Temperature-Induced Desorption of Methyl tert-Butyl Ether Confined on ZSM-5: An In Situ Synchrotron XRD Powder Diffraction Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Experimental
2.3. Instrumentation
3. Results and Discussion
3.1. Adsorption from Aqueous Solutions
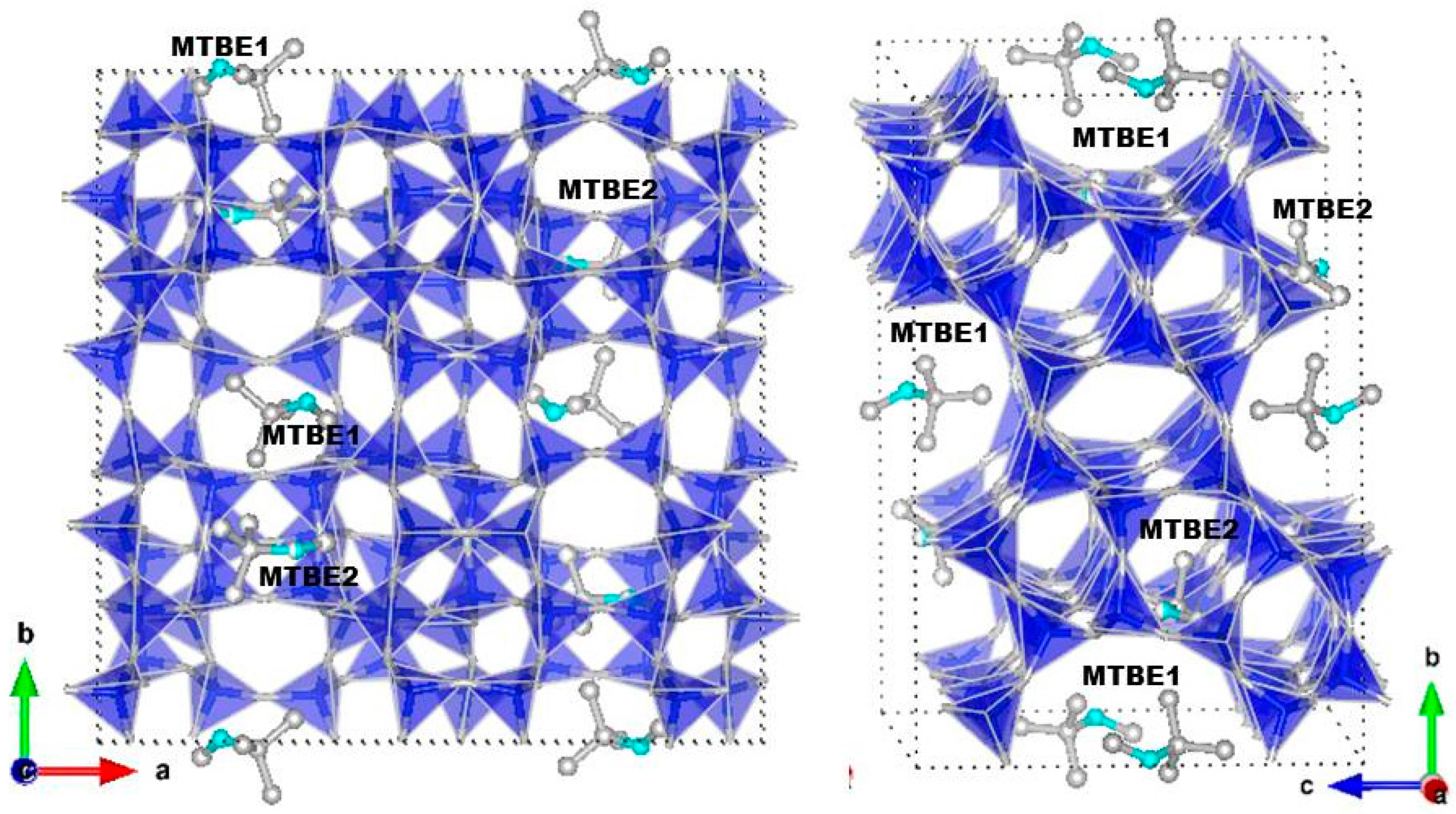
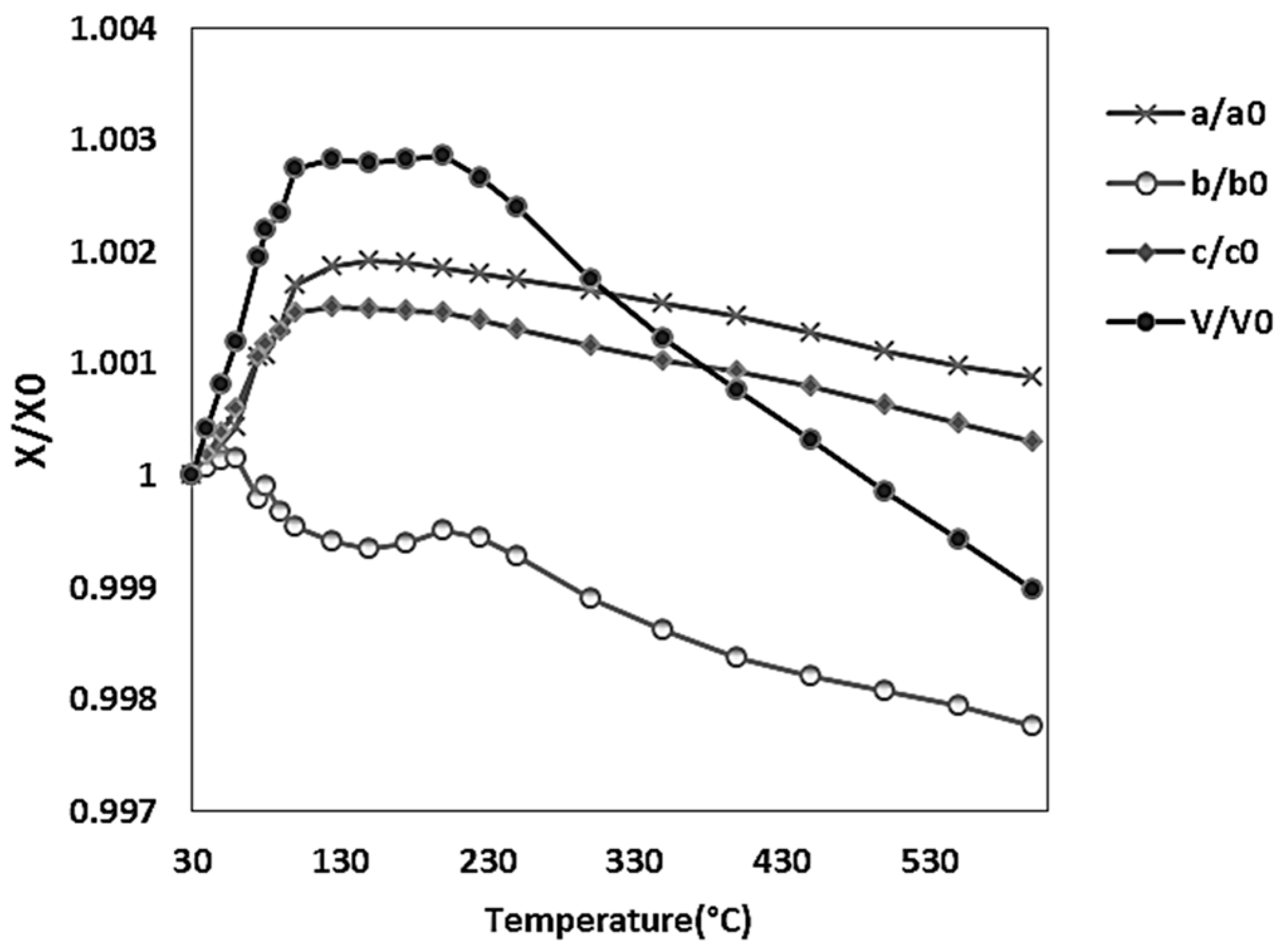
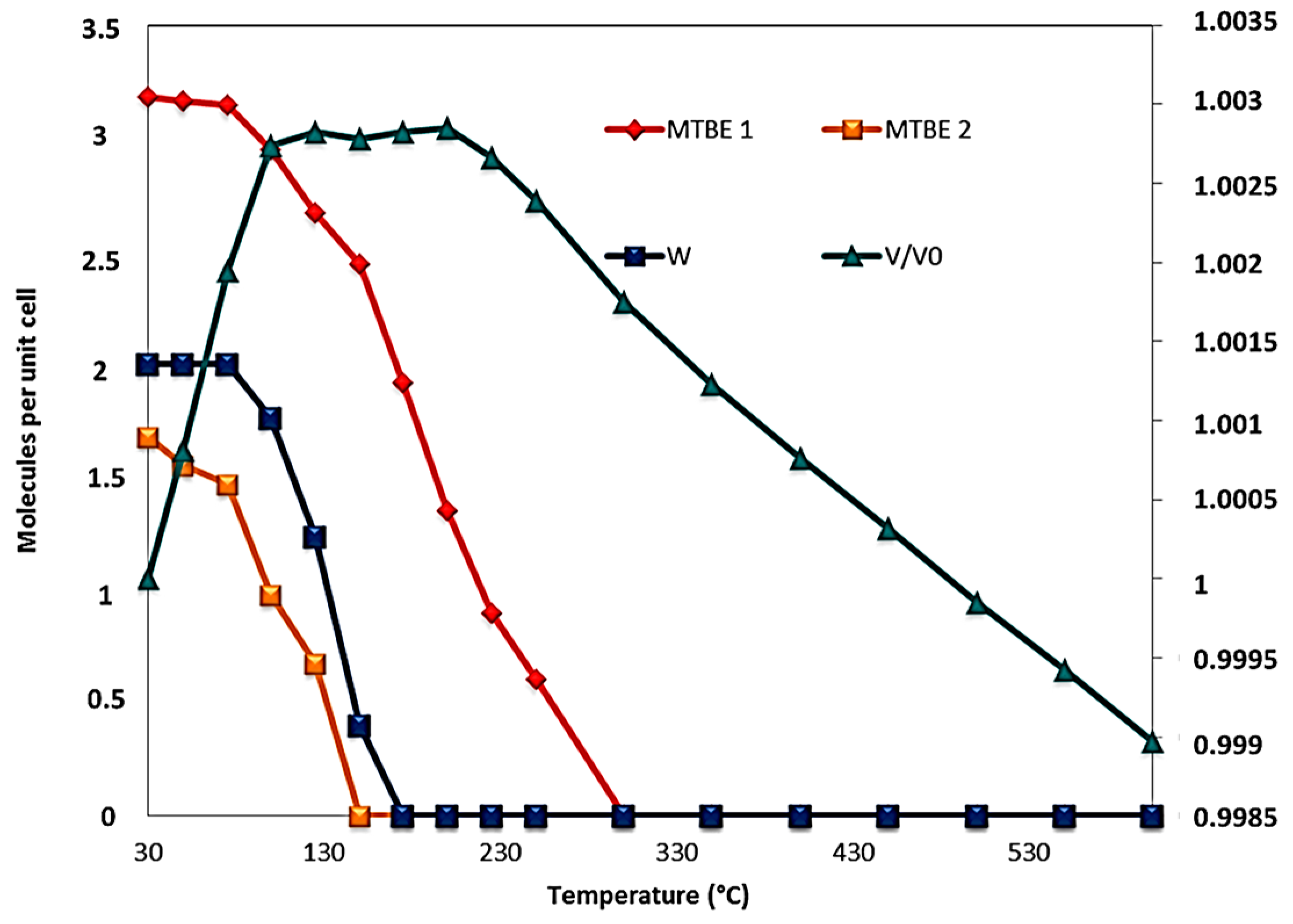
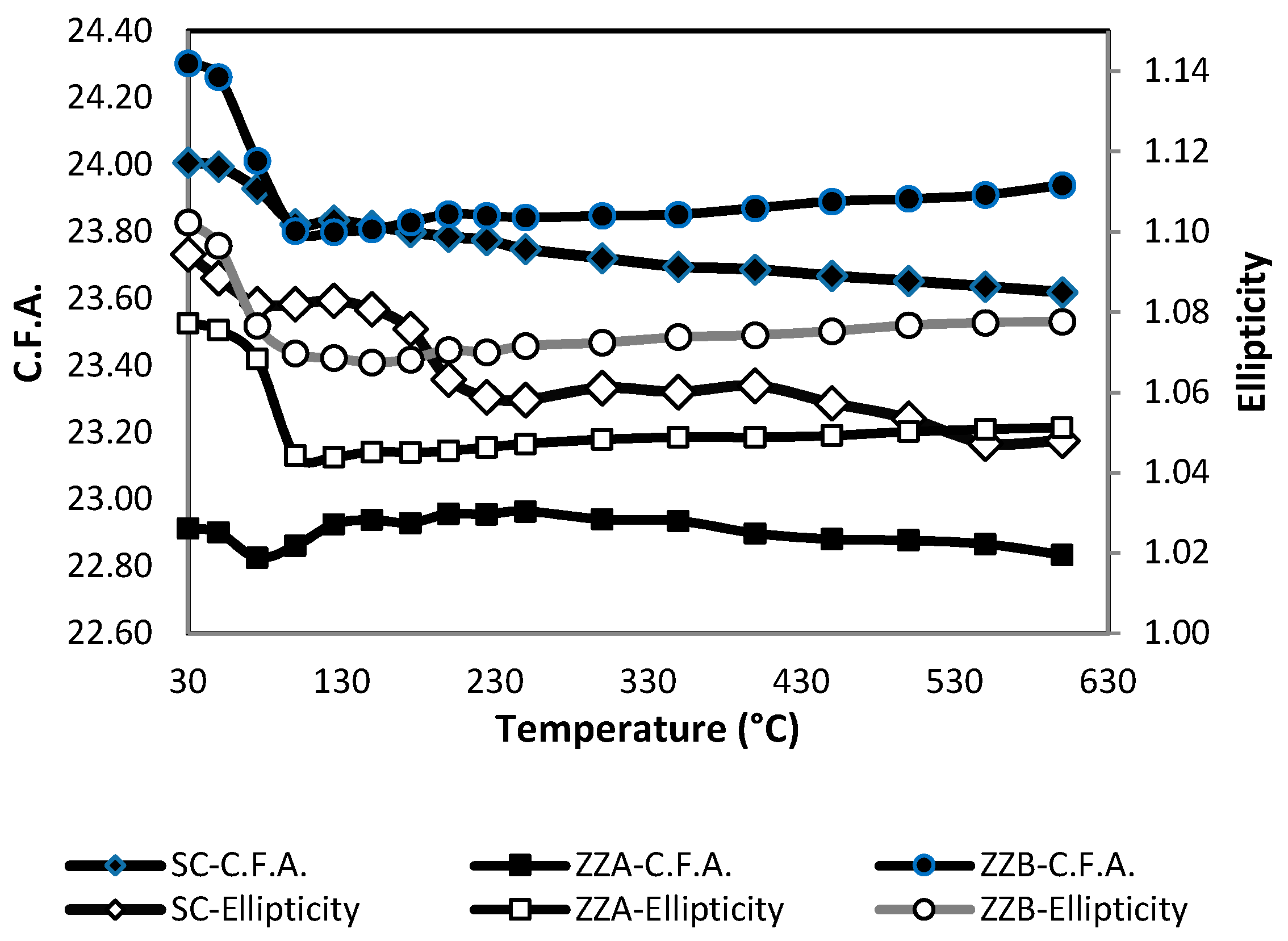
3.2. Structural Analyses
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chong, M.N.; Jin, B.; Chow, C.W.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.; Pankow, J.; Bender, D.; Price, C.; Zogorski, J. MTBE—To What Extent Will Past Releases Contaminate Community Water Supply Wells? Environ. Sci. Technol. 2000, 34, 210A–217A. [Google Scholar] [CrossRef] [PubMed]
- Amberg, A.; Rosner, E.; Dekant, W. Toxicokinetics of methyl tert-butyl ether and its metabolites in humans after oral exposure. Toxicol. Sci. 2001, 61, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Mackay, D.; Shiu, W.Y.; Ma, K.C.; Lee, S.C. Illustrated Handbook of Physical–Chemical Properties and Environmental Fate for Organic Chemicals—Volatile Organic Chemicals; Taylor & Francis Group, LLC: Boca Raton, FL, USA, 2006; Volume 3, pp. 1–925. [Google Scholar]
- Squillace, P.; Pankow, J.; Kortes, N.; Zogorski, J. Review of the environmental behavior and fate of methyl tert-butyl ether. Environ. Toxicol. Chem. 2009, 16, 1836–1844. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Drinking Water Advisory: Consumer Acceptability Advice and Health Effects Analysis on Methyl Tertiary-Butyl Ether (MTBE); EPA-822-F-97-009; US Environmental Protection Agency: Washington, DC, USA, 1997; pp. 11–13.
