3.3.1. XRD and SEM Findings
The mineralogical composition of the crystalline phases of the starting CW and ACW materials is shown in
Table 2.
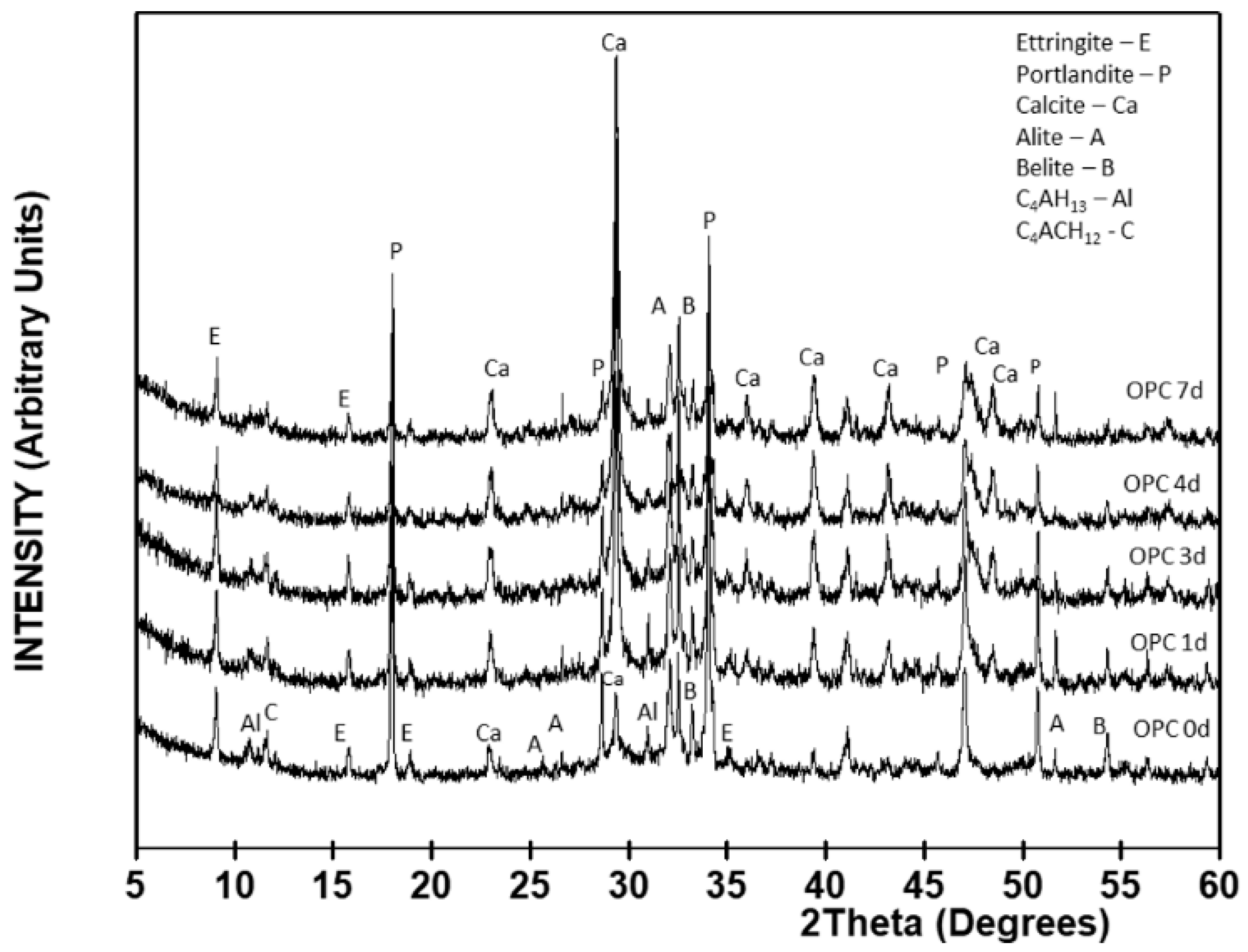
The crystalline mineralogical phases observed in the 28 days cured (
t = 0) OPC paste shown in
Figure 4 included alite (C
3S, A), belite (C
2S, B), calcite (Ca), portlandite (P), ettringite (E), tetracalcium aluminate hydrate (C
4AH
13, Al), and monocarboaluminate hydrate (C
4AcH
12, C).
