Higher Availability of Long-Chain Monounsaturated Fatty Acids in Preterm than in Full-Term Human Milk
Abstract
:1. Introduction
2. Materials and Methods
2.1. Analytical Method
2.2. Statistical Analysis
3. Results
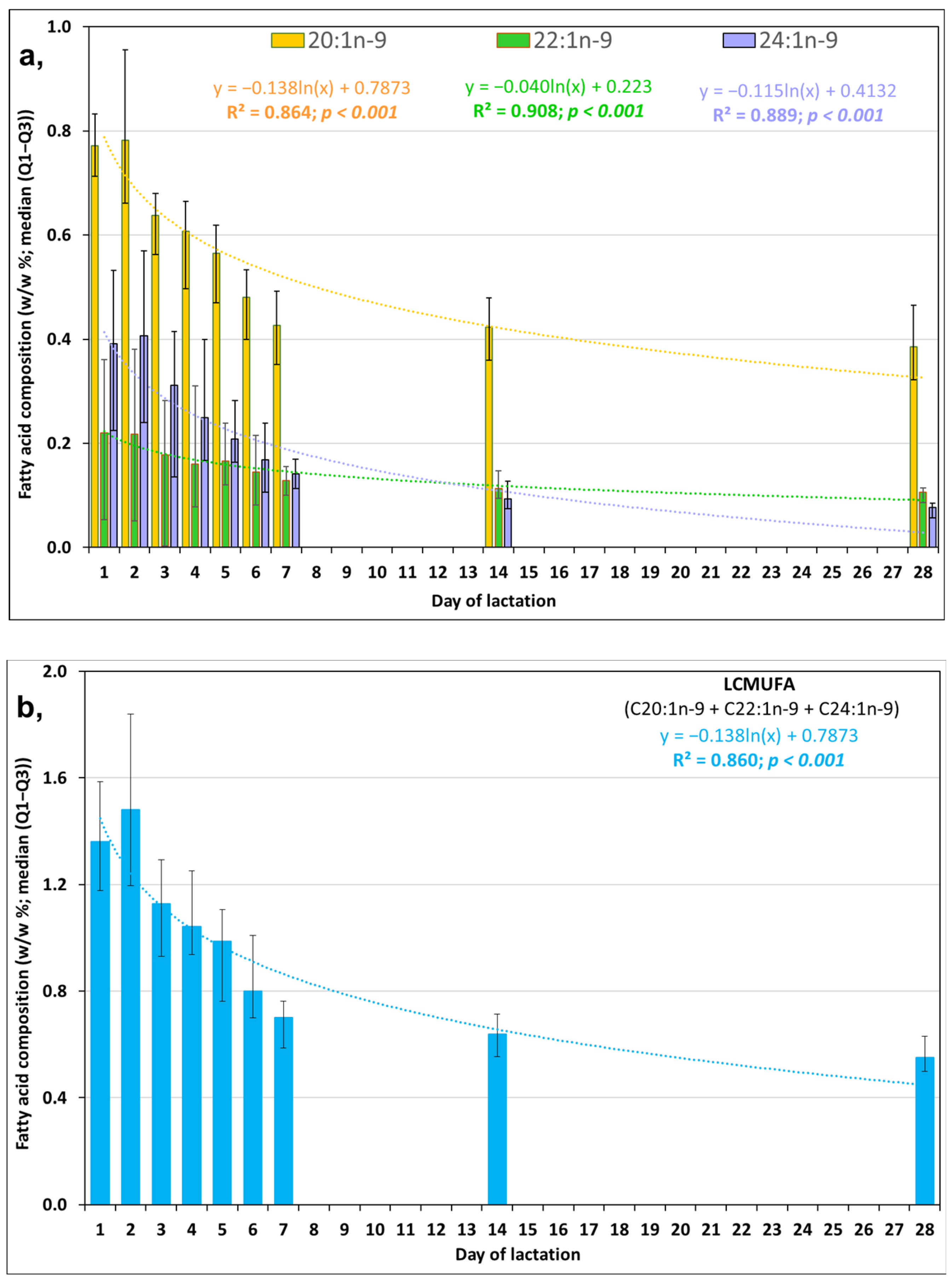
3.1. Day-to-Day Changes in LCMUFAs in FT HM during the First Week of Life
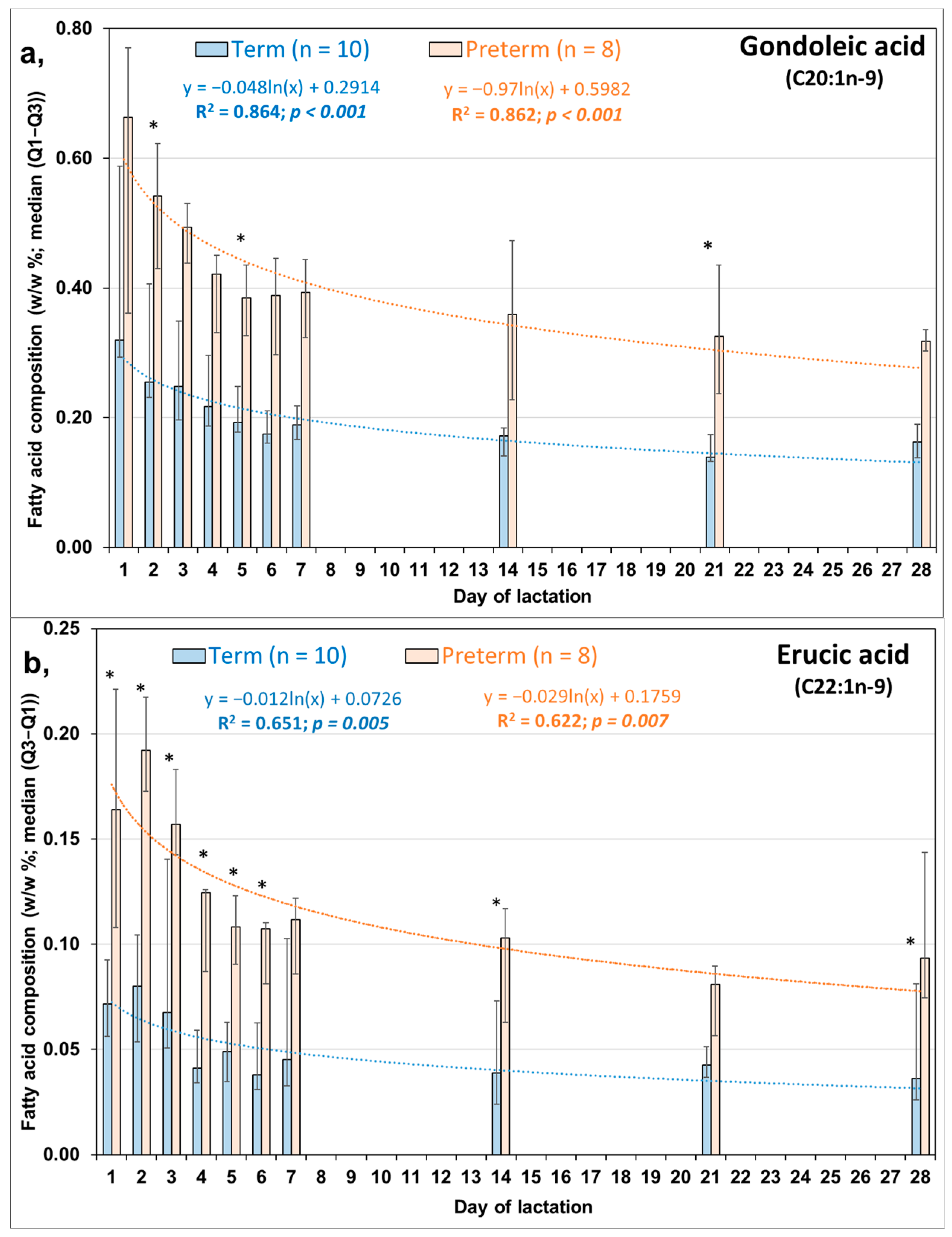
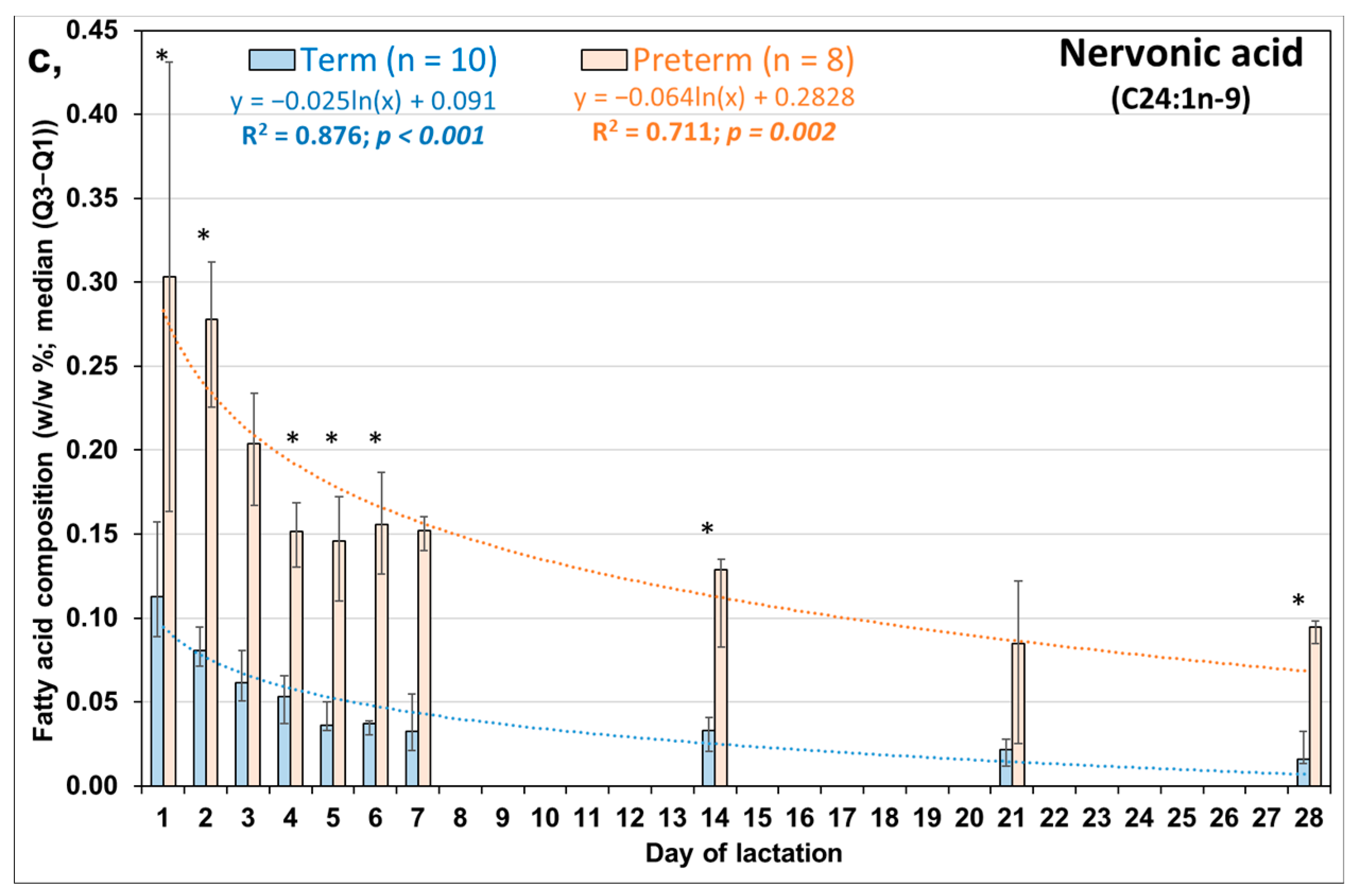
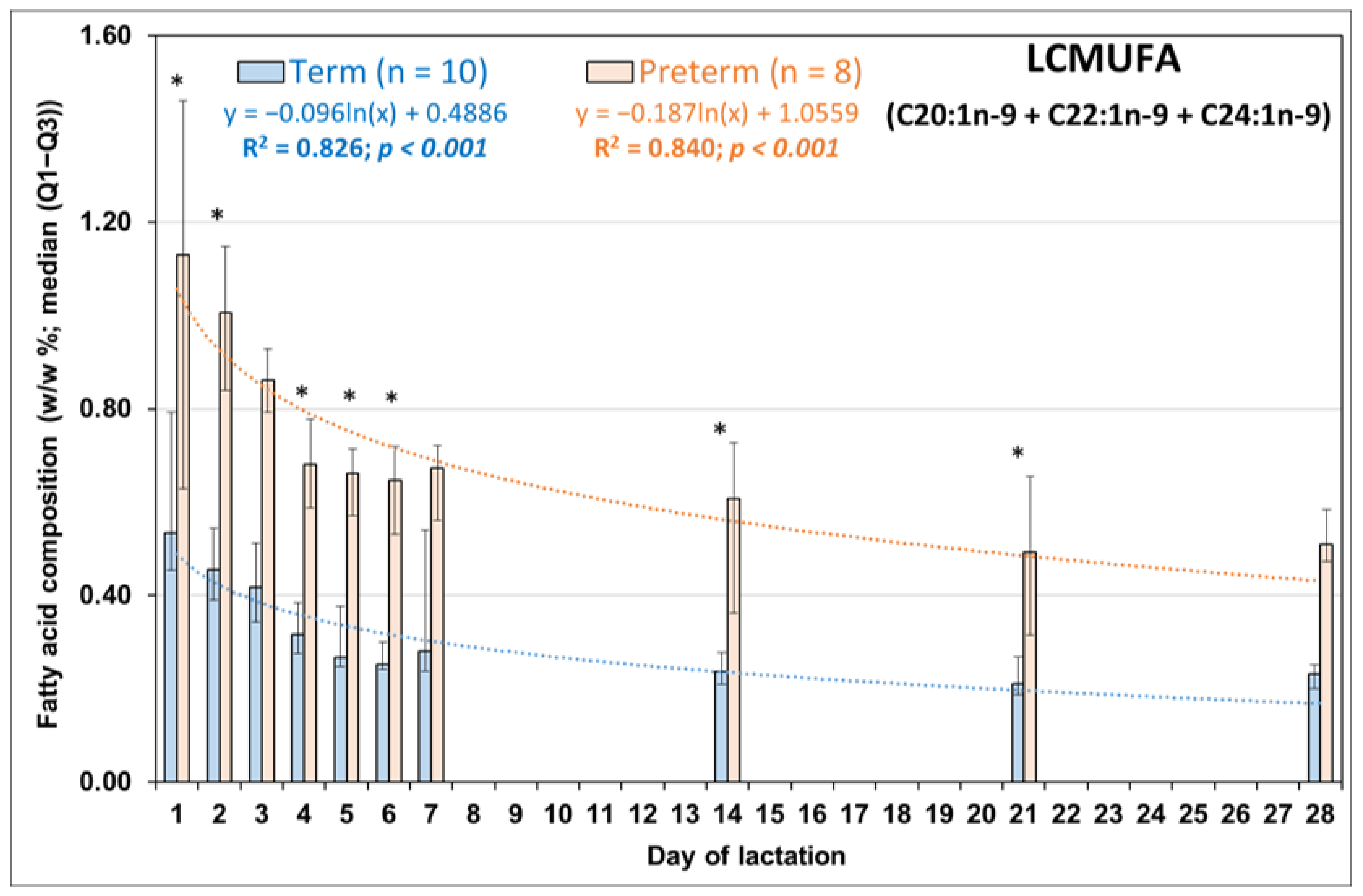
3.2. Comparison of Day-to-Day Changes in LCMUFAs in PT and FT HM during the First Week of Life
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Uauy, R.; Hoffman, D.R.; Peirano, P.; Birch, D.G.; Birch, E.E. Essential fatty acids in visual and brain development. Lipids 2001, 36, 885–895. [Google Scholar] [CrossRef] [PubMed]
- Amminger, G.P.; Schafer, M.R.; Klier, C.M.; Slavik, J.M.; Holzer, I.; Holub, M.; Goldstone, S.; Whitford, T.J.; McGorry, P.D.; Berk, M. Decreased nervonic acid levels in erythrocyte membranes predict psychosis in help-seeking ultra-high-risk individuals. Mol. Psychiatry 2012, 17, 1150–1152. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, E.C.; Farquharson, J.; Logan, R.W.; Howatson, A.G.; Patrick, W.J.; Weaver, L.T.; Cockburn, F. Infant cerebellar gray and white matter fatty acids in relation to age and diet. Lipids 1999, 34, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Bitsanis, D.; Crawford, M.A.; Moodley, T.; Holmsen, H.; Ghebremeskel, K.; Djahanbakhch, O. Arachidonic acid predominates in the membrane phosphoglycerides of the early and term human placenta. J. Nutr. 2005, 135, 2566–2571. [Google Scholar] [CrossRef]
- Babin, F.; Sarda, P.; Limasset, B.; Descomps, B.; Rieu, D.; Mendy, F.; Crastes de Paulet, A. Nervonic acid in red blood cell sphingomyelin in premature infants: An index of myelin maturation? Lipids 1993, 28, 624–630. [Google Scholar] [CrossRef]