- Gaya, U.I.; Abdullah, A.H. Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: A review of fundamentals, progress and problems. J. Photochem. Photobiol. C Photochem. Rev. 2008, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Costa, A.A.; Wilson, W.B.; Wang, H.; Campiglia, A.D.; Dias, J.A.; Dias, S.C.L. Comparison of BEA, USY and ZSM-5 for the quantitative extraction of polycyclic aromatic hydrocarbons from water samples. Microporous Mesoporous Mater. 2012, 149, 186–192. [Google Scholar] [CrossRef]
- Milestone, N.B.; Bibby, D.M. Concentration of alcohols by adsorption on silicalite. J. Chem. Technol. Biotechnol. 1981, 31, 732–736. [Google Scholar] [CrossRef]
- Grose, R.W.; Flanigen, E.M. Novel Zeolite Compositions and Processes for Preparing and Using Same. U.S. Patent 4,257,885, 24 March 1981. [Google Scholar]
- Yazaydin, A.O.; Thompson, R.W. Molecular simulation of the adsorption of MTBE in silicalite, mordenite, and zeolite beta. J. Phys. Chem. B 2006, 110, 14458–14462. [Google Scholar] [CrossRef] [PubMed]
- Abu-Lail, L.; Bergendahl, J.A.; Thompson, R.W. Adsorption of methyl tertiary butyl ether on granular zeolites: Batch and column studies. J. Hazard. Mater. 2010, 178, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.A. Removal of MTBE and other organic contaminants from water by sorption to high silica zeolites. Environ. Sci. Technol. 2000, 34, 725–727. [Google Scholar] [CrossRef]