The semi-quantitative XRD findings (
Table 3) for the crystalline phases in the OPC paste denoted a clear change in the mineralogical phases from the third day of carbonation. In the first 24 h of accelerated carbonation, the content of all the phases except calcite declined and remained flat until the end of day 3. At that time calcite, the main mineralogical phase, accounted for 56% of the total, portlandite for 14% and C
2S for 11%. From the fourth to the seventh day, none of the crystalline phases was affected by CO
2, an indication that the carbonation front had stabilized in the cement matrix.
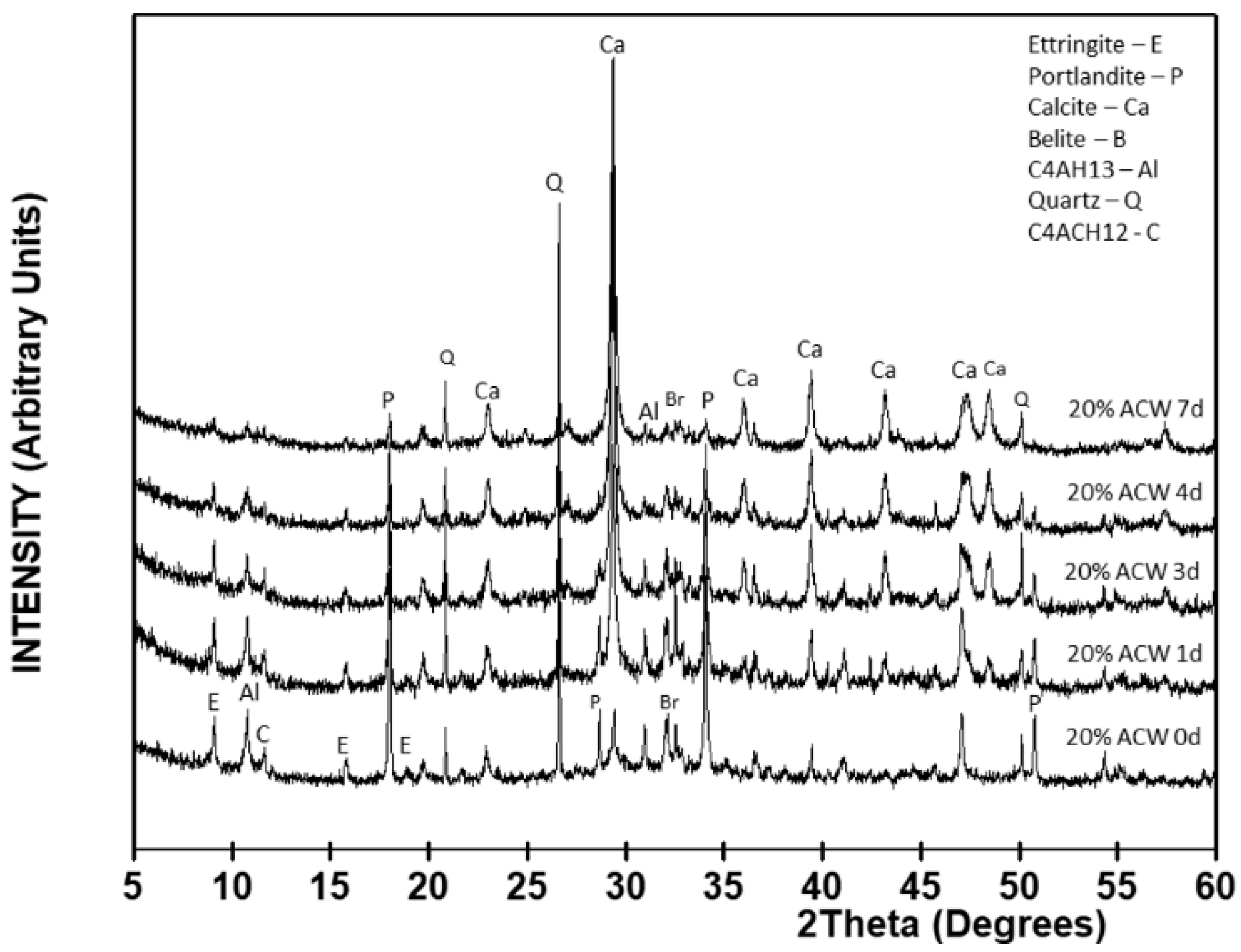
With the addition of 20% ACW to the OPC (
Figure 5 and
Table 4), the intensity patterns varied from the findings for the control in all the reflections except in the line attributed to quartz, which appeared in the 20% ACW only. Excepting calcite and quartz, content declined gradually in all the crystalline mineralogical phases to practically disappear after 7 days of exposure. At 84% of the total 7 day crystalline phases, calcite content was 59% greater in the blended cement paste than in the OPC paste. This finding clearly shows that the presence of 20% ACW hastened the decomposition of these hydrated phases, as they had been identified in the OPC.