- Kjellberg, E.; Roswall, J.; Bergman, S.; Strandvik, B.; Dahlgren, J. Serum n-6 and n-9 fatty acids correlate with serum igf-1 and growth up to 4 months of age in healthy infants. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 141–146. [Google Scholar] [CrossRef]
- Martínez, M.; Mougan, I. Fatty acid composition of human brain phospholipids during normal development. J. Neurochem. 1998, 71, 2528–2533. [Google Scholar] [CrossRef]
- Song, W.; Zhang, K.; Xue, T.; Han, J.; Peng, F.; Ding, C.; Lin, F.; Li, J.; Sze, F.T.A.; Gan, J.; et al. Cognitive improvement effect of nervonic acid and essential fatty acids on rats ingesting acer truncatum bunge seed oil revealed by lipidomics approach. Food Funct. 2022, 13, 2475–2490. [Google Scholar] [CrossRef]
- Yu, J.; Yuan, T.; Zhang, X.; Jin, Q.; Wei, W.; Wang, X. Quantification of nervonic acid in human milk in the first 30 days of lactation: Influence of lactation stages and comparison with infant formulae. Nutrients 2019, 11, 1892. [Google Scholar] [CrossRef]
- Aydin, İ.; Turan, Ö.; Aydin, F.N.; Koç, E.; Hirfanoğlu, İ.M.; Akyol, M.; Öztosun, M.; Akgül, E.Ö.; Demirin, H.; Kiliç, S.; et al. Comparing the fatty acid levels of preterm and term breast milk in turkish women. Turk. J. Med. Sci. 2014, 44, 305–310. [Google Scholar] [CrossRef]
- Bobiński, R.; Mikulska, M.; Mojska, H.; Simon, M. Comparison of the fatty acid composition of transitional and mature milk of mothers who delivered healthy full-term babies, preterm babies and full-term small for gestational age infants. Eur. J. Clin. Nutr. 2013, 67, 966–971. [Google Scholar] [CrossRef]
- Kuipers, R.S.; Luxwolda, M.F.; Dijck-Brouwer, D.A.; Muskiet, F.A. Fatty acid compositions of preterm and term colostrum, transitional and mature milks in a sub-saharan population with high fish intakes. Prostaglandins Leukot. Essent. Fat. Acids 2012, 86, 201–207. [Google Scholar] [CrossRef]
