Prismatic to Asbestiform Offretite from Northern Italy: Occurrence, Morphology and Crystal-Chemistry of a New Potentially Hazardous Zeolite
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Morphology
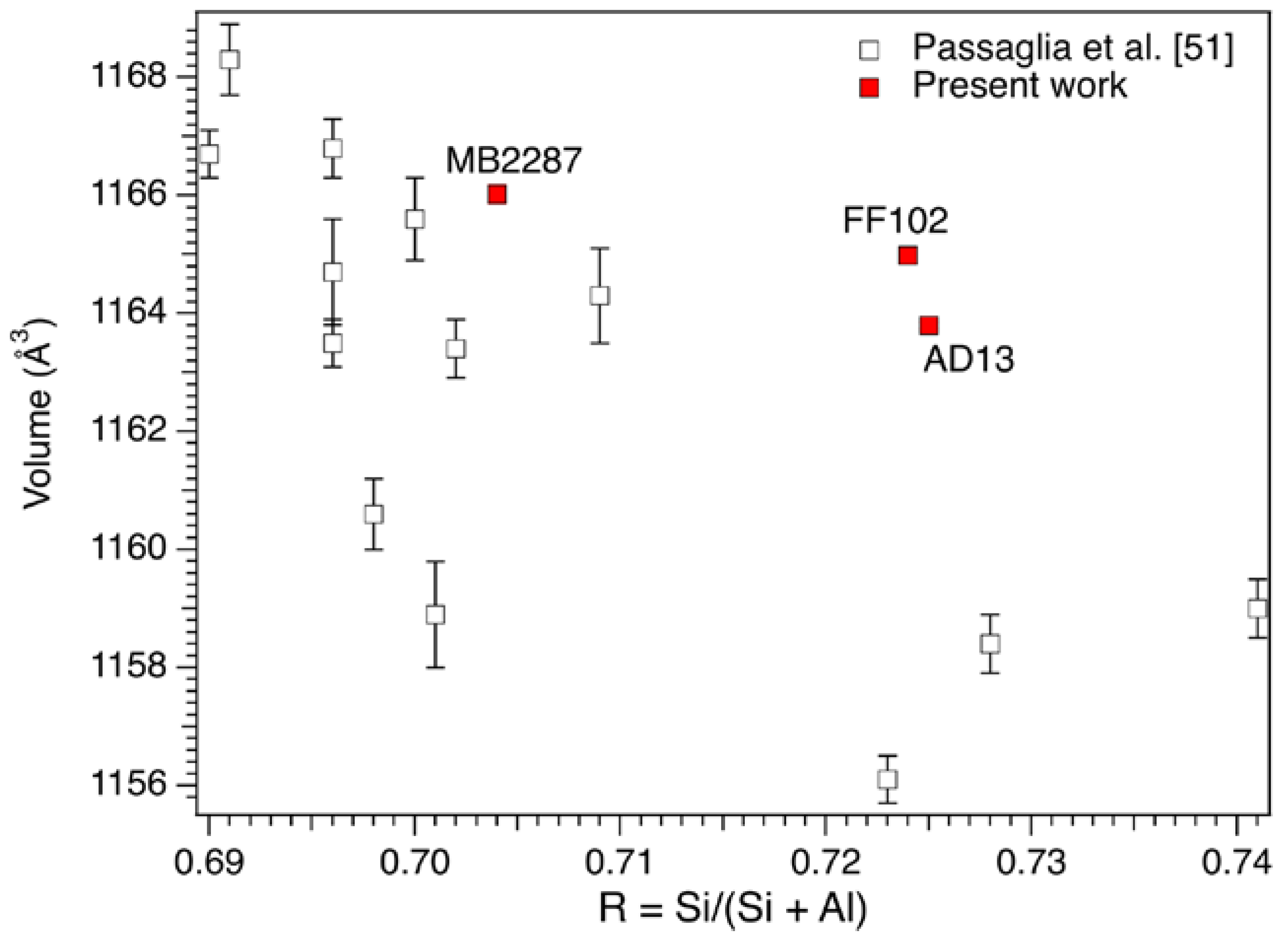
3.2. Mineralogical Composition and Structural Data
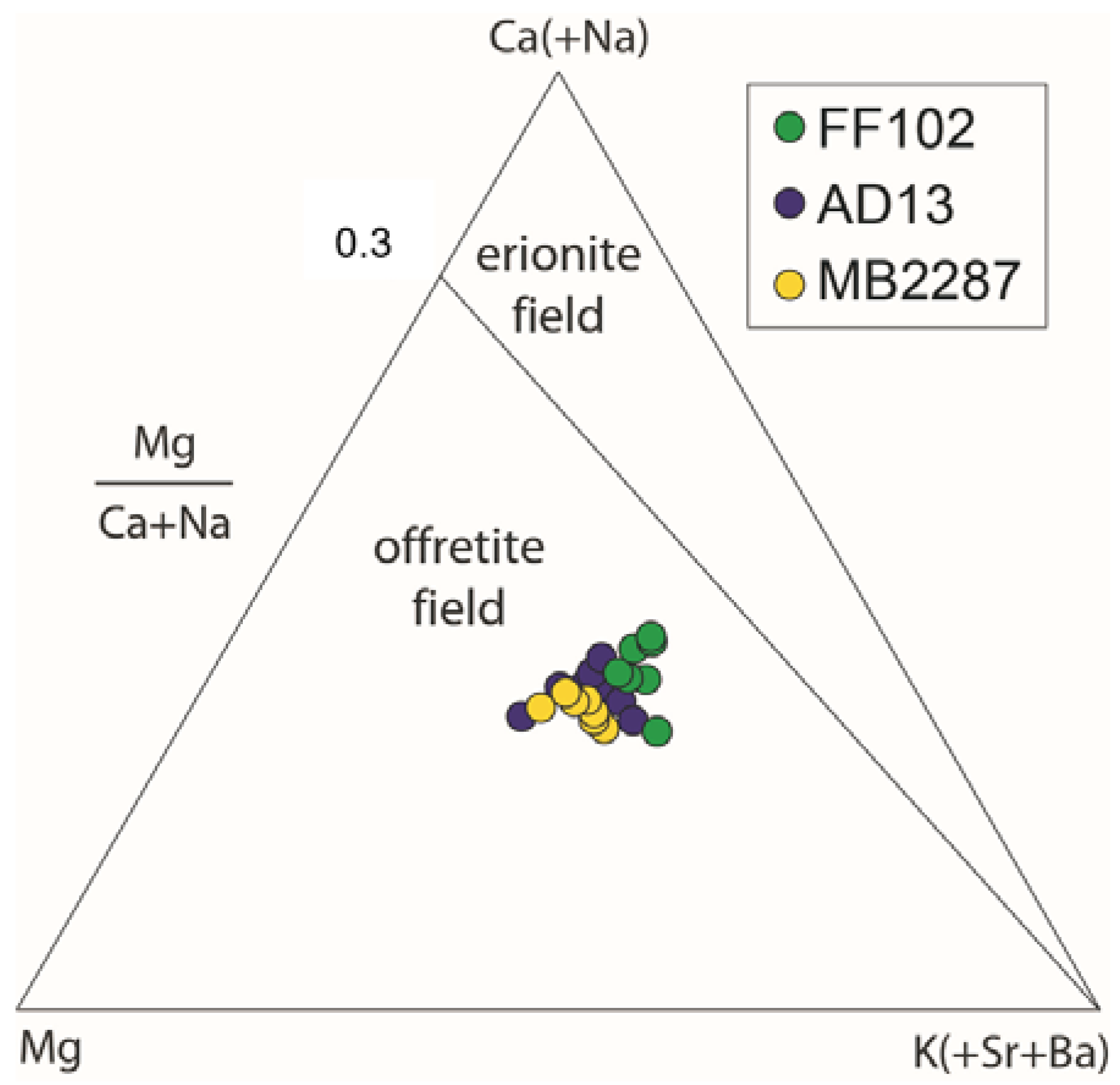
3.3. Chemical Data
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Andujar, P.; Lacourt, A.; Brochard, P.; Pairon, J.-C.; Jaurand, M.-C.; Jean, D. Five years update on relationships between malignant pleural mesothelioma and exposure to asbestos and other elongated mineral particles. J. Toxicol. Environ. Health B 2016, 19, 151–172. [Google Scholar] [CrossRef] [PubMed]
- Gualtieri, A.F. Mineral Fibres: Crystal Chemistry, Chemical-Physical Properties, Biological Interaction and Toxicity; EMU Notes in Mineralogy; European Mineralogical Union and the Mineralogical Society of Great Britain and Ireland: London, UK, 2017; Volume 18. [Google Scholar]
- Stanton, M.F.; Layard, M.; Tegeris, A.; Miller, E.; May, M.; Morgan, E.; Smith, A. Relation of particles dimension to carcinogenicity in amphibole asbestoses and other fibrous minerals. J. Nat. Cancer Inst. 1981, 67, 965–975. [Google Scholar] [PubMed]
- Lee, K.P. Lung response to particulates with emphasis on asbestos and other fibrous dust. Crit. Rev. Toxicol. 1985, 14, 33–86. [Google Scholar] [CrossRef] [PubMed]
- Aust, A.E.; Cook, P.M.; Dodson, R.D. Morphological and chemical mechanisms of elongated mineral particle toxicities. J. Toxicol. Environ. Health B 2011, 14, 40–75. [Google Scholar] [CrossRef] [PubMed]
- Boulanger, G.; Andujar, P.; Pairon, J.C.; Billon-Galland, M.A.; Dion, C.; Dumortier, P.; Brochard, P.; Sobaszek, A.; Bartsch, P.; Paris, C.; et al. Quantification of short and long asbestos fibers to assess asbestos exposure: A review of fiber size toxicity. Environ. Health 2014, 13, 59. [Google Scholar] [CrossRef] [PubMed]
- Belluso, E.; Cavallo, A.; Halterman, D. Crystal habit of mineral fibres. In Mineral Fibres: Crystal Chemistry, Chemical-Physical Properties, Biological Interaction and Toxicity; EMU Notes in Mineralogy; Gualtieri, A.F., Ed.; European Mineralogical Union and the Mineralogical Society of Great Britain and Ireland: London, UK, 2017; Volume 18, pp. 65–110. [Google Scholar]
- Hesterberg, T.W.; Chase, G.; Axten, C.; Miller, W.C.; Musselman, R.P.; Kamstrup, O.; Hadley, J.; Morscheidt, C.; Bernstein, D.M.; Thevenaz, P. Biopersistence of synthetic vitreous fibers and amosite asbestos in the rat lung following inhalation. Toxicol. Appl. Pharmacol. 1998, 151, 262–275. [Google Scholar] [CrossRef] [PubMed]
- Maxim, D.L.; Hadley, J.G.; Potter, R.M.; Niebo, R. The role of fiber durability/biopersistence of silica-based synthetic vitreous fibers and their influence on toxicology. Regul. Toxicol. Pharmacol. 2006, 46, 42–62. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, D.M.; Chevalier, J.; Smith, P. Comparison of Calidria chrysotile asbestos to pure tremolite: Final results of the inhalation biopersistence and histopathology examination following short-term exposure. Inhal. Toxicol. 2005, 17, 427–449. [Google Scholar] [CrossRef] [PubMed]