- Rossner, A.; Knappe, D.R. MTBE adsorption on alternative adsorbents and packed bed adsorber performance. Water Res. 2008, 42, 2287–2299. [Google Scholar] [CrossRef] [PubMed]
- Martucci, A.; Braschi, I.; Marchese, L.; Quartieri, S. Recent advances in clean-up strategies of waters polluted with sulfonamide antibiotics: A review of sorbents and related properties. Mineral. Mag. 2014, 78, 1115–1140. [Google Scholar] [CrossRef]
- Braschi, I.; Blasioli, S.; Gigli, L.; Gessa, C.E.; Alberti, A.; Martucci, A. Removal of sulfonamide antibiotics from water: Evidence of adsorption into an organophilic zeolite Y by its structural modifications. J. Hazard. Mater. 2010, 17, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Martucci, A.; Pasti, L.; Marchetti, N.; Cavazzini, A.; Dondi, F.; Alberti, A. Adsorption of pharmaceuticals from aqueous solutions on synthetic zeolites. Microporous Mesoporous Mater. 2012, 148, 174–183. [Google Scholar] [CrossRef]
- Pasti, L.; Martucci, A.; Nassi, M.; Cavazzini, A.; Alberti, A.; Bagatin, R. The role of water in DCE adsorption from aqueous solutions onto hydrophobic zeolites. Microporous Mesoporous Mater. 2012, 160, 182–193. [Google Scholar] [CrossRef]
- Pasti, L.; Sarti, E.; Cavazzini, A.; Marchetti, N.; Dondi, F.; Martucci, A. Factors affecting drug adsorption on beta zeolites. J. Sep. Sci. 2013, 36, 1604–1611. [Google Scholar] [CrossRef] [PubMed]
- Braschi, I.; Martucci, A.; Blasioli, S.; Mzini, L.L.; Ciavatta, C.; Cossi, M. Effect of humic monomers on the adsorption of sulfamethoxazole sulfonamide antibiotic into a high silica zeolite Y: An interdisciplinary study. Chemosphere 2016, 155, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Leardini, L.; Martucci, A.; Braschi, I.; Blasioli, S.; Quartieri, S. Regeneration of high-silica zeolites after sulfamethoxazole antibiotic adsorption: A combined in situ high-temperature synchrotron X-ray powder diffraction and thermal degradation study. Mineral. Mag. 2014, 78, 1141–1160. [Google Scholar] [CrossRef]
- Grima, J.N.; Zammit, V.; Gatt, R.M. Negative Thermal Expansion. Xjenza 2006, 11, 17–29. [Google Scholar]
- Bull, I.; Lightfoot, P.; Villaescusa, L.A.; Bull, L.M. An X-ray Diffraction and MAS NMR Study of the Thermal Expansion Properties of Calcined Siliceous Ferrierite. J. Am. Chem. Soc. 2003, 125, 4342–4349. [Google Scholar] [CrossRef] [PubMed]
- Woodcock, D.A.; Lightfoot, P.; Wright, P.A.; Villaescusa, L.A.; Dìaz-Cabañasb, M.J.; Camblorb, M.A. Strong negative thermal expansion in the siliceous zeolites ITQ-1,ITQ-3 and SSZ-23. J. Mater. Chem. 1999, 9, 349–351. [Google Scholar] [CrossRef]
- Alberti, A.; Martucci, A. Phase transformations and structural modifications induced by heating in microporous materials. Stud. Surf. Sci. Catal. 2005, 155, 19–43. [Google Scholar]
- Alberti, A.; Martucci, A. Reconstructive phase transitions in microporous materials: Rules and factors affecting them. Microporous Mesoporous Mater. 2011, 141, 192–198. [Google Scholar] [CrossRef]
- Cruciani, G. Zeolites upon heating: Factors governing their thermal stability and structural changes. J. Phys. Chem. Solids 2006, 67, 1973–1994. [Google Scholar] [CrossRef]
