Prediction of Soluble Al2O3 in Calcined Kaolin Using Infrared Spectroscopy and Multivariate Calibration
Abstract
:1. Introduction
2. Methods
2.1. Calcination of Kaolin and Samples
2.2. Soluble Al2O3
2.3. Infrared Spectra Collection and Processing
2.4. Regression Methods
2.4.1. Partial Least Squares Regression
2.4.2. Support Vector Regression
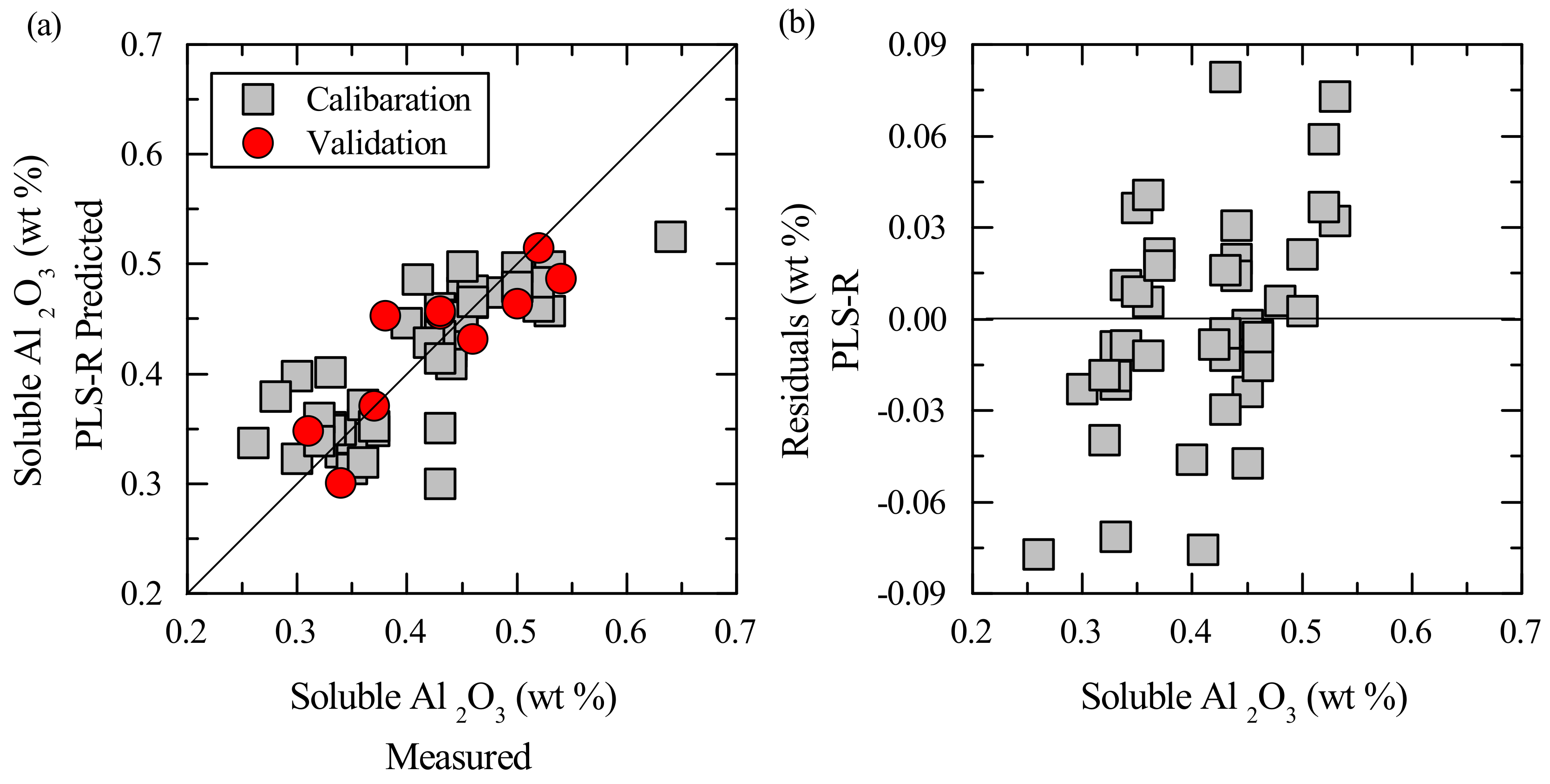
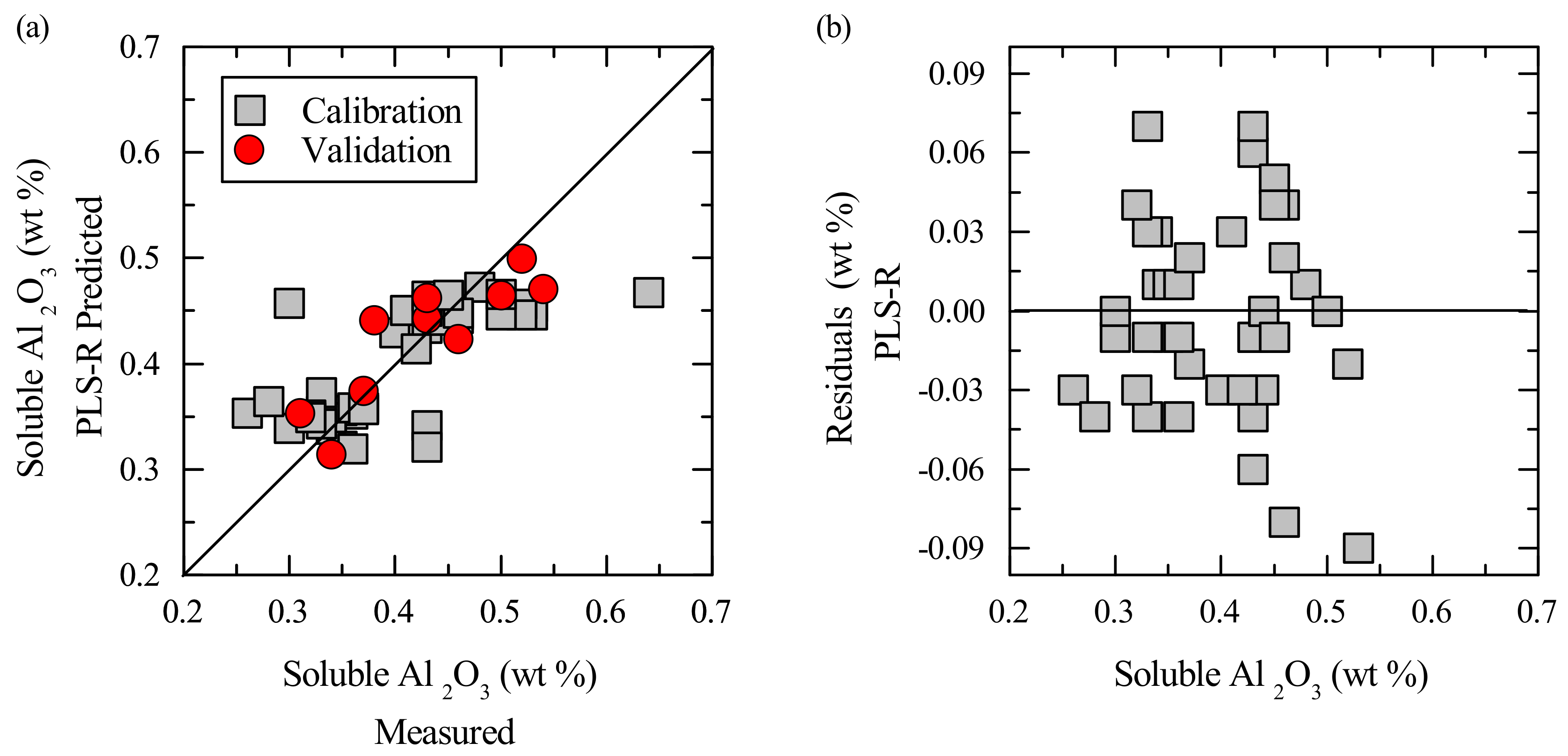
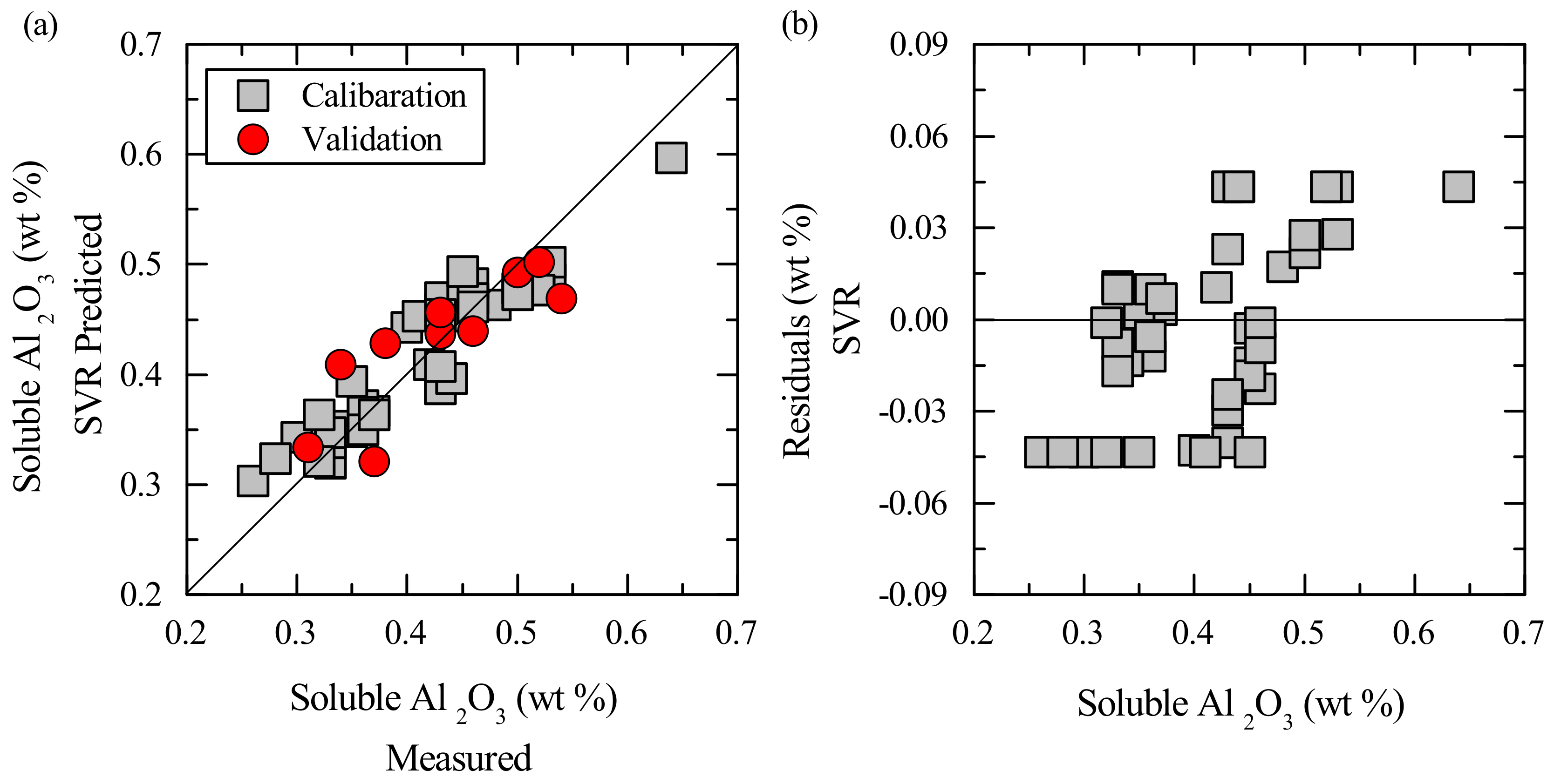
3. Results
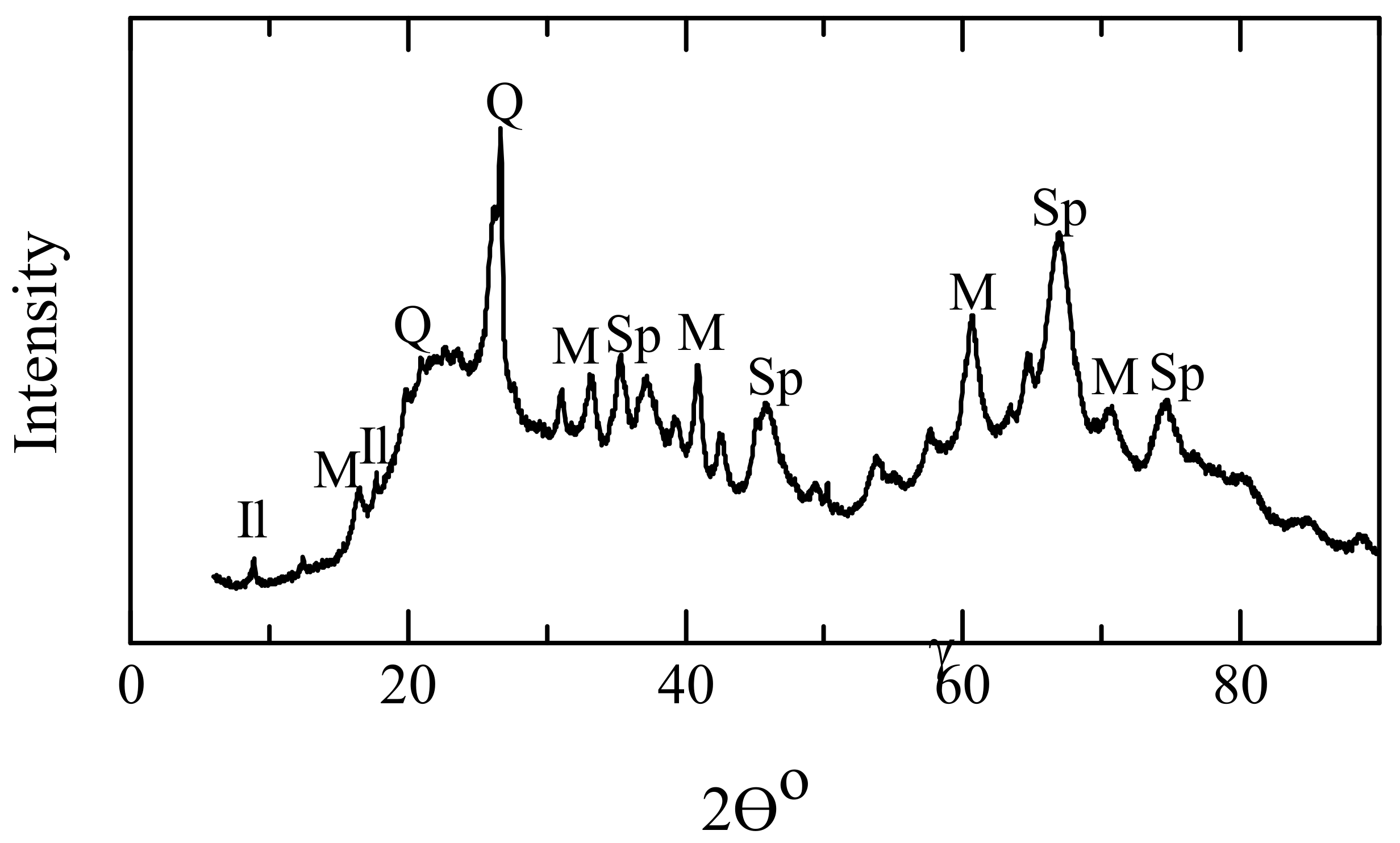
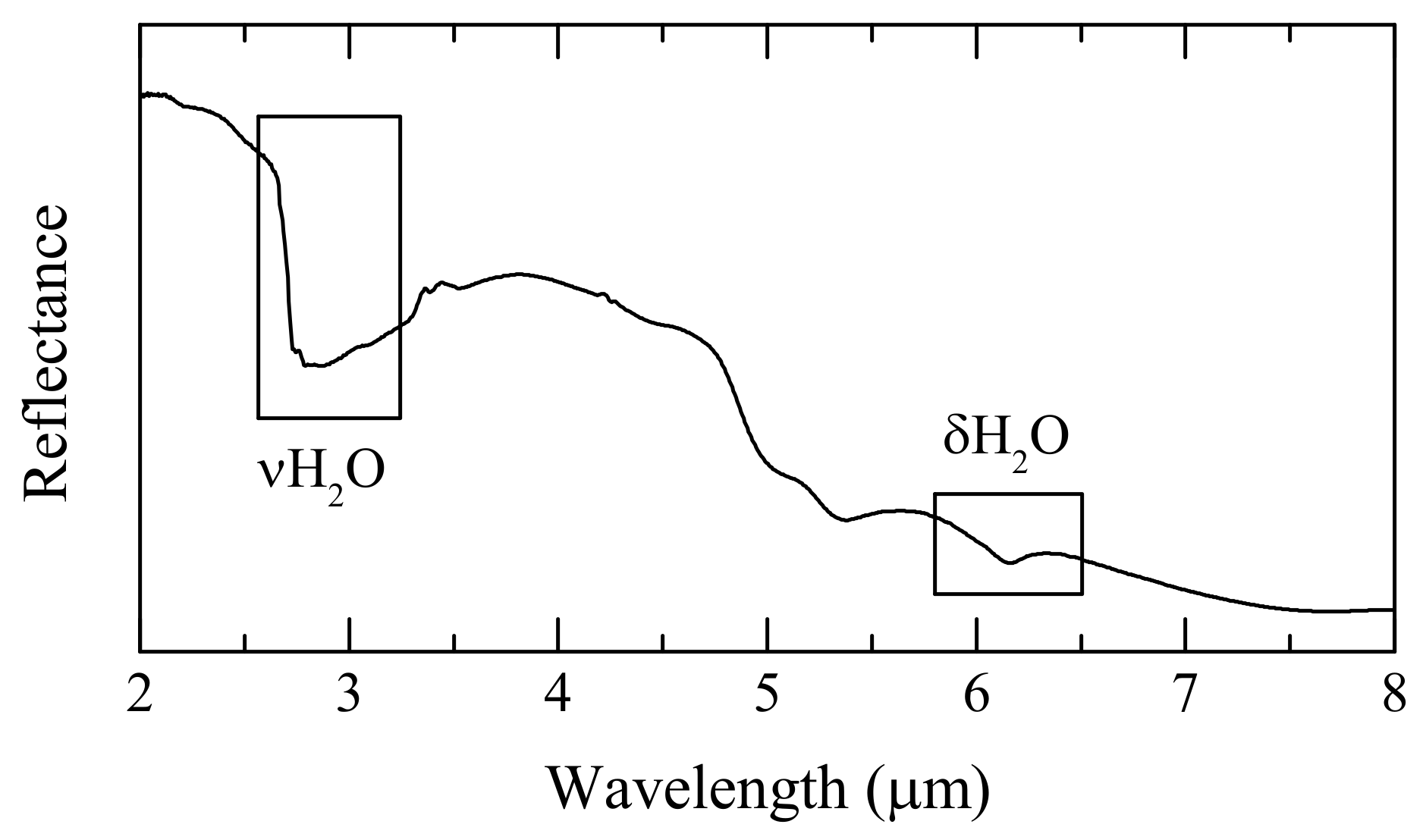
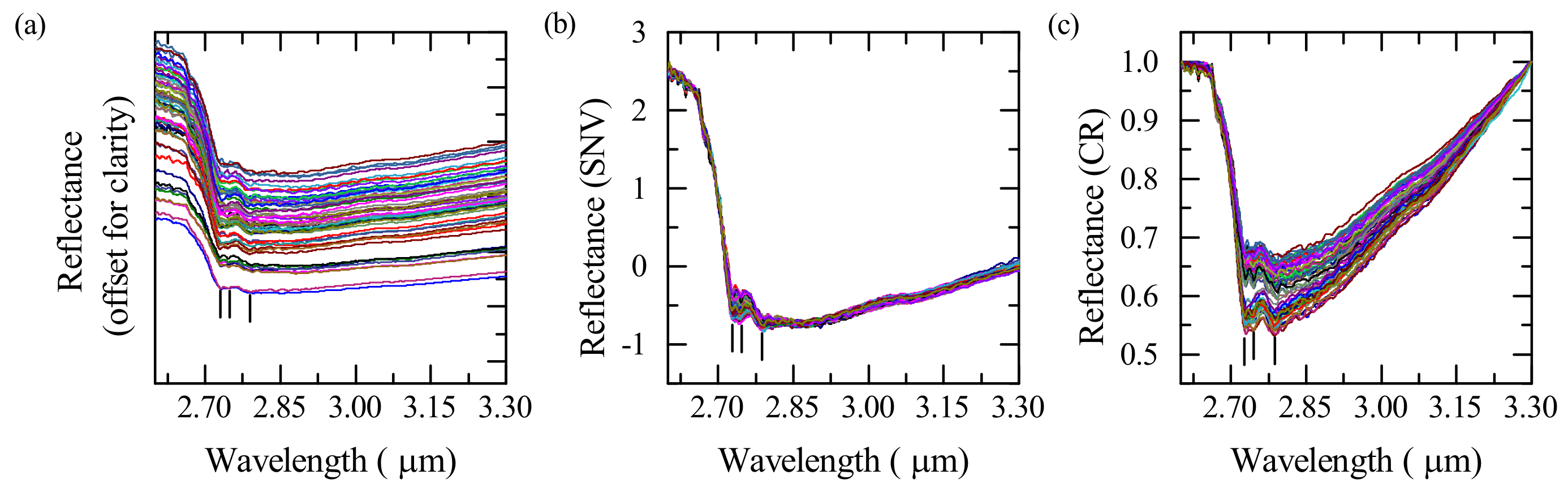
3.1. Spectral Processing
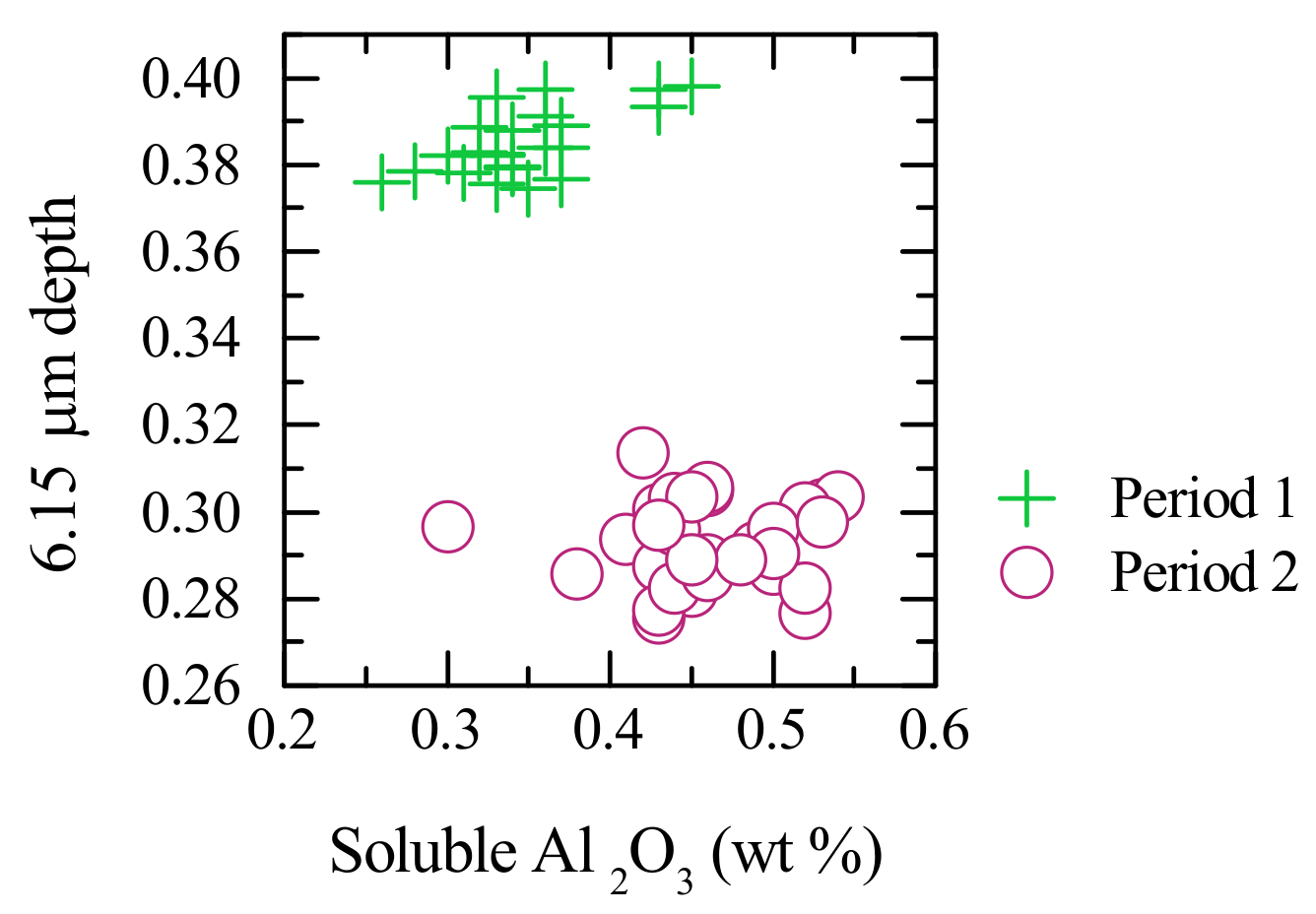
3.2. Multivariate Calibration
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Murray, H.H. Chapter 5 Kaolin Applications. Dev. Clay Sci. 2006, 2, 85–109. [Google Scholar]
- Gualtieri, A.; Bellotto, M. Modelling the structure of the metastable phases in the reaction sequence kaolinite-mullite by X-ray scattering experiments. Phys. Chem. Miner. 1998, 25, 442–452. [Google Scholar] [CrossRef]
- Chakraborty, A.K. New Data on Thermal Effects of Kaolinite in the High temperature Region. J. Therm. Anal. Calorim. 2003, 71, 799–808. [Google Scholar] [CrossRef]