- Muhle, H.; Bellmann, B.; Pott, F. Durability of various mineral fibres in rat lungs, mechanisms in fibre carcinogenesis. In NATO ASI Series A: Life Sciences; Brown, R.C., Hoskins, J.A., Johnson, N.F., Eds.; Plenum Press: New York, NY, USA, 1991; Volume 223, pp. 181–187. [Google Scholar]
- Ballirano, P.; Pacella, A.; Cremisini, C.; Nardi, E.; Fantauzzi, M.; Atzei, D.; Rossi, A.; Cametti, G. Fe (II) segregation at a specific crystallographic site of fibrous erionite: A first step toward the understanding of the mechanisms inducing its carcinogenicity. Microporous Mesoporous Mater. 2015, 211, 49–63. [Google Scholar] [CrossRef]
- Crovella, S.; Bianco, A.M.; Vuch, J.; Zupin, L.; Moura, R.R.; Trevisan, E.; Schneider, M.; Brollo, A.; Nicastro, E.M.; Cosenzi, A.; et al. Iron signature in asbestos-induced malignant pleural mesothelioma: A population-based autopsy study. J. Toxicol. Environ. Health A 2016, 79, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Pacella, A.; Fantauzzi, M.; Atzei, D.; Cremisini, C.; Nardi, E.; Montereali, M.R.; Rossi, A.; Ballirano, P. Iron within the erionite cavity and its potential role in inducing its toxicity: Evidences of Fe (III) segregation as extra-framework cation. Microporous Mesoporous Mater. 2017, 237, 168–179. [Google Scholar] [CrossRef]
- Hochella, M.F. Surface chemistry, structure, and reactivity of hazardous mineral dust. In Health Effects of Mineral Dusts; Reviews in Mineralogy and Geochemistry; Guthrie, G.D., Mossman, B.T., Eds.; Bookcrafters: Chelsea, MI, USA, 1993; Volume 28, pp. 275–308. [Google Scholar]
- Bloise, A.; Barca, D.; Gualtieri, A.F.; Pollastri, S.; Belluso, E. Trace elements in hazardous mineral fibres. Environ. Pollut. 2016, 216, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Pollastri, S.; Gualtieri, A.F.; Gualtieri, M.L.; Hanuskova, M.; Cavallo, A.; Gaudino, G. The Zeta potential of mineral fibres. J. Hazard. Mater. 2014, 276, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, M.; Giordani, M.; Dogan, M.; Cangiotti, M.; Avella, G.; Giorgi, R.; Dogan, A.U.; Ottaviani, M.F. Morpho-chemical characterization and surface properties of carcinogenic zeolite fibers. J. Hazard. Mater. 2016, 306, 140–148. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Asbestos and other Natural Mineral Fibers; Environmental Health Criteria: Geneva, Switzerland, 1986; Volume 53, pp. 69–107. [Google Scholar]
- National Institute for Occupational Safety and Health (NIOSH). Asbestos Fibers and Other Elongate Mineral Particles: State of the Science and Roadmap for Research; Current Intelligence Bulletin 62, Version 4; Department of Health and Human Services: Cincinnati, OH, USA, 2011.
- Gianfagna, A.; Ballirano, P.; Bellatreccia, F.; Bruni, B.; Paoletti, L.; Oberti, R. Characterisation of amphibole fibres linked to mesothelioma in the area of Biancavilla, eastern Sicily, Italy. Mineral. Mag. 2003, 67, 1221–1229. [Google Scholar] [CrossRef]