- Moltó-Puigmartí, C.; Castellote, A.I.; Carbonell-Estrany, X.; López-Sabater, M.C. Differences in fat content and fatty acid proportions among colostrum, transitional, and mature milk from women delivering very preterm, preterm, and term infants. Clin. Nutr. 2011, 30, 116–123. [Google Scholar] [CrossRef]
- Rueda, R.; Ramírez, M.; García-Salmerón, J.L.; Maldonado, J.; Gil, A. Gestational age and origin of human milk influence total lipid and fatty acid contents. Ann. Nutr. Metab. 1998, 42, 12–22. [Google Scholar] [CrossRef]
- Thakkar, S.K.; De Castro, C.A.; Beauport, L.; Tolsa, J.F.; Fischer Fumeaux, C.J.; Affolter, M.; Giuffrida, F. Temporal progression of fatty acids in preterm and term human milk of mothers from switzerland. Nutrients 2019, 11, 112. [Google Scholar] [CrossRef]
- Molnár, S.; Oláh, S.; Burus, I.; Decsi, T. Fatty acid composition of colostrum and mature human milk in hungary. Orv. Hetil. 2002, 143, 1015–1019. [Google Scholar]
- Minda, H.; Kovács, A.; Funke, S.; Szász, M.; Burus, I.; Molnár, S.; Marosvölgyi, T.; Decsi, T. Changes of fatty acid composition of human milk during the first month of lactation: A day-to-day approach in the first week. Ann. Nutr. Metab. 2004, 48, 202–209. [Google Scholar] [CrossRef]
- Kovács, A.; Funke, S.; Marosvölgyi, T.; Burus, I.; Decsi, T. Fatty acids in early human milk after preterm and full-term delivery. J. Pediatr. Gastroenterol. Nutr. 2005, 41, 454–459. [Google Scholar] [CrossRef]
- Mihályi, K.; Györei, E.; Szabó, E.; Marosvölgyi, T.; Lohner, S.; Decsi, T. Contribution of n-3 long-chain polyunsaturated fatty acids to human milk is still low in hungarian mothers. Eur. J. Pediatr. 2015, 174, 393–398. [Google Scholar] [CrossRef]