These findings are consistent with reports by Pizzol et al. [
43] for hybrid fibre cement composites exposed to accelerated carbonation for 10 h at 60 °C and 90% RH, in which no crystalline ettringite, monosulfate or monocarboaluminate was identified in the carbonated composite.
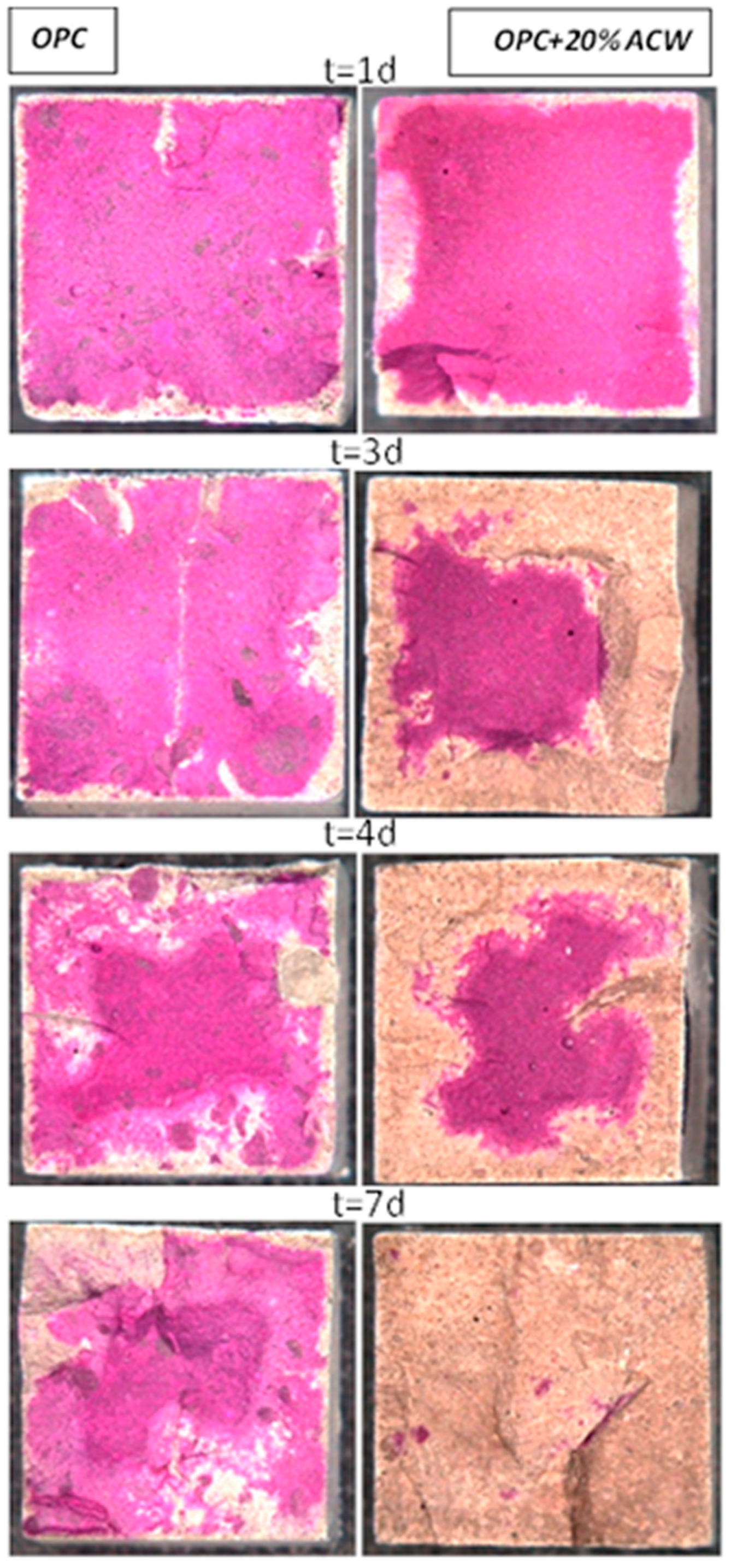
The present findings show that the addition of 20% ACW to OPC hastens carbonation. They attest not only to portlandite carbonation but also to the decalcification of calcium-bearing phases such as ettringite, monocarboaluminate, tetracalcium aluminate and even dicalcium silicate (C2S) as a result of their reaction with CO2.