- Andreozzi, G.B.; Ballirano, P.; Gianfagna, A.; Mazziotti-Tagliani, S.; Pacella, A. Structural and spectroscopic characterization of a suite of fibrous amphiboles with high environmental and health relevance from Biancavilla (Sicily, Italy). Am. Mineral. 2009, 94, 1333–1340. [Google Scholar] [CrossRef]
- Gazzano, E.; Riganti, C.; Tomatis, M.; Turci, F.; Bosia, A.; Fubini, B.; Ghigo, D. Potential toxicity of nonregulated asbestiform minerals: Balangeroite from the Western Alps. Part 3: Depletion of antioxidant defenses. J. Toxicol. Environ. Health A 2005, 68, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Groppo, C.; Tomatis, M.; Turci, F.; Gazzano, E.; Ghigo, D.; Compagnoni, R.; Fubini, B. Potential Toxicity of Nonregulated Asbestiform Minerals: Balangeroite From the Western Alps. Part 1: Identification and Characterization. J. Toxicol. Environ. Health A 2005, 68, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Price, B. Industrial-grade talc exposure and the risk of mesothelioma. Crit. Rev. Toxicol. 2010, 40, 513–530. [Google Scholar] [CrossRef] [PubMed]
- García Romero, E.; Suarez, M. Sepiolite-palygorskite: Textural study and genetic considerations. Appl. Clay Sci. 2013, 86, 129–144. [Google Scholar] [CrossRef]
- Mattioli, M.; Giardini, L.; Roselli, C.; Desideri, D. Mineralogical characterization of commercial clays used in cosmetics and possible risk for health. Appl. Clay Sci. 2016, 119, 449–454. [Google Scholar] [CrossRef]
- Larson, D.; Powers, A.; Ambrosi, J.-P.; Tanjia, M.; Napolitano, A.; Flores, E.G.; Baumann, F.; Pellegrini, L.; Jennings, C.J.; Buck, B.J.; et al. Investigating palygorskite’s role in the development of mesothelioma in southern Nevada: Insights into fiber-induced carcinogenicity. J. Toxicol. Environ. Health B 2016, 19, 213–230. [Google Scholar] [CrossRef] [PubMed]
- Ballirano, P.; Bloise, A.; Gualtieri, A.F.; Lezzerini, M.; Pacella, A.; Perchiazzi, N.; Dogan, M.; Dogan, A.U. The crystal structure of mineral fibres. In Mineral Fibres: Crystal Chemistry, Chemical-Physical Properties, Biological Interaction and Toxicity; Gualtieri, A.F., Ed.; EMU Notes in Mineralogy; European Mineralogical Union and the Mineralogical Society of Great Britain and Ireland: London, UK, 2017; Volume 18, pp. 17–64. [Google Scholar]
- Baris, I.; Artvinli, M.; Saracci, R.; Simonato, L.; Pooley, F.; Skidmore, J.; Wagner, C. Epidemiological and environmental evidence of the health effects of exposure to erionite fibres: A four-year study in the Cappadocian region of Turkey. Int. J. Cancer 1987, 39, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Carbone, M.; Emri, S.; Dogan, A.U.; Steele, I.; Tuncer, M.; Pass, H.I.; Baris, Y.I. A mesothelioma epidemic in Cappadocia: Scientific developments and unexpected social outcomes. Nat. Rev. Cancer 2007, 7, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Demirer, E.; Ghattas, C.F.; Radwan, M.O.; Elamin, E.M. Clinical and prognostic features of erionite-induced malignant mesothelioma. Yonsei Med. J. 2015, 56, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Coffin, D.L.; Cook, P.M.; Creason, J.P. Relative mesothelioma induction in rats by mineral fibers: Comparison with residual pulmonary mineral fiber number and epidemiology. Inhal. Toxicol. 1992, 4, 273–300. [Google Scholar] [CrossRef]