- Raclot, T.; Leray, C.; Bach, A.C.; Groscolas, R. The selective mobilization of fatty acids is not based on their positional distribution in white-fat-cell triacylglycerols. Biochem. J. 1995, 311 Pt 3, 911–916. [Google Scholar] [CrossRef]
- Tsutsumi, R.; Yamasaki, Y.; Takeo, J.; Miyahara, H.; Sebe, M.; Bando, M.; Tanba, Y.; Mishima, Y.; Takeji, K.; Ueshima, N.; et al. Long-chain monounsaturated fatty acids improve endothelial function with altering microbial flora. Transl. Res. 2021, 237, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.H.; Bando, M.; Sakurai, T.; Chen, Y.; Emma-Okon, B.; Wilhite, B.; Fukuda, D.; Vaisman, B.; Pryor, M.; Wakabayashi, Y.; et al. Long-chain monounsaturated fatty acid-rich fish oil attenuates the development of atherosclerosis in mouse models. Mol. Nutr. Food Res. 2016, 60, 2208–2218. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Y.; Su, D.; Lv, X.; Wang, M.; Ding, D.; Ma, J.; Xia, M.; Wang, D.; Yang, Y.; et al. The opposite associations of long-chain versus very long-chain monounsaturated fatty acids with mortality among patients with coronary artery disease. Heart 2014, 100, 1597–1605. [Google Scholar] [CrossRef] [PubMed]
- Sala-Vila, A.; Castellote, A.I.; Rodriguez-Palmero, M.; Campoy, C.; López-Sabater, M.C. Lipid composition in human breast milk from granada (spain): Changes during lactation. Nutrition 2005, 21, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Giuffrida, F.; Cruz-Hernandez, C.; Bertschy, E.; Fontannaz, P.; Masserey Elmelegy, I.; Tavazzi, I.; Marmet, C.; Sanchez-Bridge, B.; Thakkar, S.K.; De Castro, C.A.; et al. Temporal changes of human breast milk lipids of chinese mothers. Nutrients 2016, 8, 715. [Google Scholar] [CrossRef]
- Bitman, J.; Wood, L.D.; Hamosh, M.; Hamosh, P.; Mehta, N.R. Comparison of the lipid composition of breast milk from mothers of term and preterm infants. Am. J. Clin. Nutr. 1983, 38, 300–312. [Google Scholar] [CrossRef]