SEM analysis of the OPC pastes carbonated for 4 days revealed the formation of incipient calcite rhombohedral crystals along the edges of the ettringite prisms and (primarily) in the voids and cavities in the C-S-H gels (
Figure 6). The EDX chemical microanalysis findings for the mineralogical phases are given in
Table 5.
The 20% replacement favoured the generation of calcite rhombohedral crystals, which formed massively on the surface and in the voids in the material carbonated for 4 days, although the crystal size was smaller in the latter (
Figure 7).
Very small calcium and calcite deposits were observed on the surface in the non-carbonated area of the 7 days OPC paste, while calcite crystals less than 2 μm in size clustered on both the surface and in the voids in the carbonated zone. The 20% replacement favoured the formation of calcite rhombi that accumulated profusely on the surface of the carbonated area of the specimen.
3.3.2. TG/DTG Analysis
The DTG analyses conducted identified hydrated phase decomposition during the 7 days accelerated carbonation trial. The following processes were clearly observed in both the OPC and the 20% ACW pastes: ettringite (Ett), C-S-H gel and aluminate hydrate (C
4AH
13) dehydroxylation, which respectively induced peaks at around 51 °C, 92 °C and 145 °C in a broad band spanning from 40 °C to 200 °C; and the loss of interlayer water in hydrated monocarboaluminate (C
4AcH
12), generating a weak band at 255 °C. The intensities of the main decomposition bands for the C-S-H gels, ettringite and C
4AH
