Removal Process of Structural Oxygen from Tetrahedrons in Muscovite during Acid Leaching of Vanadium-Bearing Shale
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Muscovite Leaching
2.3. Structure Calculations
2.4. Reaction Rate Model
3. Results and Discussion
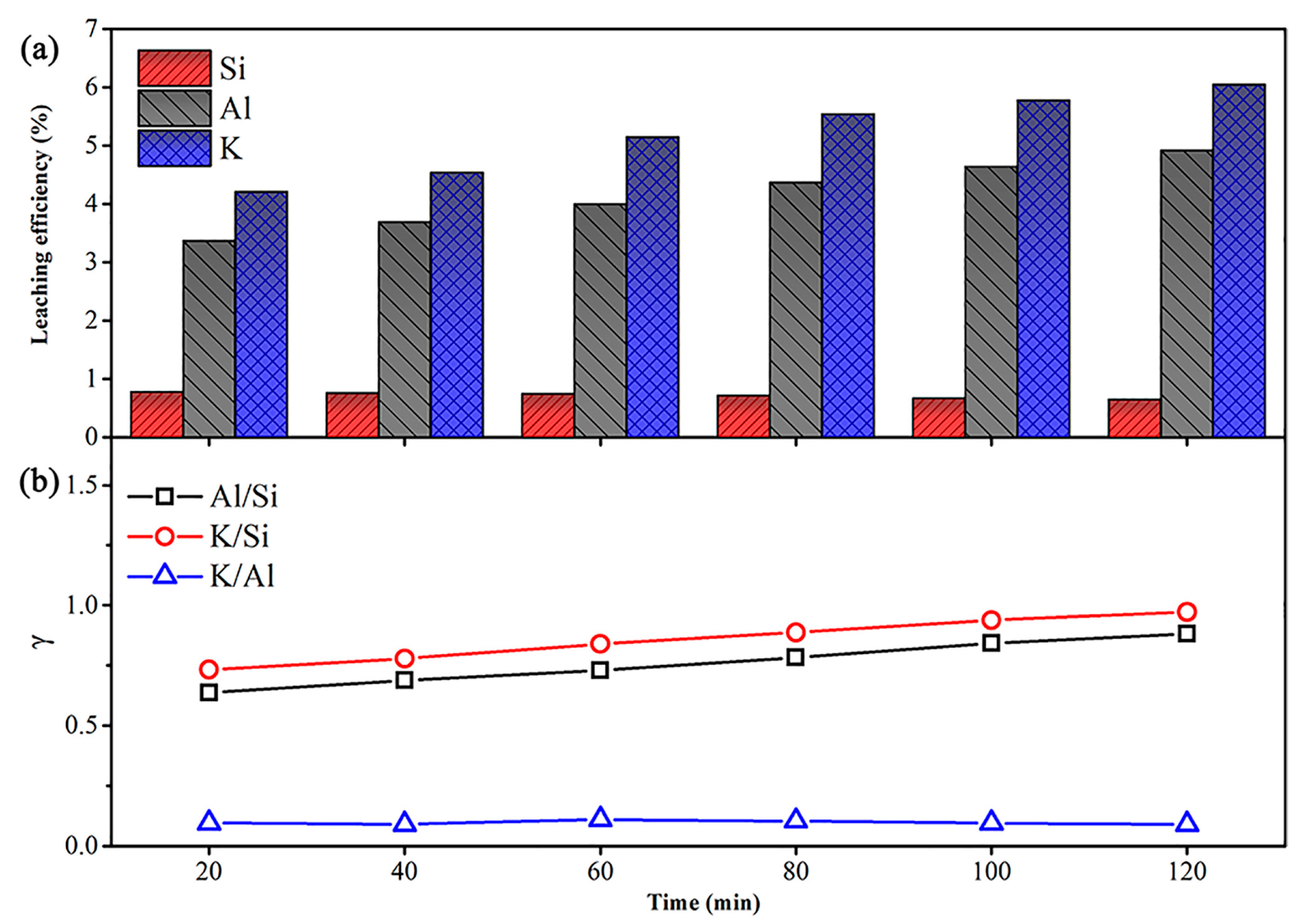
3.1. Element Dissolution of Muscovite
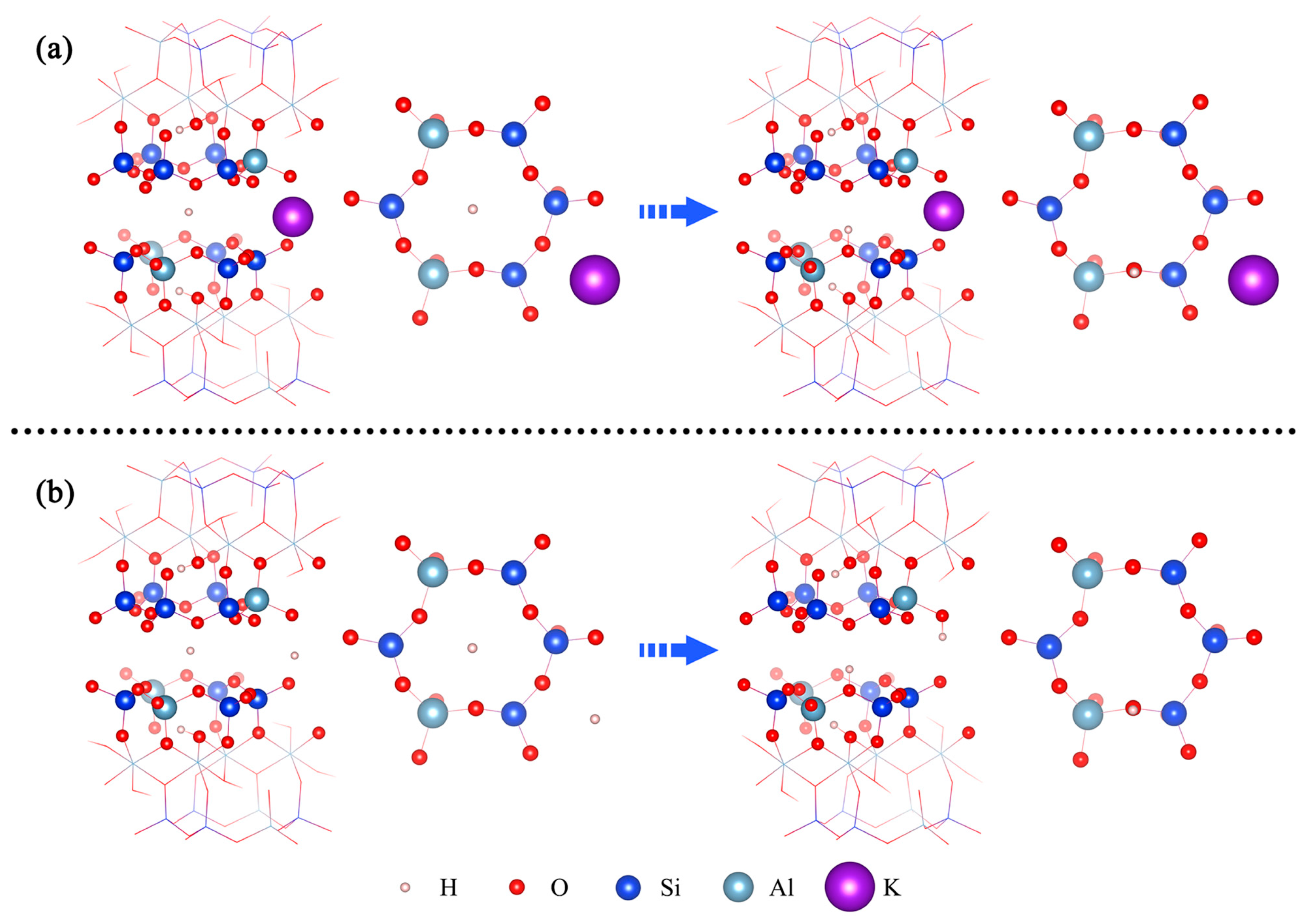
3.2. Formation of the 001 Surface Hydroxyl
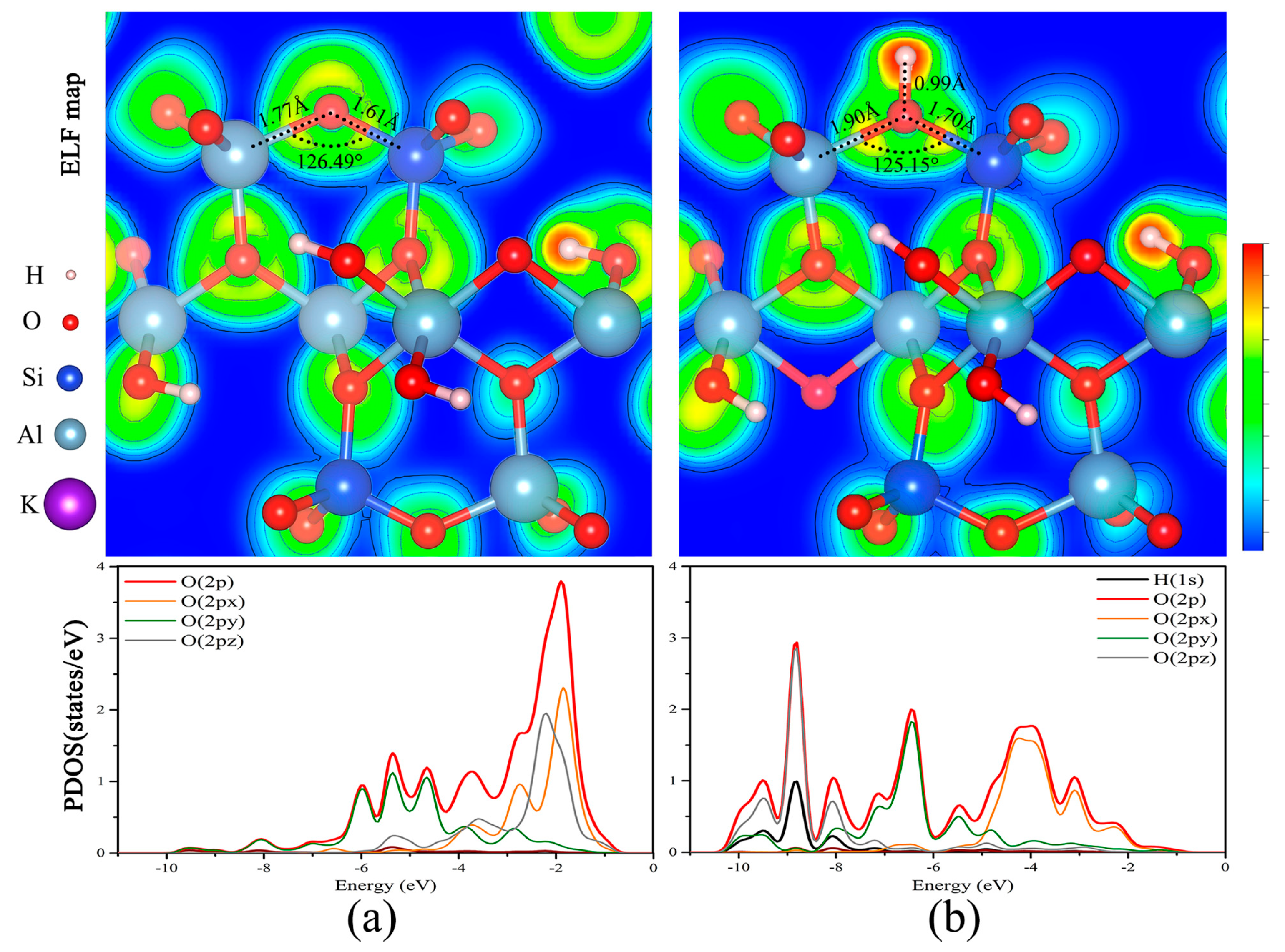
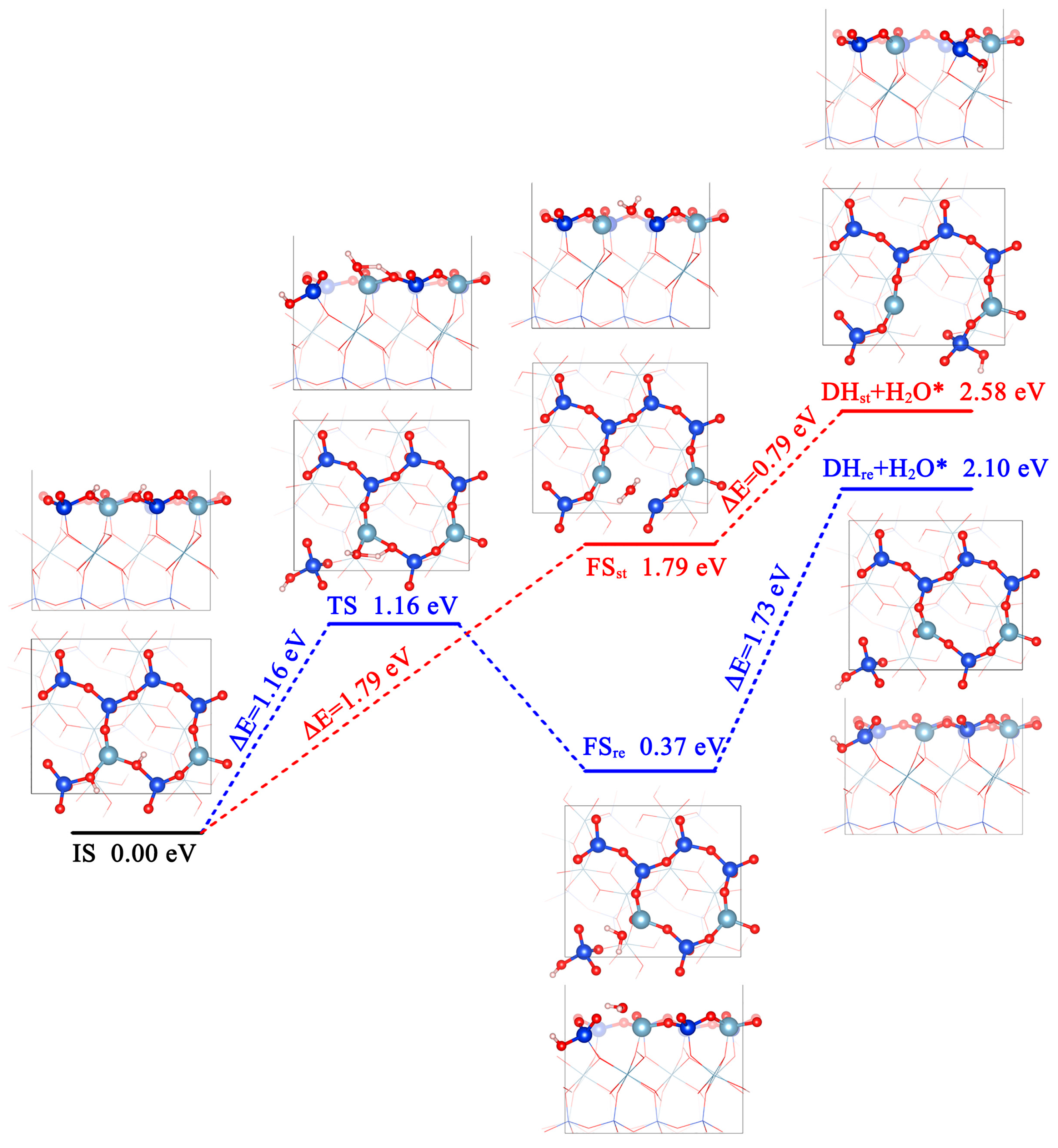
3.3. Fracture of Oxygen Framework by Dehydroxylation
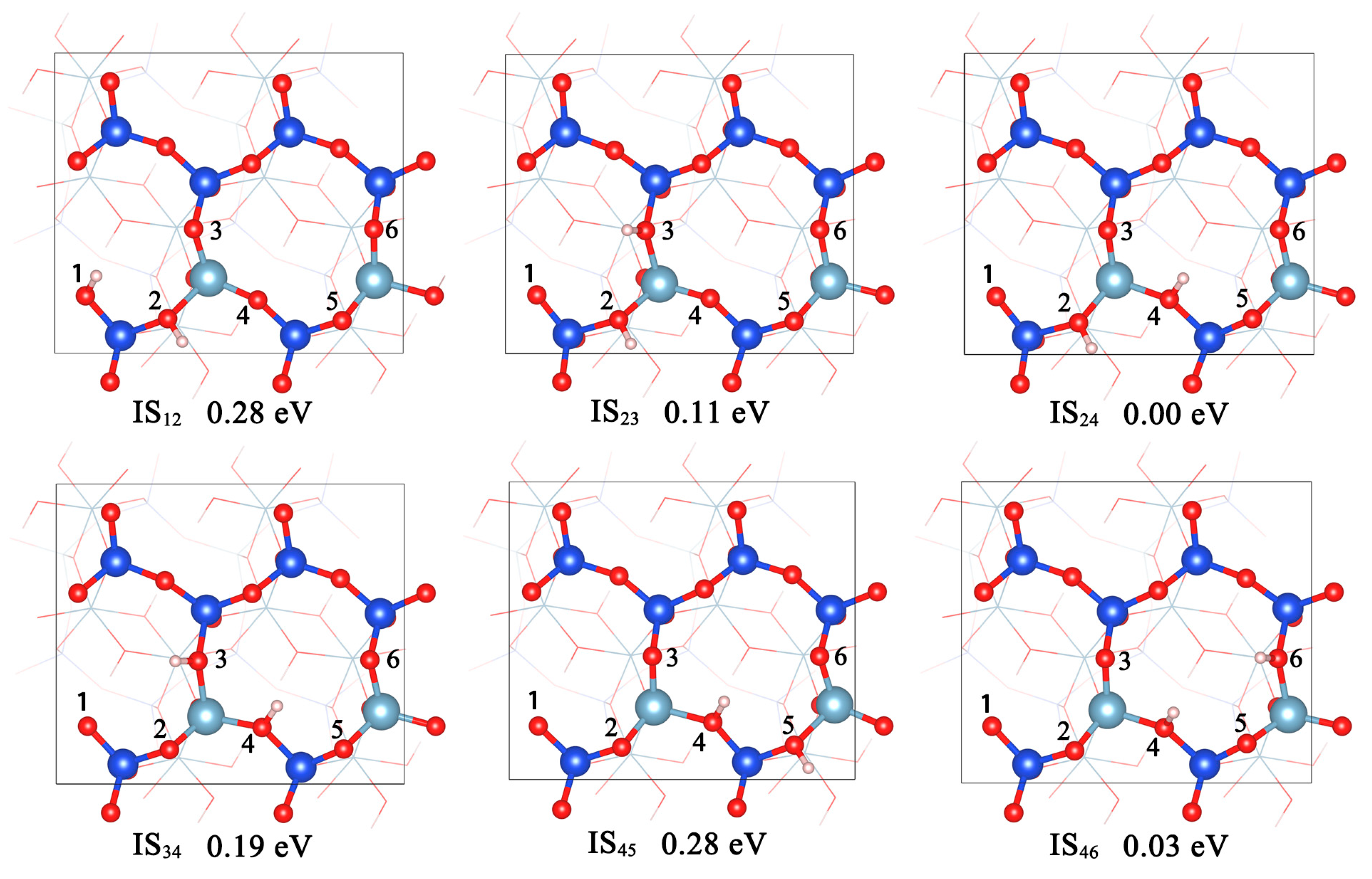
3.3.1. Reaction Mechanisms of Dehydroxylation
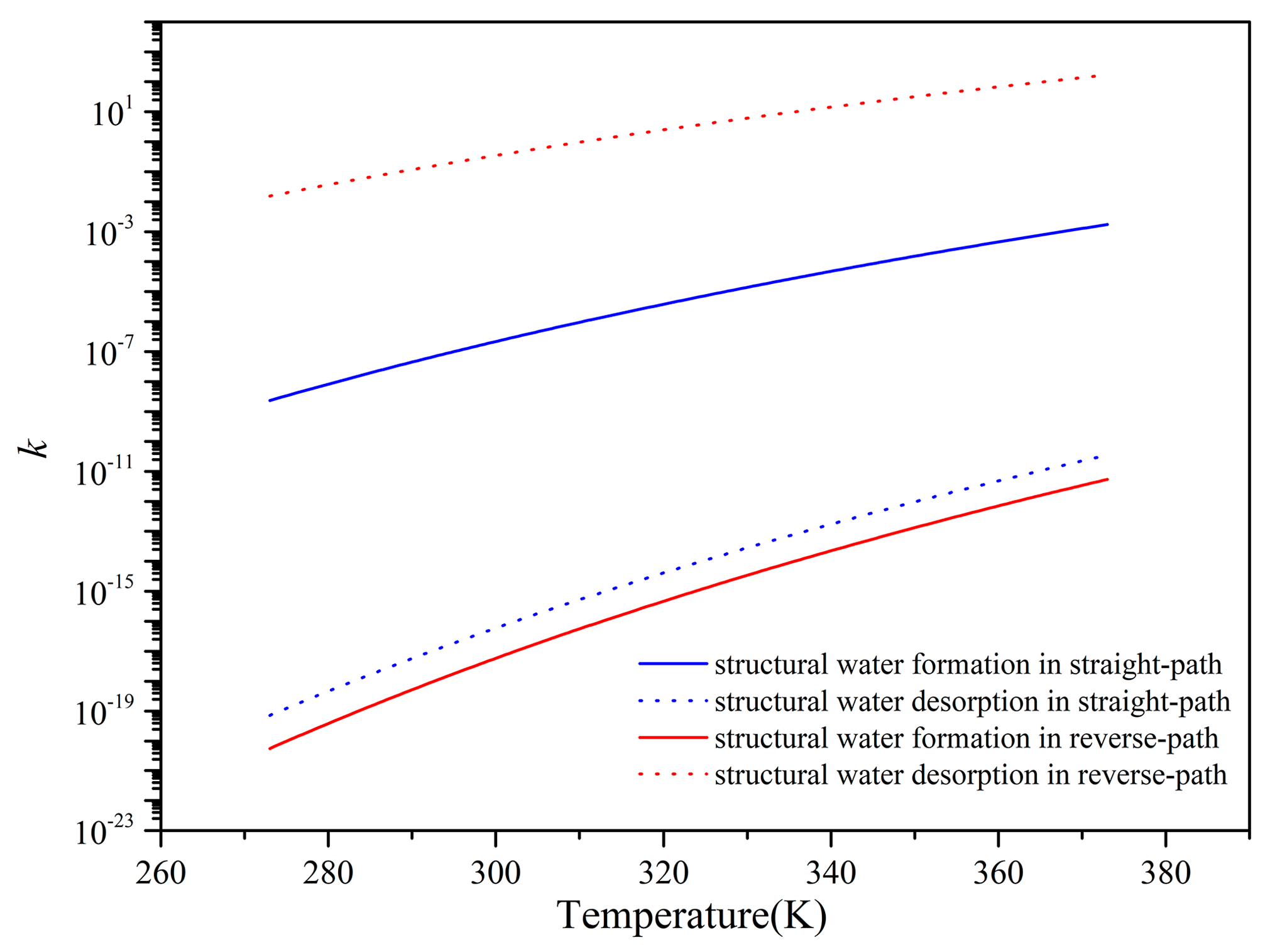
3.3.2. Reaction Rates of Dehydroxylation
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Koust, S.; Reinecke, B.N.; Adamsen, K.C.; Beinik, I.; Handrup, K.; Li, Z.S.; Moses, P.G.; Schnadt, J.; Lauritsen, J.V.; Wendt, S. Coverage-dependent oxidation and reduction of vanadium supported on anatase TiO2(101). J. Catal. 2018, 360, 118–126. [Google Scholar] [CrossRef]