- Ptáček, P.; Šoukal, F.; Opravil, T.; Nosková, M.; Havlica, J.; Brandštetr, J. Mid-infrared spectroscopic study of crystallization of cubic spinel phase from metakaolin. J. Solid State Chem. 2011, 184, 2661–2667. [Google Scholar] [CrossRef]
- Thomas, R.; Grose, D.; Obaje, G.; Taylor, R.; Rowson, N.; Blackburn, S. Residence time investigation of a multiple hearth kiln using mineral tracers. Chem. Eng. Process. Process Intensif. 2009, 48, 950–954. [Google Scholar] [CrossRef]
- Hulbert, S.F.; Huff, D.E. Kinetics of alumina removal from a calcined kaolin with nitric, sulphuric and hydrochloric acids. Clay Miner. 1970, 8, 337–345. [Google Scholar] [CrossRef]
- Phillips, C.; Wills, K. A laboratory study of the extraction of alumina of smelter grade from China clay micaceous residues by a nitric acid route. Hydrometallurgy 1982, 9, 15–28. [Google Scholar] [CrossRef]
- Ruiz-Santaquiteria, C.; Skibsted, J. Reactivity of Heated Kaolinite from a Combination of Solid State NMR and Chemical Methods. In RILEM Bookseries; Springer: Amsterdam, The Netherlands, 2015; pp. 125–132. [Google Scholar]
- Taylor, R.J. Treatment of Metakaolin. United Kingdom WO2005108295 A1, 17 November 2005. [Google Scholar]
- Thomas, R. High Temperature Processing of Kaolinitic Materials. Ph.D. Thesis, University of Birmingham, Birmingham, UK, 2010. [Google Scholar]