13 declined with carbonation time, more notably in the 20% ACW blended cement. That finding ratified the effect of carbonation (decalcification) not only on portlandite, but also on other hydrated phases [
14,
44,
45]. The intensity of the peak centred over 435 °C generated by portlandite decomposition declined as decarbonation advanced, with only traces of the mineral appearing in the 7 days 20% ACW paste. A band at 650 °C to 750 °C, peaking at 733 °C and increasing in intensity with time, was attributed to carbonate decomposition.
Whilst both carbonated cements underwent gradual weight loss between 450 °C and 650 °C, this loss was much more significant in the cement containing the 20% replacement. Further to findings reported by Black et al. [
16], this observation might be associated with the formation of an amorphous calcium carbonate hydrate generated by the decalcification of the calcium hydrate phases, primarily C-S-H gels (the majority phase in cement hydration).
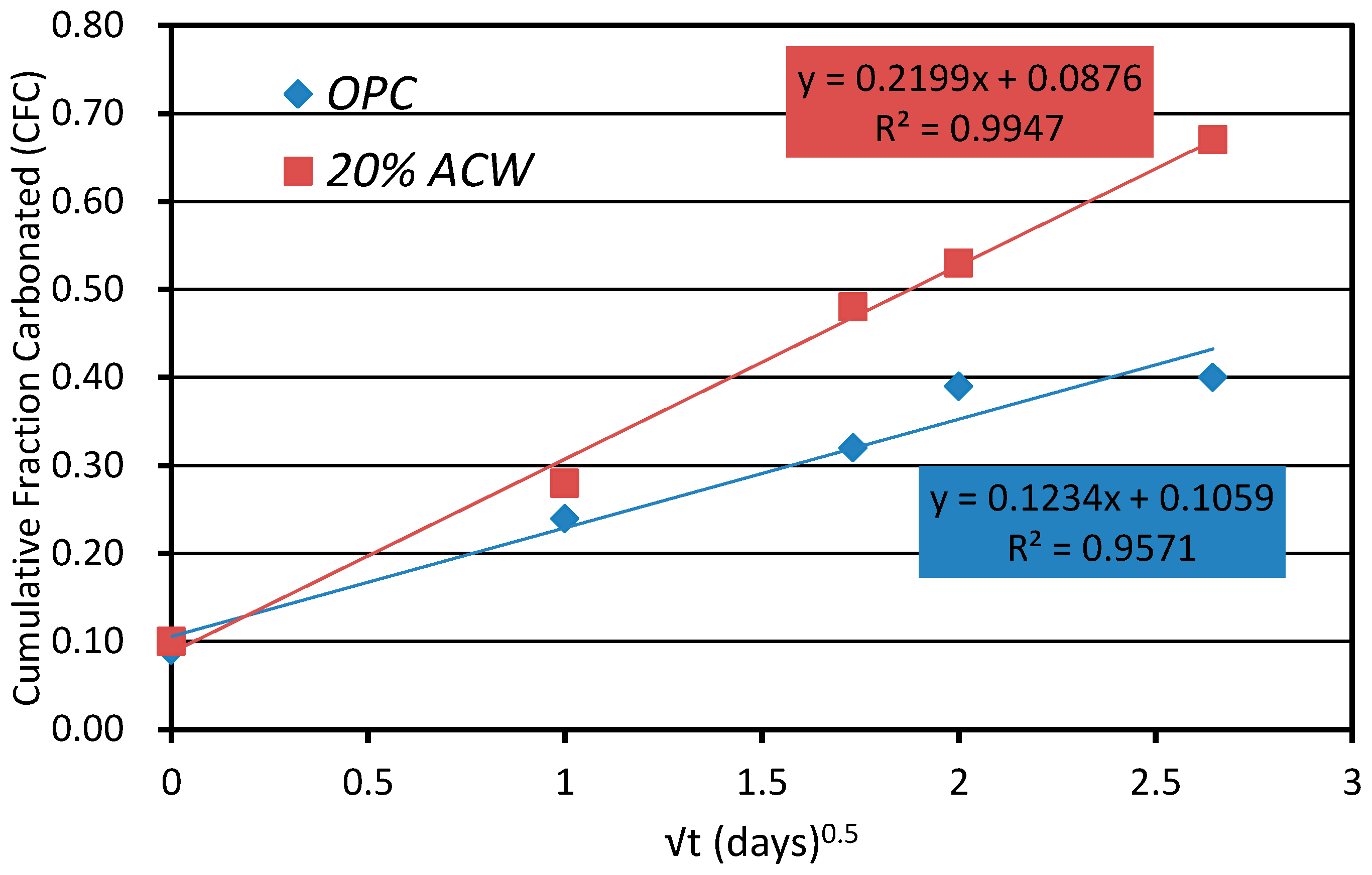
Table 6 gives the Ca(OH)
2, CaCO
3, cumulative fraction carbonated (CFC) and chemically combined water contents calculated from the weight loss detected in the TG analyses (of the residue). The CFC was estimated by dividing the respective carbonate content (expressed as CaO) by the CaO in the anhydrous cement and the pozzolan [
40]. The plot of this parameter against the square root of time (up to 7 days) proved to be linear (
Figure 8). Based on the slopes of these lines, the 20% ACW cement carbonated at a 78% higher rate than the OPC paste, concurring with the trend illustrated in
Figure 2.