- Bitman, J.; Wood, L.D.; Mehta, N.R.; Hamosh, P.; Hamosh, M. Comparison of the phospholipid composition of breast milk from mothers of term and preterm infants during lactation. Am. J. Clin. Nutr. 1984, 40, 1103–1119. [Google Scholar] [CrossRef]
- Hopperton, K.E.; Pitino, M.A.; Chouinard-Watkins, R.; Shama, S.; Sammut, N.; Bando, N.; Williams, B.A.; Walton, K.; Kiss, A.; Unger, S.L.; et al. Determinants of fatty acid content and composition of human milk fed to infants born weighing <1250 g. Am. J. Clin. Nutr. 2021, 114, 1523–1534. [Google Scholar] [CrossRef]
- Floris, L.M.; Stahl, B.; Abrahamse-Berkeveld, M.; Teller, I.C. Human milk fatty acid profile across lactational stages after term and preterm delivery: A pooled data analysis. Prostaglandins Leukot. Essent. Fat. Acids 2020, 156, 102023. [Google Scholar] [CrossRef]
- Berenhauser, A.C.; Pinheiro do Prado, A.C.; da Silva, R.C.; Gioielli, L.A.; Block, J.M. Fatty acid composition in preterm and term breast milk. Int. J. Food Sci. Nutr. 2012, 63, 318–325. [Google Scholar] [CrossRef]
- Marín, M.C.; Sanjurjo, A.L.; Sager, G.; Margheritis, C.; de Alaniz, M.J. Fatty acid composition of human milk from mothers of preterm and full-term infants. Arch. Argent. Pediatr. 2009, 107, 315–320. [Google Scholar] [CrossRef]
- Jang, S.H.; Lee, B.S.; Park, J.H.; Chung, E.J.; Um, Y.S.; Lee-Kim, Y.C.; Kim, E.A. Serial changes of fatty acids in preterm breast milk of korean women. J. Hum. Lact. 2011, 27, 279–285. [Google Scholar] [CrossRef]
- Nyuar, K.B.; Min, Y.; Ghebremeskel, K.; Khalil, A.K.; Elbashir, M.I.; Cawford, M.A. Milk of northern sudanese mothers whose traditional diet is high in carbohydrate contains low docosahexaenoic acid. Acta Paediatr. 2010, 99, 1824–1827. [Google Scholar] [CrossRef]