- Glass, H.J. Geometallurgy—Driving Innovation in the Mining Value Chain. In Proceedings of the Third AusIMM International Geometallurgy Conference (GeoMet) 2016, Perth, Australia, 15–17 June 2016; The Australasian Institute of Mining and Metallurgy: Melbourne, Australia, 2016; pp. 21–28. [Google Scholar]
- Eskelinen, A.; Zakharov, A.; Jämsä-Jounela, S.L.; Hearle, J. Dynamic modeling of a multiple hearth furnace for kaolin calcination. AIChE J. 2015, 61, 3683–3698. [Google Scholar] [CrossRef]
- Guatame-García, A.; Buxton, M.; Deon, F.; Lievens, C.; Hecker, C. Towards an On-line Characterisation of Kaolin Calcination Process Using Short-Wave Infrared Spectroscopy. Miner. Process. Extr. Metall. Rev. 2018, in press. [Google Scholar]
- Percival, H.J.; Duncan, J.F.; Foster, P.K. Interpretation of the Kaolinite-Mullite Reaction Sequence from Infrared Absorption Spectra. J. Am. Ceram. Soc. 1974, 57, 57–61. [Google Scholar] [CrossRef]
- Frost, R.L.; Makó, E.; Kristóf, J.; Kloprogge, J.T. Modification of kaolinite surfaces through mechanochemical treatment—A mid-IR and near-IR spectroscopic study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2002, 58, 2849–2859. [Google Scholar] [CrossRef]
- Drits, V.A.; Derkowski, A.; Sakharov, B.A.; Zviagina, B.B. Experimental evidence of the formation of intermediate phases during transition of kaolinite into metakaolinite. Am. Mineral. 2016, 101, 2331–2346. [Google Scholar] [CrossRef]