- Motola, M.; Satrapinskyy, L.; Caplovicova, M.; Roch, T.; Gregor, M.; Grancic, B.; Gregus, J.; Caplovic, L.; Plesch, G. Enhanced photocatalytic activity of hydrogenated and vanadium doped TiO2 nanotube arrays grown by anodization of sputtered Ti layers. Appl. Surf. Sci. 2018, 434, 1257–1265. [Google Scholar] [CrossRef]
- Crans, D.C.; Yang, L.N.; Haase, A.; Yang, X.G. Health benefits of vanadium and its potential as an anticancer agent. Met. Ions Life Sci. 2018, 18, 251–279. [Google Scholar]
- Marques, M.P.M.; Gianolio, D.; Ramos, S.; Batista de Carvalho, L.A.E.; Aureliano, M. An EXAFS approach to the study of polyoxometalate-protein interactions: The case of decavanadate-actin. Inorg. Chem. 2017, 56, 10893–10903. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.M.; Bao, S.X.; Liu, T.; Chen, T.J.; Huang, J. The technology of extracting vanadium from stone coal in China: History, current status and future prospects. Hydrometallurgy 2011, 109, 116–124. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Hu, Y.J.; Bao, S.X. Vanadium emission during roasting of vanadium-bearing stone coal in chlorine. Miner. Eng. 2012, 30, 95–98. [Google Scholar] [CrossRef]
- Ye, P.H.; Wang, X.W.; Wang, M.Y.; Fan, Y.Y.; Xiang, X.Y. Recovery of vanadium from stone coal acid leaching solution by coprecipitation, alkaline roasting and water leaching. Hydrometallurgy 2012, 117, 108–115. [Google Scholar] [CrossRef]
- Cai, Z.L.; Zhang, Y.M. Phase transformations of vanadium recovery from refractory stone coal by novel NaOH molten roasting and water leaching technology. RSC Adv. 2017, 7, 36917–36922. [Google Scholar] [CrossRef]
- Hu, K.L.; Liu, X.H.; Li, Q.G. Extracting vanadium from stone coal by a cyclic alkaline leaching method. Metall. Mater. Trans. B 2017, 48, 1342–1347. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Y.M.; Liu, T.; Huang, J.; Zhao, J.; Zhang, G.B.; Liu, J. A mechanism of calcium fluoride-enhanced vanadium leaching from stone coal. Int. J. Miner. Process. 2015, 145, 87–93. [Google Scholar] [CrossRef]
