by antagonizing MDM2-mediated ubiquitination of DPP4 in human valvular interstitial cells. Eur. Heart J. 2021, 42, 2935–2951. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Maguire-Nguyen, K.K.; Rando, T.A.; Zasloff, M.A.; Strange, K.B.; Yin, V.P. The protein tyrosine phosphatase 1B inhibitor MSI-1436 stimulates regeneration of heart and multiple other tissues. NPJ Regen. Med. 2017, 2, 4. [Google Scholar] [CrossRef] [PubMed]
- Münch, J.; González-Rajal, A.; de la Pompa, J.L. Notch regulates blastema proliferation and prevents differentiation during adult zebrafish fin regeneration. Development 2013, 140, 1402–1411. [Google Scholar] [CrossRef] [PubMed]
- Kujawski, S.; Lin, W.; Kitte, F.; Börmel, M.; Fuchs, S.; Arulmozhivarman, G.; Vogt, S.; Theil, D.; Zhang, Y.; Antos, C.L. Calcineurin regulates coordinated outgrowth of zebrafish regenerating fins. Dev. Cell 2014, 28, 573–587. [Google Scholar] [CrossRef] [PubMed]
- McMillan, S.C.; Zhang, J.; Phan, H.E.; Jeradi, S.; Probst, L.; Hammerschmidt, M.; Akimenko, M.A. A regulatory pathway involving retinoic acid and calcineurin demarcates and maintains joint cells and osteoblasts in regenerating fin. Development 2018, 145, dev161158. [Google Scholar] [CrossRef]
- Zhang, Y.; Roos, M.; Himburg, H.; Termini, C.M.; Quarmyne, M.; Li, M.; Zhao, L.; Kan, J.; Fang, T.; Yan, X.; et al. PTPσ inhibitors promote hematopoietic stem cell regeneration. Nat. Commun. 2019, 10, 3667. [Google Scholar] [CrossRef]
- Zhou, S.; Shen, D.; Wang, Y.; Gong, L.; Tang, X.; Yu, B.; Gu, X.; Ding, F. microRNA-222 targeting PTEN promotes neurite outgrowth from adult dorsal root ganglion neurons following sciatic nerve transection. PLoS ONE 2012, 7, e44768. [Google Scholar] [CrossRef]
- Xing, X.; Guo, S.; Zhang, G.; Liu, Y.; Bi, S.; Wang, X.; Lu, Q. miR-26a-5p protects against myocardial ischemia/reperfusion injury by regulating the PTEN/PI3K/AKT signaling pathway. Braz. J. Med. Biol. Res. 2020, 53, e9106. [Google Scholar] [CrossRef]
- Simpson, L.M.; Fulcher, L.J.; Sathe, G.; Brewer, A.; Zhao, J.F.; Squair, D.R.; Crooks, J.; Wightman, M.; Wood, N.T.; Gourlay, R.; et al. An affinity-directed phosphatase, AdPhosphatase, system for targeted protein dephosphorylation. Cell Chem. Biol. 2023, 30, 188–202.e6. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Liu, C.; Zhao, L.; Zhang, X.; Su, Y. The Emerging Role of Protein Phosphatase in Regeneration. Life 2023, 13, 1216. https://doi.org/10.3390/life13051216
Zhang M, Liu C, Zhao L, Zhang X, Su Y. The Emerging Role of Protein Phosphatase in Regeneration. Life. 2023; 13(5):1216. https://doi.org/10.3390/life13051216
Chicago/Turabian StyleZhang, Meiling, Chenglin Liu, Long Zhao, Xuejiao Zhang, and Ying Su. 2023. "The Emerging Role of Protein Phosphatase in Regeneration" Life 13, no. 5: 1216. https://doi.org/10.3390/life13051216