- Chryssikos, G.; Gates, W. Spectral Manipulation and Introduction to Multivariate Analysis. Dev. Clay Sci. 2017, 8, 64–106. [Google Scholar]
- Haavisto, O.; Hyötyniemi, H. Recursive multimodel partial least squares estimation of mineral flotation slurry contents using optical reflectance spectra. Anal. Chim. Acta 2009, 642, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Andrés, J.; Bona, M. Analysis of coal by diffuse reflectance near-infrared spectroscopy. Anal. Chim. Acta 2005, 535, 123–132. [Google Scholar] [CrossRef]
- Bona, M.; Andrés, J. Coal analysis by diffuse reflectance near-infrared spectroscopy: Hierarchical cluster and linear discriminant analysis. Talanta 2007, 72, 1423–1431. [Google Scholar] [CrossRef] [PubMed]
- Filgueiras, P.R.; Sad, C.M.; Loureiro, A.R.; Santos, M.F.; Castro, E.V.; Dias, J.C.; Poppi, R.J. Determination of API gravity, kinematic viscosity and water content in petroleum by ATR-FTIR spectroscopy and multivariate calibration. Fuel 2014, 116, 123–130. [Google Scholar] [CrossRef]
- Wold, S.; Sjöström, M.; Eriksson, L. PLS-regression: a basic tool of chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Devos, O.; Ruckebusch, C.; Durand, A.; Duponchel, L.; Huvenne, J.P. Support vector machines (SVM) in near infrared (NIR) spectroscopy: Focus on parameters optimization and model interpretation. Chemom. Intell. Lab. Syst. 2009, 96, 27–33. [Google Scholar] [CrossRef]