- Leardini, L.; Martucci, A.; Alberti, A.; Cruciani, G. Template burning effects on stability and boron coordination in boron levyne studied by in situ time resolved synchrotron powder diffraction. Microporous Mesoporous Mater. 2013, 167, 117–126. [Google Scholar] [CrossRef]
- Martucci, A.; Leardini, L.; Nassi, M.; Sarti, E.; Bagatin, R.; Pasti, L. Removal of emerging organic contaminants from aqueous systems: Adsorption and location of methyl-tertiary-butylether on synthetic ferrierite. Mineral. Mag. 2014, 78, 1161–1175. [Google Scholar] [CrossRef]
- Arletti, R.; Martucci, A.; Alberti, A.; Pasti, L.; Nassi, M.; Bagatin, R. Location of MTBE and toluene in the channel system of the zeolite mordenite: Adsorption and host–guest interactions. J. Solid State Chem. 2012, 194, 135–142. [Google Scholar] [CrossRef]
- Martucci, A.; Braschi, I.; Bisio, C.; Sarti, E.; Rodeghero, E.; Bagatin, R.; Pasti, L. Influence of water on the retention of methyl tertiary-butyl ether by high silica ZSM-5 and Y zeolites: A multidisciplinary study on the adsorption from liquid and gas phase. RSC Adv. 2015, 5, 86997–87006. [Google Scholar] [CrossRef]
- Knappe, D.R.U.; Campos, A.A.R. Effectiveness of high-silica zeolites for the adsorption of methyl tertiary-butyl ether from natural water. Water Sci. Technol. Water Supply 2005, 5, 83–91. [Google Scholar]
- Centi, G.; Grande, A.; Perathoner, S. Catalytic conversion of MTBE to biodegradable chemicals in contaminated water. Catal. Today 2002, 75, 69–76. [Google Scholar] [CrossRef]