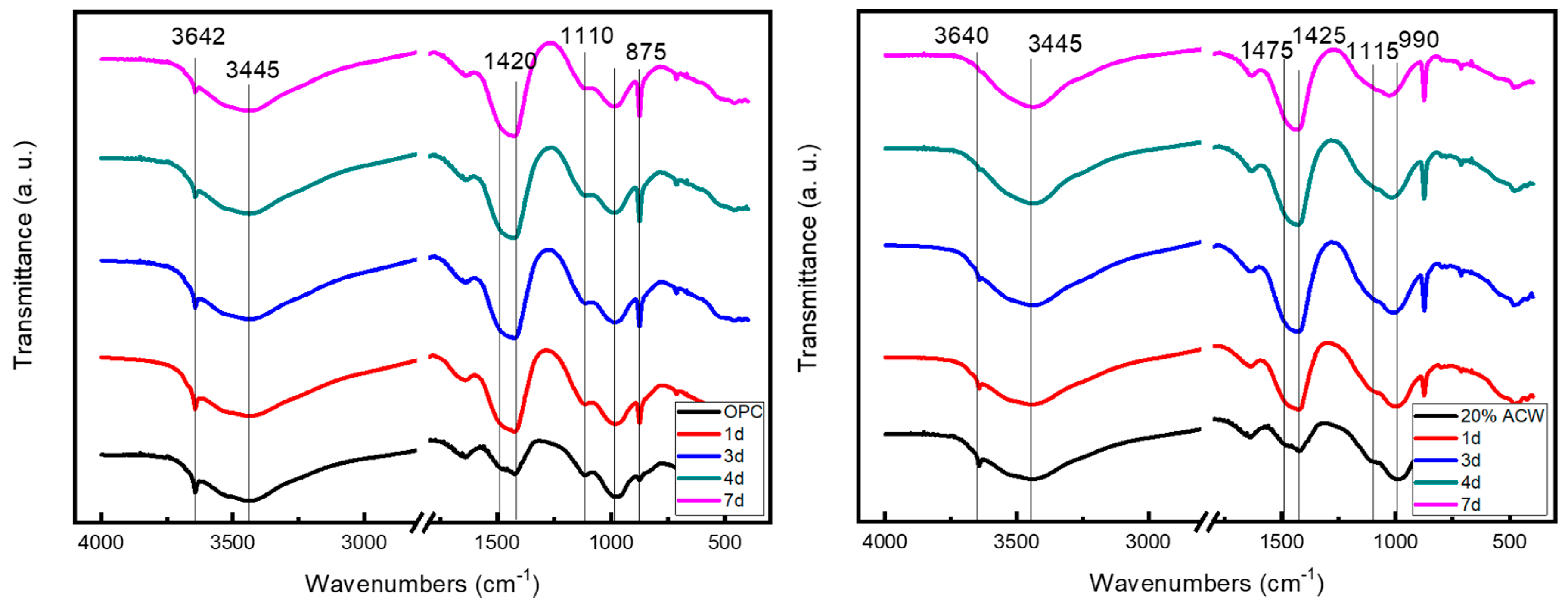
3.3.3. FT-IR and Micro-Raman Findings
The FT-IR spectrum for the non-carbonated OPC specimen exhibited a sharply defined absorption band at 3644 cm
−1, denoting the presence of portlandite and/or ettringite; a shoulder at 3520 cm
−1, the region corresponding to OH vibrations in aluminates; and a band at 3440 cm
−1 associated with OH vibrations in the C-S-H gel. At lower frequencies, an incipient absorption band near 1420 cm
−1 was attributable to carbonates. The sulfate bands at 1115 cm
−1 (ν S-O) and the Si-O vibration bands at around 975 cm
−1 were typical of hydrated OPC paste. The band assigned to portlandite was present through the seventh day of hydration, despite the progressive rise in the intensity of the bands attributed to carbonates (compare to DTG results in
Table 5). The FT-IR spectra for the two cement pastes are shown in
Figure 9 by time of exposure to CO
2.
The O-H bands attributable to portlandite and/or ettringite on the spectrum for the cured, non-carbonated 20% ACW cement were less intense than the analogous bands on the spectrum for OPC paste at t = 0 (which is consistent with the DTG results:
Table 6). The S-O vibration-induced absorption band was broader and located at a lower frequency (1100 cm
−1) on the 20% ACW than on the OPC paste spectrum. In contrast, the Si-O group absorption band appeared at a higher frequency, around 990 cm
−1. This finding may be an indication of C-S-H gel decalcification [
46].
The absorption band attributable to the OH present in portlandite and ettringite was less intense on FT-IR spectra for the 1 day OPC and 20% ACW carbonated samples and in general declined proportionally with carbonation time.
The intensity of the bands at around 3520 cm−1 (sh) and 3440 cm−1 also waned with carbonation time. At lower frequencies, a very intense band at around 1420 cm−1 generated by CO32− groups denoted the presence of calcium carbonate, primarily as calcite, although a shoulder at 1470 cm−1 attested to the existence of aragonite. The absorption bands at around 875 cm−1, assigned to calcite, and 850 cm−1, to aragonite, supported this interpretation.
The effect of CO2 on the 20% ACW paste was less straightforward. The OH absorption band at 3644 cm−1 dropped significantly with carbonation time, disappearing altogether from spectrum on day 7. Band intensity at 3520 (cm−1) (sh) and 3440 cm−1 also declined with carbonation time; the shoulder, specifically, was absent from the 4 days spectrum.