- Li, H.; Liang, Y.; Xu, Q. Support vector machines and its applications in chemistry. Chemom. Intell. Lab. Syst. 2009, 95, 188–198. [Google Scholar] [CrossRef]
- Brereton, R.G.; Lloyd, G.R. Support Vector Machines for classification and regression. Analyst 2010, 135, 230–267. [Google Scholar] [CrossRef] [PubMed]
- Savitzky, A.; Golay, M.J.E. Smoothing and Differentiation of Data by Simplified Least Squares Procedures. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar] [CrossRef]
- Barnes, R.J.; Dhanoa, M.S.; Lister, S.J. Standard Normal Variate Transformation and De-Trending of Near-Infrared Diffuse Reflectance Spectra. Appl. Spectrosc. 1989, 43, 772–777. [Google Scholar] [CrossRef]
- Clark, R.N.; Roush, T.L. Reflectance spectroscopy: Quantitative analysis techniques for remote sensing applications. J. Geophys. Res. 1984, 89, 6329–6340. [Google Scholar] [CrossRef]
- Stevens, A.; Ramirez-Lopez, L. An introduction to the prospectr package. In R Package Vignette; R Foundation for Statistical Computing; Vienna, Austria, 2014. [Google Scholar]
- Höskuldsson, A. PLS regression methods. J. Chemom. 1988, 2, 211–228. [Google Scholar] [CrossRef]