- Hu, P.C.; Zhang, Y.M.; Huang, J.; Liu, T.; Yuan, Y.Z.; Xue, N.N. Eco-friendly leaching and separation of vanadium over iron impurity from vanadium-bearing shale using oxalic acid as a leachant. ACS Sustain. Chem. Eng. 2018, 6, 1900–1908. [Google Scholar] [CrossRef]
- Yuan, Y.Z.; Zhang, Y.M.; Liu, T.; Chen, T.J. Comparison of the mechanisms of microwave roasting and conventional roasting and of their effects on vanadium extraction from stone coal. Int. J. Min. Met. Mater. 2015, 22, 476–482. [Google Scholar] [CrossRef]
- Xue, N.N.; Zhang, Y.M.; Liu, T.; Huang, J.; Liu, H.; Chen, F. Mechanism of vanadium extraction from stone coal via hydrating and hardening of anhydrous calcium sulfate. Hydrometallurgy 2016, 166, 48–56. [Google Scholar] [CrossRef]
- Crundwell, F.K. The mechanism of dissolution of minerals in acidic and alkaline solutions: Part II application to a new theory to silicates, aluminosilicates and quartz. Hydrometallurgy 2014, 149, 265–275. [Google Scholar] [CrossRef]
- Crundwell, F.K. The mechanism of dissolution of minerals in acidic and alkaline solutions: Part VI a molecular viewpoint. Hydrometallurgy 2016, 161, 34–44. [Google Scholar] [CrossRef]
- Hernández-Haro, N.; Ortega-Castro, J.; Valle, C.P.D.; Muñoz-Santiburcio, D.; Sainz-Díaz, C.I.; Hernández-Laguna, A. Computational study of the elastic behavior of the 2M1 muscovite-paragonite series. Am. Miner. 2013, 98, 651–664. [Google Scholar] [CrossRef]
- Ortega-Castro, J.; Hernández-Haro, N.; Hernández-Laguna, A.; Sainz-Díaz, C.I. DFT calculation of crystallographic properties of dioctahedral 2:1 phyllosilicates. Clay Miner. 2008, 43, 351–361. [Google Scholar] [CrossRef]
- Ulian, G.; Valdrè, G. Density functional investigation of the thermo-physical and thermo-chemical properties of 2M1 muscovite. Am. Miner. 2015, 100, 935–944. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for open-shell transition metals. Phys. Rev. B 1993, 48, 13115–13118. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Wang, Y. Generalized gradient approximation for the exchange-correlation hole of a many-electron system. Phys. Rev. B 1996, 54, 16533–16539. [Google Scholar] [CrossRef]
- Perdew, J.P.; Chevary, J.A.; Vosko, S.H.; Jackson, K.A.; Pederson, M.R.; Singh, D.J.; Fiolhais, C. Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B 1992, 46, 6671–6687. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Zheng, Q.S.; Zhang, Y.M.; Liu, T.; Huang, J.; Xue, N.N.; Shi, Q.H. Optimal location of vanadium in muscovite and its geometrical and electronic properties by DFT calculation. Minerals 2017, 7, 32. [Google Scholar] [CrossRef]
- Henkelman, G.; Jónsson, H. Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points. J. Chem. Phys. 2000, 113, 9978–9985. [Google Scholar] [CrossRef]
- Henkelman, G.; Uberuaga, B.P.; Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 2000, 113, 9901–9904. [Google Scholar] [CrossRef]
- Sholl, D.S.; Steckel, J.A. Density Functional Theory—A Practical Introduction; John Wiley & Sons: Hoboken, NJ, USA, 2009; pp. 131–158. [Google Scholar]
- Moro, D.; Ulian, G.; Valdrè, G. Nanoscale cross-correlated AFM, Kelvin probe, elastic modulus and quantum mechanics investigation of clay mineral surfaces: The case of chlorite. Appl. Clay Sci. 2016, 131, 175–181. [Google Scholar] [CrossRef]
- Valdrè, G.; ToSoni, S.; Moro, D. Zeolitic-type Brønsted-Lowry sites distribution imaged on clinochlore. Am. Miner. 2011, 96, 1461–1466. [Google Scholar] [CrossRef]
- Molina-Montes, E.; Donadio, D.; Hernández-Laguna, A.; Sainz-Díaz, C.I.; Parrinello, M. DFT Research on the Dehydroxylation Reaction of Pyrophyllite 1. First-Principle Molecular Dynamics Simulations. J. Phys. Chem. B 2008, 112, 7051–7060. [Google Scholar] [CrossRef] [PubMed]
- Molina-Montes, E.; Donadio, D.; Hernández-Laguna, A.; Sainz-Díaz, C.I.; Parrinello, M. DFT Research on the Dehydroxylation Reaction of Pyrophyllite 2. Characterization of Reactants, Intermediates, and Transition States along the Reaction Path. J. Phys. Chem. A 2008, 112, 6373–6383. [Google Scholar] [CrossRef] [PubMed]

| K | Na | Al | Mg | Si | Fe | Ti |
|---|---|---|---|---|---|---|
| 7.50 | 0.39 | 17.22 | 0.18 | 22.00 | 3.15 | 0.64 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Q.; Zhang, Y.; Liu, T.; Huang, J.; Xue, N. Removal Process of Structural Oxygen from Tetrahedrons in Muscovite during Acid Leaching of Vanadium-Bearing Shale. Minerals 2018, 8, 208. https://doi.org/10.3390/min8050208
Zheng Q, Zhang Y, Liu T, Huang J, Xue N. Removal Process of Structural Oxygen from Tetrahedrons in Muscovite during Acid Leaching of Vanadium-Bearing Shale. Minerals. 2018; 8(5):208. https://doi.org/10.3390/min8050208
Chicago/Turabian StyleZheng, Qiushi, Yimin Zhang, Tao Liu, Jing Huang, and Nannan Xue. 2018. "Removal Process of Structural Oxygen from Tetrahedrons in Muscovite during Acid Leaching of Vanadium-Bearing Shale" Minerals 8, no. 5: 208. https://doi.org/10.3390/min8050208