- Sacchetto, V.; Gatti, G.; Paul, G.; Braschi, I.; Berlier, G.; Cossi, M.; Bisio, C. The interactions of methyl tert-butyl ether on high silica zeolites: A combined experimental and computational study. Phys. Chem. Chem. Phys. 2013, 15, 13275–13287. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tuan, V.A.; Noble, R.D.; Falconer, J.L. MTBE adsorption on all-silica β zeolite. Environ. Sci. Technol. 2003, 37, 4007–4010. [Google Scholar] [CrossRef] [PubMed]
- Baerlocher, C.; Meir, W.M.; Olson, O.H. Atlas of Zeolite Framework Types, 5th ed.; Elsevier Science: New York, NY, USA, 2001. [Google Scholar]
- Larson, A.C.; von Dreele, R.B. GSAS, General Structure Analysis System; LANSCE, MS-H805; Los Alamos National Laboratory: Los Alamos, NM, USA, 1994. [Google Scholar]
- Toby, B.H. EXPGUI, a graphical user interface for GSAS. J. Appl. Crystallogr. 2001, 34, 210–213. [Google Scholar] [CrossRef]
- Martucci, A.; Pasti, L.; Nassi, M.; Alberti, A.; Arletti, R.; Bagatin, R.; Sticca, R. Adsorption mechanism of 1,2-dichloroethane into an organophilic zeolite mordenite: A combined diffractometric and gas chromatographic study. Microporous Mesoporous Mater. 2012, 151, 358–367. [Google Scholar] [CrossRef]
- Degen, T.; Sadki, M.; Bron, E.; König, U.; Nénert, G. The HighScore suite. Powder Diffr. 2014, 29, S13–S18. [Google Scholar] [CrossRef]
- Ardit, M.; Martucci, A.; Cruciani, G. Monoclinic–orthorhombic phase transition in ZSM-5 zeolite: Spontaneous strain variation and thermodynamic properties. J. Phys. Chem. C 2015, 119, 7351–7359. [Google Scholar] [CrossRef]
- Martucci, A.; Rodeghero, E.; Pasti, L.; Bosi, V.; Cruciani, G. Adsorption of 1,2-dichloroethane on ZSM-5 and desorption dynamics by in situ synchrotron powder X-ray diffraction. Microporous Mesoporous Mater. 2015, 215, 175–182. [Google Scholar] [CrossRef]