- Cortes, C.; Vapnik, V. Support-vector networks. Mach. Learn. 1995, 20, 273–297. [Google Scholar] [CrossRef]
- Smola, A.J.; Schölkopf, B. A tutorial on support vector regression. Stat. Comput. 2004, 14, 199–222. [Google Scholar] [CrossRef]
- Mevik, B.H.; Wehrens, R. The pls Package: Principal Component and Partial Least Squares Regression in R. J. Stat. Softw. 2007, 18, 23. [Google Scholar] [CrossRef]
- Chang, C.C.; Lin, C.J. LIBSVM: A library for support vector machines. ACM Trans. Intell. Syst. Technol. 2001, 2, 27. [Google Scholar] [CrossRef]
- Meyer, D. Support Vector Machines: The Interface to libsvm in package e1071. In R Package Vignette; R Foundation for Statistical Computing; Vienna, Austria, 2017. [Google Scholar]
- Aines, R.D.; Rossman, G.R. Water in minerals? A peak in the infrared. J. Geophys. Res. 1984, 89, 4059–4071. [Google Scholar] [CrossRef]
- Liu, X. DRIFTS Study of Surface of γ-Alumina and Its Dehydroxylation. J. Phys. Chem. C 2008, 112, 5066–5073. [Google Scholar] [CrossRef]
- Johnston, C. Infrared Studies of Clay Mineral-Water Interactions. Dev. Clay Sci. 2017, 8, 288–309. [Google Scholar]
- Yitagesu, F.A.; van der Meer, F.; van der Werff, H.; Hecker, C. Spectral characteristics of clay minerals in the 2.5–14 μm wavelength region. Appl. Clay Sci. 2011, 53, 581–591. [Google Scholar] [CrossRef]