- Jiang, J.; Wu, K.; Yu, Z.; Ren, Y.; Zhao, Y.; Jiang, Y.; Xu, X.; Li, W.; Jin, Y.; Yuan, J.; et al. Changes in fatty acid composition of human milk over lactation stages and relationship with dietary intake in chinese women. Food Funct. 2016, 7, 3154–3162. [Google Scholar] [CrossRef]
- Sánchez-Hernández, S.; Esteban-Muñoz, A.; Giménez-Martínez, R.; Aguilar-Cordero, M.J.; Miralles-Buraglia, B.; Olalla-Herrera, M. A comparison of changes in the fatty acid profile of human milk of spanish lactating women during the first month of lactation using gas chromatography-mass spectrometry. A comparison with infant formulas. Nutrients 2019, 11, 3055. [Google Scholar] [CrossRef]
- Wu, K.; Gao, R.; Tian, F.; Mao, Y.; Wang, B.; Zhou, L.; Shen, L.; Guan, Y.; Cai, M. Fatty acid positional distribution (sn-2 fatty acids) and phospholipid composition in chinese breast milk from colostrum to mature stage. Br. J. Nutr. 2019, 121, 65–73. [Google Scholar] [CrossRef]
- Ortega, D.F.; Reyes, S.C.; Suárez, R.D.; González, Q.S.; Rosales, C.J. Fatty acid concentration analysis in colostrum and transitional milk. An. Esp. Pediatr. 1997, 46, 455–459. [Google Scholar]
- Khor, G.L.; Tan, S.S.; Stoutjesdijk, E.; Ng, K.W.T.; Khouw, I.; Bragt, M.; Schaafsma, A.; Dijck-Brouwer, D.A.J.; Muskiet, F.A.J. Temporal changes in breast milk fatty acids contents: A case study of malay breastfeeding women. Nutrients 2020, 13, 101. [Google Scholar] [CrossRef]
- Li, J.; Fan, Y.; Zhang, Z.; Yu, H.; An, Y.; Kramer, J.K.; Deng, Z. Evaluating the trans fatty acid, cla, pufa and erucic acid diversity in human milk from five regions in china. Lipids 2009, 44, 257–271. [Google Scholar] [CrossRef]
- Ntoumani, E.; Strandvik, B.; Sabel, K.G. Nervonic acid is much lower in donor milk than in milk from mothers delivering premature infants—of neglected importance? Prostaglandins Leukot. Essent. Fat. Acids 2013, 89, 241–244. [Google Scholar] [CrossRef]