- Bertino, P.; Marconi, A.; Palumbo, L.; Bruni, B.M.; Barbone, D.; Germano, S.; Dogan, A.U.; Tassi, G.F.; Porta, C.; Mutti, L.; Gaudino, G. Erionite and asbestos differently cause transformation of human mesothelial cells. Int. J. Cancer 2007, 121, 2766–2774. [Google Scholar] [CrossRef] [PubMed]
- Hillegass, J.M.; Miller, J.M.; MacPherson, M.B.; Westbom, C.M.; Sayan, M.; Thompson, J.K.; Macura, S.L.; Perkins, T.N.; Beuschel, S.L.; Alexeeva, V.; et al. Asbestos and erionite prime and activate the NLRP3 inflammasome that stimulates autocrine cytokine release in human mesothelial cells. Part. Fibre Toxicol. 2013, 10, 39. [Google Scholar] [CrossRef] [PubMed]
- De Assis, L.V.M.; Locatelli, J.; Isoldi, M.C. The role of key genes and pathways involved in the tumorigenesis of malignant mesothelioma. Biochim. Biophys. Acta 2014, 1845, 232–247. [Google Scholar] [CrossRef] [PubMed]
- Zebedeo, C.N.; Davis, C.; Pena, C.; Ng, K.W.; Pfau, J.C. Erionite induces production of autoantibodies and IL-17 in C57BL/6 mice. Toxicol. Appl. Pharmacol. 2014, 275, 257–264. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer (IARC). IARC Monographs on the Evaluation of the Carcinogenic Risk to Humans; Overall Eval. Carcinog. Updating IARC Monographs Vol. 1 to 42; IARC: Lyon, France, 1987; Supplement 7; p. 150. [Google Scholar]
- International Agency for Research on Cancer (IARC). IARC Monographs on the evaluation of the carcinogenic risk to humans. Arsen. Met. Fibres Dusts 2011, 100, 311–316. [Google Scholar]
- Van Gosen, B.S.; Blitz, T.A.; Plumlee, G.S.; Meeker, G.P.; Pierson, M.P. Geologic occurrences of erionite in the United States: An emerging national public health concern for respiratory disease. Environ. Geochem. Health 2013, 35, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Saini-Eidukat, B.; Triplet, J.W. Erionite and offretite from the Killdeer Mountains, Dunn County, North Dakota, USA. Am. Mineral. 2014, 99, 8–15. [Google Scholar] [CrossRef]
- Baumann, F.; Carbone, M. Environmental risk of mesothelioma in the United States: An emerging concern-epidemiological issues. J. Toxicol. Environ. Health B 2016, 19, 231–249. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Guerrero, M.A.; Carrasco-Núñez, G. Environmental occurrence, origin, physical and geochemical properties, and carcinogenic potential of erionite near San Miguel de Allende, Mexico. Environ. Geochem. Health 2014, 36, 517–529. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Guerrero, M.A.; Carrasco-Núñez, G.; Barragán-Campos, H.; Ortega, M.R. High incidence of lung cancer and malignant mesothelioma linked to erionite fibre exposure in a rural community in Central Mexico. Occup. Environ. Med. 2015, 72, 216–218. [Google Scholar] [CrossRef] [PubMed]