| Al2O3 | SiO2 | K2O | Fe2O3 | TiO2 | CaO | MgO | Na2O | LOI | Soluble Al | |
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 42.27 | 54.41 | 1.58 | 0.69 | 0.11 | 0.11 | 0.31 | 0.09 | 0.26 | 0.41 |
| Standard Deviation | 0.33 | 0.20 | 0.14 | 0.07 | 0.07 | 0.21 | 0.03 | 0.02 | 0.05 | 0.08 |
| Minimum | 41.07 | 53.85 | 1.25 | 0.58 | 0.04 | 0.04 | 0.26 | 0.05 | 0.17 | 0.26 |
| Median | 42.23 | 54.47 | 1.61 | 0.67 | 0.07 | 0.05 | 0.32 | 0.09 | 0.27 | 0.43 |
| Maximum | 42.91 | 54.69 | 1.80 | 0.78 | 0.32 | 1.31 | 0.35 | 0.13 | 0.37 | 0.64 |
| Scenario | LV | RMSECV | RMSEC | RMSEP | ||
|---|---|---|---|---|---|---|
| Range | Processing | (wt %) | (wt %) | (wt %) | ||
| 2.6 to 3.3 | raw | 3 | 0.051 | 0.044 | 0.046 | 0.62 |
| SNV | 3 | 0.051 | 0.040 | 0.054 | 0.62 | |
| CR | 2 | 0.050 | 0.044 | 0.055 | 0.57 | |
| 5.7 to 6.5 | raw | 3 | 0.051 | 0.047 | 0.053 | 0.60 |
| SNV | 2 | 0.050 | 0.041 | 0.046 | 0.64 | |
| CR | 1 | 0.050 | 0.047 | 0.051 | 0.52 | |
| Scenario | LV | RMSECV | RMSEC | RMSEP | ||
|---|---|---|---|---|---|---|
| Range | Processing | (wt %) | (wt %) | (wt %) | ||
| 2.6 to 3.3 | raw | 3 | 0.053 | 0.034 | 0.046 | 0.62 |
| SNV | 4 | 0.053 | 0.036 | 0.061 | 0.62 | |
| CR | 3 | 0.050 | 0.036 | 0.056 | 0.57 | |