- Rodeghero, E.; Martucci, A.; Cruciani, G.; Bagatin, R.; Sarti, E.; Bosi, V.; Pasti, L. Kinetics and dynamic behaviour of toluene desorption from ZSM-5 using in situ high-temperature synchrotron powder X-ray diffractionand chromatographic techniques. Catal. Today 2016, 227, 118–125. [Google Scholar] [CrossRef]
- Bhange, D.S.; Ramaswamy, V. High temperature thermal expansion behavior of silicalite-1 molecular sieve: In Situ HTXRD study. Microporous Mesoporous Mater. 2007, 103, 235–242. [Google Scholar] [CrossRef]
- Bhange, D.S.; Ramaswamy, V. Enhanced negative thermal expansion in MFI molecular sieves by varying framework composition. Microporous Mesoporous Mater. 2010, 130, 322–326. [Google Scholar] [CrossRef]
- Villaescusa, L.A.; Lightfoot, P.; Teat, S.J.; Morris, R.E. Variable-Temperature Microcrystal X-ray Diffraction Studies of Negative Thermal Expansion in the Pure Silica Zeolite IFR. J. Am. Chem. Soc. 2001, 123, 5453–5459. [Google Scholar] [CrossRef] [PubMed]
- Milanesio, M.; Artioli, G.; Gualtieri, A.F.; Palin, L.; Lamberti, C. Template burning inside TS-1 and Fe-MFI molecular sieves: An in situ XRPD study. J. Am. Chem. Soc. 2003, 125, 14549–14558. [Google Scholar] [CrossRef] [PubMed]
- Martucci, A.; de Lourdes Guzman-Castillo, M.; Di Renzo, F.; Fajula, F.; Alberti, A. Reversible channel deformation of zeolite omega during template degradation highlighted by in situ time-resolved synchrotron powder diffraction. Microporous Mesoporous Mater. 2007, 104, 257–268. [Google Scholar] [CrossRef]