- Strandvik, B.; Ntoumani, E.; Lundqvist-Persson, C.; Sabel, K.G. Long-chain saturated and monounsaturated fatty acids associate with development of premature infants up to 18 months of age. Prostaglandins Leukot. Essent. Fat. Acids 2016, 107, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.Y.; Kwan, K.Y.; Tong, K.K.; Ratnayake, W.M.; Li, H.Q.; Leung, S.S. Breast milk fatty acid composition: A comparative study between hong kong and chongqing chinese. Lipids 1997, 32, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Yuhas, R.; Pramuk, K.; Lien, E.L. Human milk fatty acid composition from nine countries varies most in dha. Lipids 2006, 41, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Fulco, A.J.; Mead, J.F. The biosynthesis of lignoceric, cerebronic, and nervonic acids. J. Biol. Chem. 1961, 236, 2416–2420. [Google Scholar] [CrossRef]
- Bettger, W.J.; DiMichelle-Ranalli, E.; Dillingham, B.; Blackadar, C.B. Nervonic acid is transferred from the maternal diet to milk and tissues of suckling rat pups. J. Nutr. Biochem. 2003, 14, 160–165. [Google Scholar] [CrossRef]
| Molnár S, 2002 [16] | Minda H, 2004 [17] | Kovács A, 2005 [18] | Mihályi K, 2015 [19] | |||
|---|---|---|---|---|---|---|
| Number of included mothers | n = 18 | n = 15 | n = 18 | n = 10 | n = 8 | n = 46 |
| Maternal age (years) | n.d. | n.d. | 29.4 ± 4.0 | 28.0 (4.5) | 30.5 (4.2) | 32.9 |
| Maternal weight (kg) | 68.4 ± 12.0 a | 60.2 ± 6.2 a | 60.5 (23.6) | 64.75 (8.7) | 60.0 (7.0) | n.d. |
| Maternal BMI (kg/m2) | 24.7 ± 3.2 a | 22.2 ± 3.3 a | 22.2 (6.8) | 24.3 (5.4) | 22.0 (3.5) | n.d. |
| Gestational age (weeks) | * | * | 39.1 ± 1.6 | 38.5 (2.7) b | 28.0 (4.2) b | >37th week |
| Birth weight (g) | * | * | 3537 ± 528 | 3375 (282) b | 1235 (420) b | 3535 ± 517 |
| Birth length (cm) | * | * | 51.3 ± 2.8 | 50.5 (2.5) b | 36.0 (4.7) b | 50.7 ± 2.3 |