As in the OPC samples, intense absorption bands generated by carbonated compounds appeared on the first day of carbonation. Their position (1420 cm−1) was indicative of calcite, an inference supported by the existence of a band at 875 cm−1. Shoulders at 1845 cm−1 and 850 cm−1 denoted the presence of small amounts of aragonite.
The absorption bands at 1100 cm−1 (ν Si-O) and 990 cm−1 (Si-O) clearly varied with carbonation time, respectively shifting to lower (1080 cm−1) and higher (1030 cm−1) frequencies and declining in intensity.
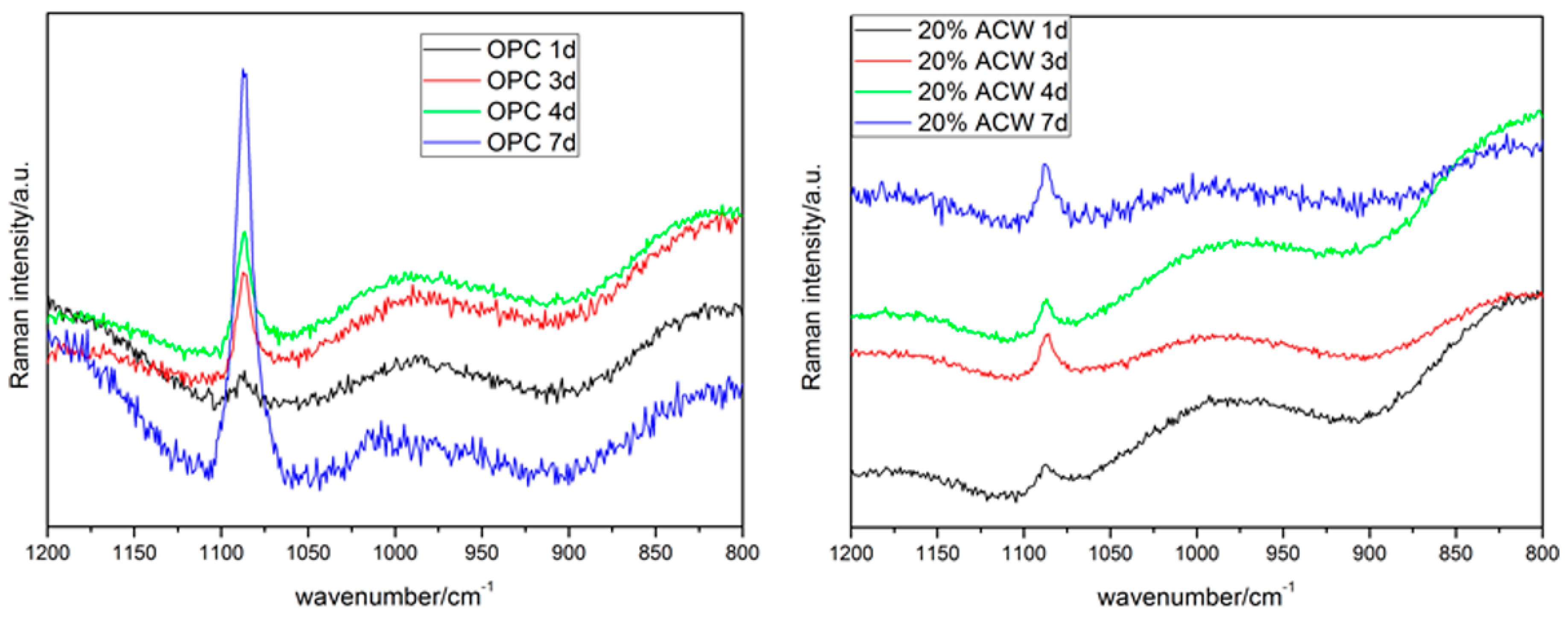
By way of summary, the FT-IR spectra showed that carbonation induced a decline in the portlandite and ettringite bands, an effect that was more visible in the 20% ACW paste. They also confirmed that calcite was the majority carbonation product. That finding was consistent with the micro-Raman results, which identified only the CaCO
3 vibration band (ν1 at 1084 cm
−1) from calcite and/or aragonite and none of the other phases detected with XRD, such as carboaluminates. Due to the intensity of sample fluorescence, no C-S-H gel signals were observed on these spectra (
Figure 10).