- Leardini, L.; Martucci, A.; Cruciani, G. The unusual thermal behaviour of boron-ZSM-5 probed by “in situ” time-resolved synchrotron powder diffraction. Microporous Mesoporous Mater. 2013, 173, 6–14. [Google Scholar] [CrossRef]
- Leardini, L.; Martucci, A.; Cruciani, G. The unusual thermal expansion of pure silica sodalite probed by in situ time-resolved synchrotron powder diffraction. Microporous Mesoporous Mater. 2012, 151, 163–171. [Google Scholar] [CrossRef]
- Leardini, L.; Quartieri, S.; Vezzalini, G.; Arletti, R. Thermal behaviour of siliceous faujasite: Further structural interpretation of negative thermal expansion. Microporous Mesoporous Mater. 2015, 202, 226–233. [Google Scholar] [CrossRef] [Green Version]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodeghero, E.; Pasti, L.; Sarti, E.; Cruciani, G.; Bagatin, R.; Martucci, A. Temperature-Induced Desorption of Methyl tert-Butyl Ether Confined on ZSM-5: An In Situ Synchrotron XRD Powder Diffraction Study. Minerals 2017, 7, 34. https://doi.org/10.3390/min7030034
Rodeghero E, Pasti L, Sarti E, Cruciani G, Bagatin R, Martucci A. Temperature-Induced Desorption of Methyl tert-Butyl Ether Confined on ZSM-5: An In Situ Synchrotron XRD Powder Diffraction Study. Minerals. 2017; 7(3):34. https://doi.org/10.3390/min7030034
Chicago/Turabian StyleRodeghero, Elisa, Luisa Pasti, Elena Sarti, Giuseppe Cruciani, Roberto Bagatin, and Annalisa Martucci. 2017. "Temperature-Induced Desorption of Methyl tert-Butyl Ether Confined on ZSM-5: An In Situ Synchrotron XRD Powder Diffraction Study" Minerals 7, no. 3: 34. https://doi.org/10.3390/min7030034