- Ilgren, E.B.; Kazemian, H.; Hoskins, J.A. Kandovan the next ‘Capadoccia’? A potential public health issue for erionite related mesothelioma risk. Epidemiol. Biostat. Public Health 2015, 12, 1–12. [Google Scholar] [CrossRef]
- Giordani, M.; Mattioli, M.; Dogan, M.; Dogan, A.U. Potential carcinogenic erionite from Lessini Mounts, NE Italy: Morphological, mineralogical and chemical characterization. J. Toxicol. Environ. Health A 2016, 79, 808–824. [Google Scholar] [CrossRef] [PubMed]
- Giordani, M.; Mattioli, M.; Ballirano, P.; Pacella, P.; Cenni, M.; Boscardin, M.; Valentini, L. Geological occurrence, mineralogical characterization and risk assessment of potentially carcinogenic erionite in Italy. J. Toxicol. Environ. Health B 2017, 20, 81–103. [Google Scholar] [CrossRef] [PubMed]
- Cangiotti, M.; Battistelli, M.; Salucci, S.; Falcieri, E.; Mattioli, M.; Giordani, M.; Ottaviani, M.F. Electron paramagnetic resonance and transmission electron microscopy study of the interactions between asbestiform zeolite fibers and model membranes. J. Toxicol. Environ. Health A 2017, 80, 171–187. [Google Scholar] [CrossRef] [PubMed]
- Cangiotti, M.; Salucci, S.; Battistelli, M.; Falcieri, E.; Mattioli, M.; Giordani, M.; Ottaviani, M.F. EPR, TEM and cell viability study of asbestiform zeolite fibers in cell media. Colloids Surf. B Biointerfaces 2018, 161, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Gottardi, G.; Galli, E. Natural Zeolites; Springer: Heidelberg, Germany, 1985. [Google Scholar]
- Passaglia, E.; Artioli, G.; Gualtieri, A. Crystal chemistry of the zeolites erionite and offretite. Am. Mineral. 1998, 83, 577–589. [Google Scholar] [CrossRef]
- Bish, D.L.; Ming, D.W. Natural Zeolites: Occurrence, Properties, Applications; Reviews in Mineralogy and Geochemistry 45; Mineralogical Society of America: Washington, DC, USA, 2001. [Google Scholar]
- Passaglia, E.; Tagliavini, A. Chabazite-offretite epitaxial overgrowths in cornubianite from Passo Forcel Rosso, Adamello, Italy. Eur. J. Mineral. 1994, 6, 379–405. [Google Scholar] [CrossRef]
- Gard, J.A.; Tait, J.M. The crystal structure of the zeolite offretite K1.1Ca1.1Mg0.7(Si12.8Al5.2O36)315.2H2O. Acta Crystallogr. B 1972, 28, 825–834. [Google Scholar] [CrossRef]
- Guastoni, A.; Dugnani, M.; Pezzotta, F.; Bardelli, G. Offretite del lago d’Arno in alta Val Saviore, Parco dell’Adamello (Bs). Atti Soc. It. Sci. Nat. Museo Civ. St. Nat. Mil. 2002, 143, 195–207. [Google Scholar]
- Mattioli, M.; Cenni, M.; Passaglia, E. Secondary mineral assemblages as indicators of multi stage alteration processes in basaltic lava flows: Evidence from the Lessini Mountains, Veneto Volcanic Province, Northern Italy. Per. Mineral. 2016, 85, 1–24. [Google Scholar]
- Boscardin, M.; Checchi, A.; Filippi, F.; Guglielmino, S.; Pegoraro, S.; Pretto, G.; Zattra, A. Offretite del Veneto. Riv. Mineral. It. 1998, 22, 25–29. [Google Scholar]
- Daleffe, A.; Boscardin, M. Offretite di Passo Roccolo tra Chiampo e S. Giovanni Ilarione (Lessini Orientali). Stud. Ric. Ass. Amici Mus. Civ. 2005, 12, 57–59. [Google Scholar]