| 5.7 to 6.5 | raw | 3 | 0.053 | 0.036 | 0.052 | 0.57 |
| SNV | 2 | 0.050 | 0.036 | 0.039 | 0.53 | |
| CR | 1 | 0.050 | 0.036 | 0.043 | 0.54 | |
| Scenario | C | SV | RMSECV | RMSEC | RMSEP | |||
|---|---|---|---|---|---|---|---|---|
| Range | Processing | (wt %) | (wt %) | (wt %) | ||||
| 2.6 to 3.3 | raw | 15.0 | 0.80 | 20 | 0.055 | 0.048 | 0.050 | 0.57 |
| SNV | 3.5 | 0.40 | 21 | 0.052 | 0.023 | 0.050 | 0.86 | |
| CR | 3.5 | 0.50 | 22 | 0.048 | 0.031 | 0.048 | 0.71 | |
| 5.7 to 6.5 | raw | 5.0 | 0.31 | 30 | 0.056 | 0.045 | 0.047 | 0.59 |
| SNV | 4.0 | 0.53 | 17 | 0.048 | 0.027 | 0.047 | 0.80 | |
| CR | 3.1 | 0.46 | 19 | 0.046 | 0.025 | 0.044 | 0.87 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guatame-Garcia, A.; Buxton, M. Prediction of Soluble Al2O3 in Calcined Kaolin Using Infrared Spectroscopy and Multivariate Calibration. Minerals 2018, 8, 136. https://doi.org/10.3390/min8040136
Guatame-Garcia A, Buxton M. Prediction of Soluble Al2O3 in Calcined Kaolin Using Infrared Spectroscopy and Multivariate Calibration. Minerals. 2018; 8(4):136. https://doi.org/10.3390/min8040136
Chicago/Turabian StyleGuatame-Garcia, Adriana, and Mike Buxton. 2018. "Prediction of Soluble Al2O3 in Calcined Kaolin Using Infrared Spectroscopy and Multivariate Calibration" Minerals 8, no. 4: 136. https://doi.org/10.3390/min8040136