| Parity (primipara/multipara) | n.d. | n.d. | 6/12 | 2/8 | 1/7 | 21/26 |
| Weight% | C20:1n-9 | C22:1n-9 | C24:1n-9 | ||||
|---|---|---|---|---|---|---|---|
| Study | Lactation Stage | PT | FT | PT | FT | PT | FT |
| Thakkar et al., 2018 [15] | C (≤7 day) | 0.76 | 0.76 | 0.19 | 0.25 | 0.30 | 0.39 |
| TM (8–14 day) | 0.60 | 0.54 | 0.19 | 0.12 | 0.13 | 0.13 | |
| MM (15–112 day) | 0.47 | 0.45 | 0.09 | 0.08 | 0.07 | 0.07 | |
| Rueda et al., 1998 [14] * | C (1–5 day) | 0.92 | 0.80 | 0.20 | 0.32 | 0.37 | 0.44 |
| TM (6–15 day) | 0.69 | 0.69 | 0.18 | 0.18 | 0.27 | 0.18 | |
| MM (16–35 day) | 0.57 | 0.54 | n.d. | 0.09 | n.d. | 0.08 | |
| Moltó-Puigmartí et al., 2011 [13] * | C (2–4 day) | 0.96 | 0.96 | 0.28 | 0.27 | 0.30 | 0.32 |
| TM (8–12 day) | 0.58 | 0.52 | 0.14 | 0.12 | 0.11 | 0.08 | |
| MM (28–32 day) | 0.48 | 0.47 | 0.10 | 0.10 | 0.05 | 0.05 | |
| Aydin et al., 2014 [10] | C (3rd day) | 1.39 | 1.21 | 0.34 | 0.33 | 0.72 | 0.78 |
| TM (7th day) | 1.23 | 1.05 | 0.3 | 0.25 | 0.77 | 0.74 | |
| MM (28th day) | 1 | 0.86 | 0.2 | 0.17 | 0.64 | 0.37 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marosvölgyi, T.; Dergez, T.; Szentpéteri, J.L.; Szabó, É.; Decsi, T. Higher Availability of Long-Chain Monounsaturated Fatty Acids in Preterm than in Full-Term Human Milk. Life 2023, 13, 1205. https://doi.org/10.3390/life13051205
Marosvölgyi T, Dergez T, Szentpéteri JL, Szabó É, Decsi T. Higher Availability of Long-Chain Monounsaturated Fatty Acids in Preterm than in Full-Term Human Milk. Life. 2023; 13(5):1205. https://doi.org/10.3390/life13051205
Chicago/Turabian StyleMarosvölgyi, Tamás, Timea Dergez, József L. Szentpéteri, Éva Szabó, and Tamás Decsi. 2023. "Higher Availability of Long-Chain Monounsaturated Fatty Acids in Preterm than in Full-Term Human Milk" Life 13, no. 5: 1205. https://doi.org/10.3390/life13051205