- Passaglia, E.; Tagliavini, A.; Gutoni, R. Offretite and other zeolites from Fittà (Verona, Italy). Neues Jahrb. Mineral. Monatshefte 1996, 418–428. [Google Scholar]
- De Vecchi, G.P.; Sedea, R. The Paleogene basalt of the Veneto region (NE Italy). Mem. Inst. Geol. Mineral. Univ. Padua 1995, 47, 253–274. [Google Scholar]
- Bonadiman, C.; Coltorti, M.; Milani, L.; Salvini, L.; Siena, F.; Tassinari, R. Metasomatism in the lithospheric mantle and its relationships to magmatism in the Veneto Volcanic Province, Italy. Per. Mineral. 2001, 70, 333–357. [Google Scholar]
- Goldstein, J.I.; Newbury, D.E.; Echlin, P.; Joy, D.C.; Roming, A.D.; Lyman, C.E.; Fiori, C.; Lifshin, E. Scanning Electron Microscopy and X-ray Microanalysis, 2nd ed.; Plenum Press: New York, NY, USA, 1992. [Google Scholar]
- Pacella, A.; Ballirano, P.; Cametti, G. Quantitative chemical analysis of erionite fibres using a micro-analytical SEM-EDX method. Eur. J. Mineral. 2016, 28, 257–264. [Google Scholar] [CrossRef]
- Passaglia, E. The crystal chemistry of chabazites. Am. Mineral. 1970, 55, 1278–1301. [Google Scholar]
- Dogan, A.U.; Dogan, M. Re-evaluation and re-classification of erionite series minerals. Environ. Geochem. Health 2008, 30, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Cametti, G.; Pacella, A.; Mura, F.; Rossi, M.; Ballirano, P. New morphological, chemical, and structural data of woolly erionite-Na from Durkee, Oregon, USA. Am. Mineral. 2013, 98, 2155–2163. [Google Scholar] [CrossRef]
- Bruker AXS. Topas V.4.2: General Profile and Structure Analysis Software for Powder Diffraction Data; Bruker AXS: Karlsruhe, Germany, 2009. [Google Scholar]
- Alberti, A.; Cruciani, G.; Galli, E.; Vezzalini, G. A re-examination of the crystal structure of the zeolite offretite. Zeolites 1996, 17, 457–461. [Google Scholar] [CrossRef]
- Ballirano, P.; Andreozzi, G.B.; Dogan, M.; Dogan, A.U. Crystal structure and iron topochemistry of erionite-K from Rome, Oregon, USA. Am. Mineral. 2009, 94, 1262–1270. [Google Scholar] [CrossRef]
- Sabine, T.M.; Hunter, B.A.; Sabine, W.R.; Ball, C.J. Analytical expressions for the transmission factor and peak shift in absorbing cylindrical specimens. J. Appl. Crystallogr. 1998, 31, 47–51. [Google Scholar] [CrossRef]
- Ballirano, P. Effects of the choice of different ionisation level for scattering curves and correction for small preferred orientation in Rietveld refinement: The MgAl2O4 test case. J. Appl. Crystallogr. 2003, 36, 1056–1061. [Google Scholar] [CrossRef]
- Katerinopoulou, A.; Balic-Zunic, T.; Lundegaard, L.F. Application of the ellipsoid modeling of the average shape of nanosized crystallites in powder diffraction. J. Appl. Crystallogr. 2012, 45, 22–27. [Google Scholar] [CrossRef]
- Jones, J.B. Al-O and Si-O tetrahedral distances in aluminosilicate framework structures. Acta Crystallogr. B 1968, 24, 355–358. [Google Scholar] [CrossRef]
- Oberdorster, G.; Castranova, V.; Asgharian, B.; Sayre, P. Inhalation exposure to carbon nanotubes (CNT) and carbon nanofibers (CNF): Methodology and dosimetry. J. Toxicol. Environ. Health B 2015, 18, 121–212. [Google Scholar] [CrossRef] [PubMed]
- Dodson, R.F.; Atkinson, M.A.L.; Levin, J.L. Asbestos fiber length as related to potential pathogenicity: A critical review. Am. J. Ind. Med. 2003, 44, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Yuen, S.R.; Ashley, R. Short, thin asbestos fibers contribute to the development of human malignant mesothelioma: Pathological evidence. Int. J. Hyg. Environ. Health 2005, 208, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Gatti, A.; Rivasi, F. Biocompatibility of micro- and nano-particles. Part I: In liver and kidney. Biomaterials 2002, 23, 2381–2387. [Google Scholar] [CrossRef]
- Bunderson-Schelvan, M.; Pfau, J.C.; Crouch, R.; Holian, A. Non-pulmonary outcomes of asbestos exposure. J. Toxicol. Environ. Health B 2011, 14, 122–152. [Google Scholar] [CrossRef] [PubMed]
- Matassa, R.; Familiari, G.; Relucenti, M.; Battaglione, E.; Downing, C.; Pacella, A.; Cametti, G.; Ballirano, P. A Deep Look Into Erionite Fibres: An Electron Microscopy Investigation of their Self-Assembly. Sci. Rep. 2015, 5, 16757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donaldson, K.; Murphy, F.A.; Duffin, R.; Poland, C.A. Asbestos, carbon nanotubes and the pleural mesothelium: A review of the hypothesis regarding the role of long fibre retention in the parietal pleura, inflammation and mesothelioma. Part. Fibre Toxicol. 2010, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, W. Modelling inhaled particle deposition in the human lung—A review. J. Aerosol Sci. 2011, 42, 693–724. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). IARC Monographs on the evaluation of the carcinogenic risk of chemicals to humans. Silica Some Silic. 1997, 42, 225–239. [Google Scholar]
- Stephenson, D.J.; Fairchild, C.I.; Buchan, R.M.; Dakins, M.E. A fiber characterization of the natural zeolite, mordenite: A potential inhalation health hazard. Aerosol Sci. Technol. 1999, 30, 467–476. [Google Scholar] [CrossRef]
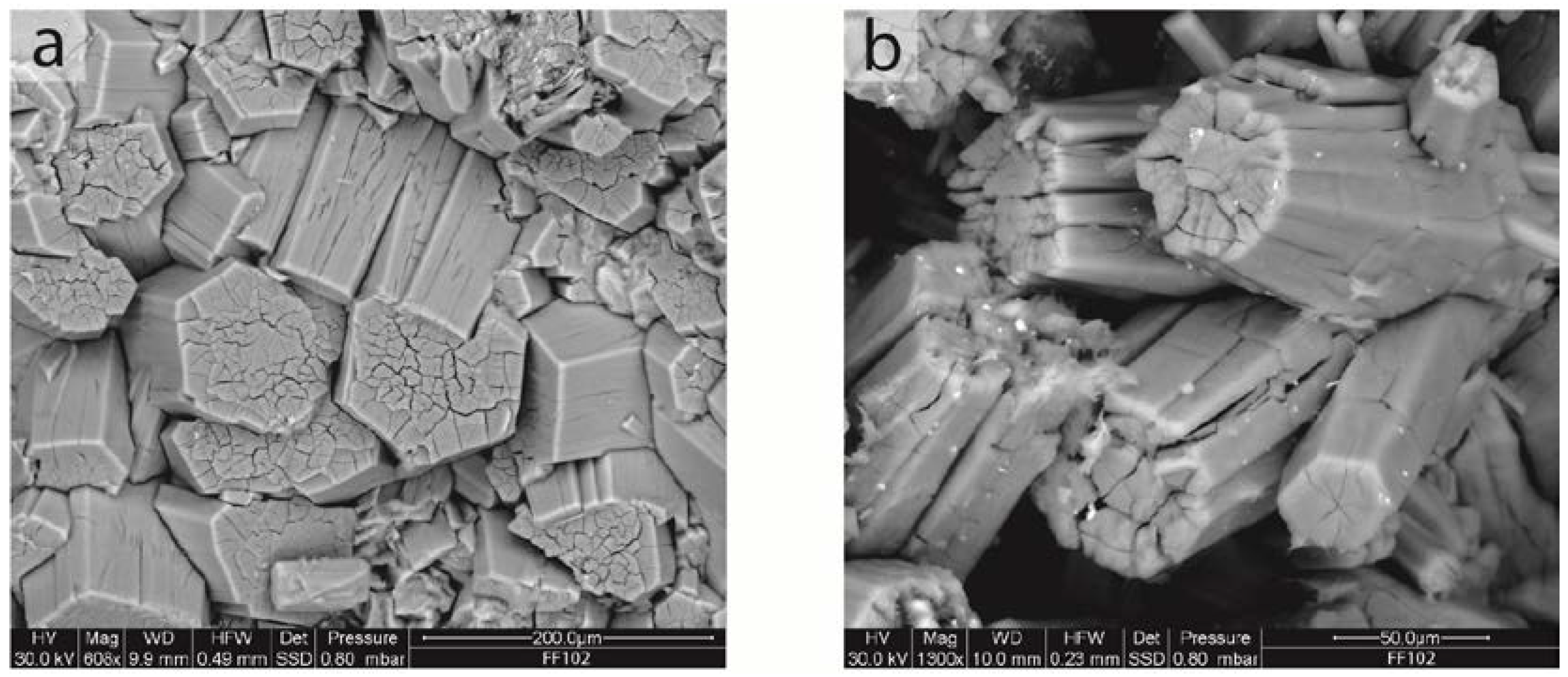

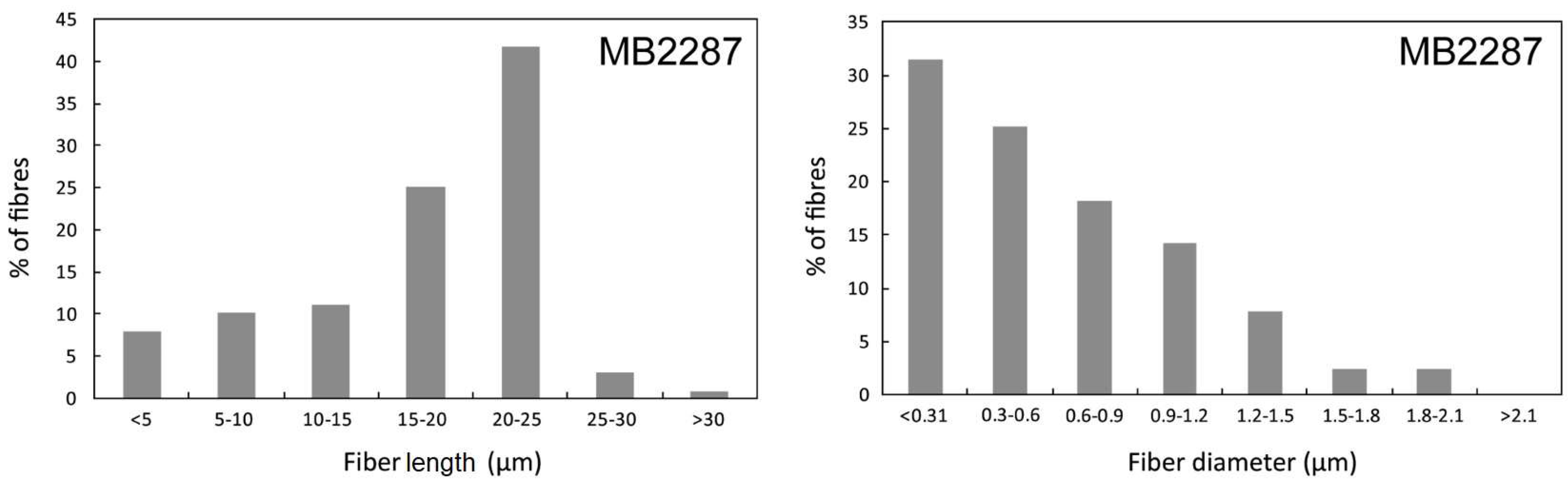
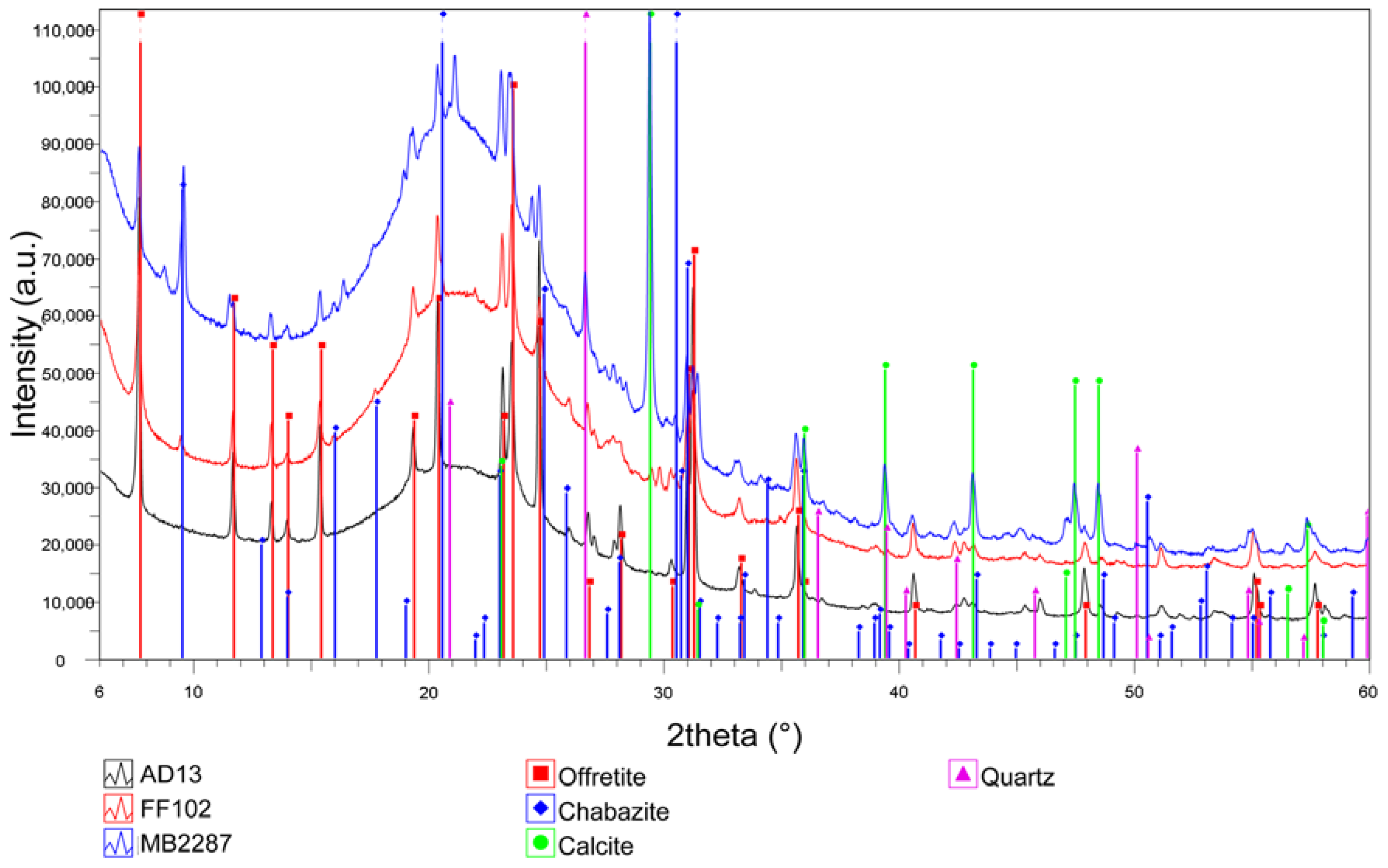

| Locality | Latitude | Longitude | Altitude (m a.s.l.) | Mineral Association | Offretite (vol %) |
|---|---|---|---|---|---|
| Marcantoni | 45.360653 | 11.174968 | 555 | CHA + OFF + ERI + CM | 60 |
| Nogare (FF102) | 45.362673 | 11.180461 | 401 | OFF + ERI + CM | 75 |
| Battistini | 45.361506 | 11.175586 | 474 | PHI/HAR + CHA + ERI + OFF + CM | 35 |
| Mattiazzi | 45.361784 | 11.180102 | 468 | OFF + ERI+ CM | 70 |
| Foscarino Mt. | 45.439427 | 11.258779 | 296 | PHI/HAR + ERI + OFF + CM | 30 |
| Colombara | 45.426723 | 11.277245 | 140 | PHI/HAR + CHA + OFF + ERI + CM | 40 |
| Fittà (AD13) | 45.453548 | 11.253994 | 281 | PHI/HAR + OFF +ERI + CM | 70 |
| Calvarina Mt. | 45.490955 | 11.269361 | 280 | NAT + ANA + ERI + OFF + CM | 15 |
| Chiereghini V. | 45.549751 | 11.219361 | 285 | PHI/HAR + NAT + OFF + CM | - |
| S.Giovanni Il. | 45.521057 | 11.236276 | 194 | ANA + ERI + OFF + PHI/HAR + CM | 30 |
| Beltrami | 45.525522 | 11.229305 | 253 | PHI/HAR + CHA + OFF + CM | 20 |
| Bagattei | 45.551624 | 11.218733 | 329 | PHI/HAR + ERI + OFF + YUG + CM | 35 |
| Soave | 45.419776 | 11.247955 | 50 | OFF + ERI + GYR + PRE + CM | 75 |
| Roncà | 45.480646 | 11.288877 | 80 | ANA + OFF + PHI/HAR + CM | 55 |
| Prun | 45.577982 | 10.951481 | 541 | CHA + OFF + ERI + CM | 60 |
| S. Cristina | 45.581039 | 10.940663 | 683 | PHI/HAR + CHA + ERI + OFF + CM | 10 |
| Saviore (MB2287) | 46.025012 | 10.255729 | 1845 | CHA + OFF + CM | 65 |
| La Trabersera | 46.024379 | 10.271761 | 1825 | CHA + OFF + PHI/HAR + CM | 45 |
| Lago d’Arno E | 46.023939 | 10.273229 | 1827 | CHA + OFF + GIS + CM | 40 |
| Sample | a (Å) | c (Å) | Volume (Å3) |
|---|---|---|---|
| FF102 | 13.3179 (5) | 7.5839 (4) | 1164.93 (13) |
| AD13 | 13.3086 (3) | 7.58724 (18) | 1163.80 (6) |
| MB2287 | 13.3216 (7) | 7.5868 (6) | 1166.02 (15) |
| Sample | FF102 | AD13 | MB2287 | |||
|---|---|---|---|---|---|---|
| Average N = 7 | σ | Average N = 8 | σ | Average N = 7 | σ | |
| SiO2 | 53.27 | 1.42 | 53.06 | 0.30 | 52.29 | 0.42 |
| Al2O3 | 17.18 | 0.91 | 17.07 | 0.33 | 18.59 | 0.38 |
| MgO | 2.04 | 0.11 | 2.65 | 0.25 | 2.98 | 0.20 |
| CaO | 4.28 | 0.57 | 4.17 | 0.36 | 3.68 | 0.22 |
| Na2O | - | - | - | - | - | - |
| K2O | 3.89 | 0.21 | 3.70 | 0.41 | 3.12 | 0.20 |
| H2O | 19.34 * | - | 19.34 * | - | 19.34 * | - |
| Total | 80.66 | - | 80.66 | - | 80.66 | - |
| Si | 13.04 | 0.29 | 13.05 | 0.09 | 12.68 | 0.10 |
| Al | 4.96 | 0.29 | 4.95 | 0.09 | 5.32 | 0.10 |
| ƩT | 18.00 | - | 18.00 | - | 18.00 | - |
| Mg | 0.75 | 0.04 | 0.97 | 0.08 | 1.08 | 0.07 |
| Ca | 1.13 | 0.15 | 1.10 | 0.13 | 0.96 | 0.06 |
| Na | - | - | - | - | - | - |
| K | 1.22 | 0.07 | 1.16 | 0.13 | 0.97 | 0.06 |
| ƩEFs.s. | 54.53 | - | 55.49 | - | 55.59 | - |
| O | 36.00 | - | 36.18 | - | 35.86 | - |
| H2O | 15.79 | 0.09 | 15.70 | 0.04 | 15.78 | 0.02 |
| R | 0.72 | 0.02 | 0.72 | 0.01 | 0.70 | 0.01 |
| E% | −0.22 | 3.12 | -6.05 | 3.75 | 5.38 | 3.52 |
| Mg/(Ca + Na) | 0.68 | - | 0.89 | - | 1.13 | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mattioli, M.; Giordani, M.; Arcangeli, P.; Valentini, L.; Boscardin, M.; Pacella, A.; Ballirano, P. Prismatic to Asbestiform Offretite from Northern Italy: Occurrence, Morphology and Crystal-Chemistry of a New Potentially Hazardous Zeolite. Minerals 2018, 8, 69. https://doi.org/10.3390/min8020069
Mattioli M, Giordani M, Arcangeli P, Valentini L, Boscardin M, Pacella A, Ballirano P. Prismatic to Asbestiform Offretite from Northern Italy: Occurrence, Morphology and Crystal-Chemistry of a New Potentially Hazardous Zeolite. Minerals. 2018; 8(2):69. https://doi.org/10.3390/min8020069
Chicago/Turabian StyleMattioli, Michele, Matteo Giordani, Pierluca Arcangeli, Laura Valentini, Matteo Boscardin, Alessandro Pacella, and Paolo Ballirano. 2018. "Prismatic to Asbestiform Offretite from Northern Italy: Occurrence, Morphology and Crystal-Chemistry of a New Potentially Hazardous Zeolite" Minerals 8, no. 2: 69. https://doi.org/10.3390/min8020069






