Effect of Silica Alumina Ratio and Thermal Treatment of Beta Zeolites on the Adsorption of Toluene from Aqueous Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Experimental
2.3. Instrumentation
3. Results and Discussion
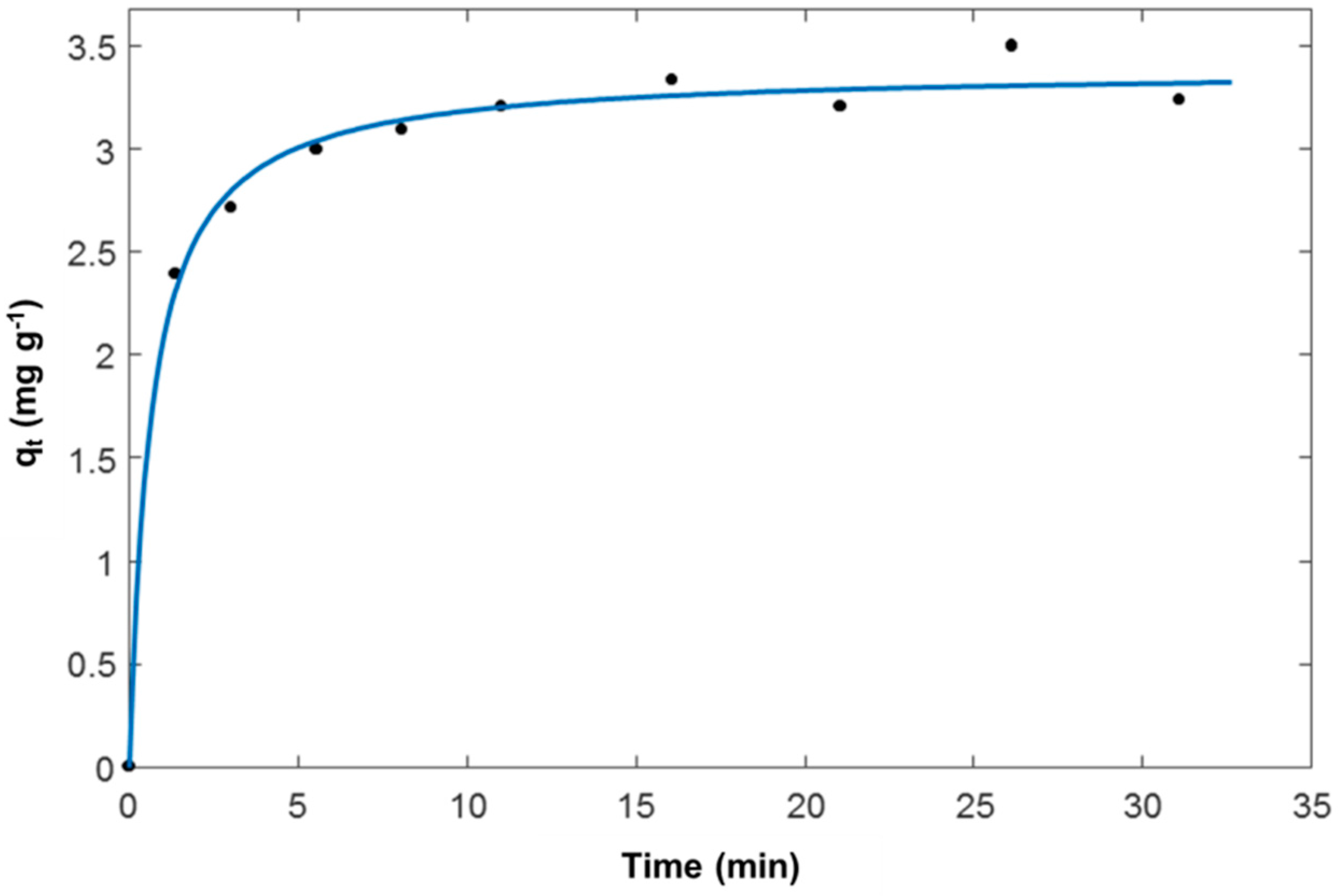
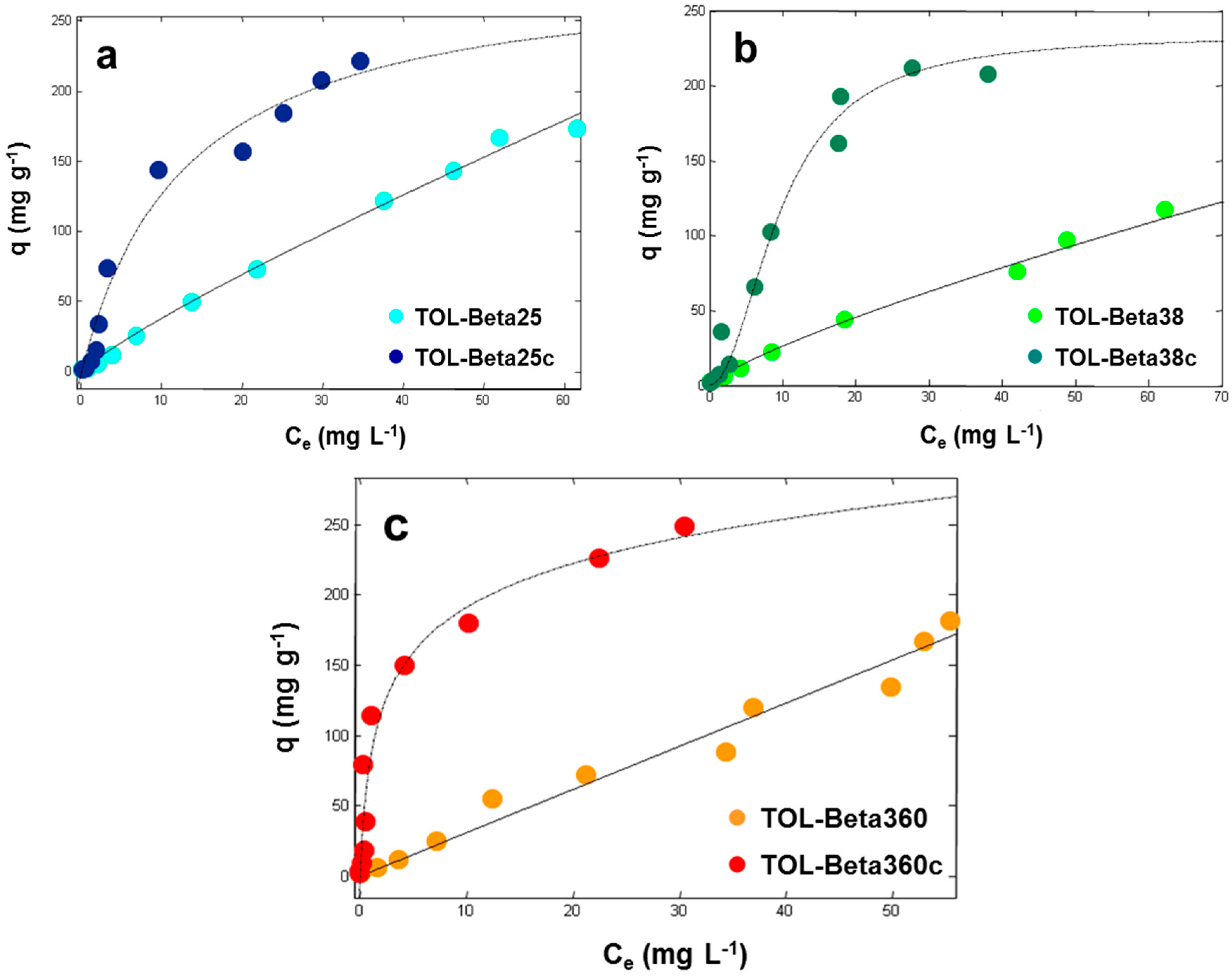
3.1. Adsorption from Aqueous Solutions
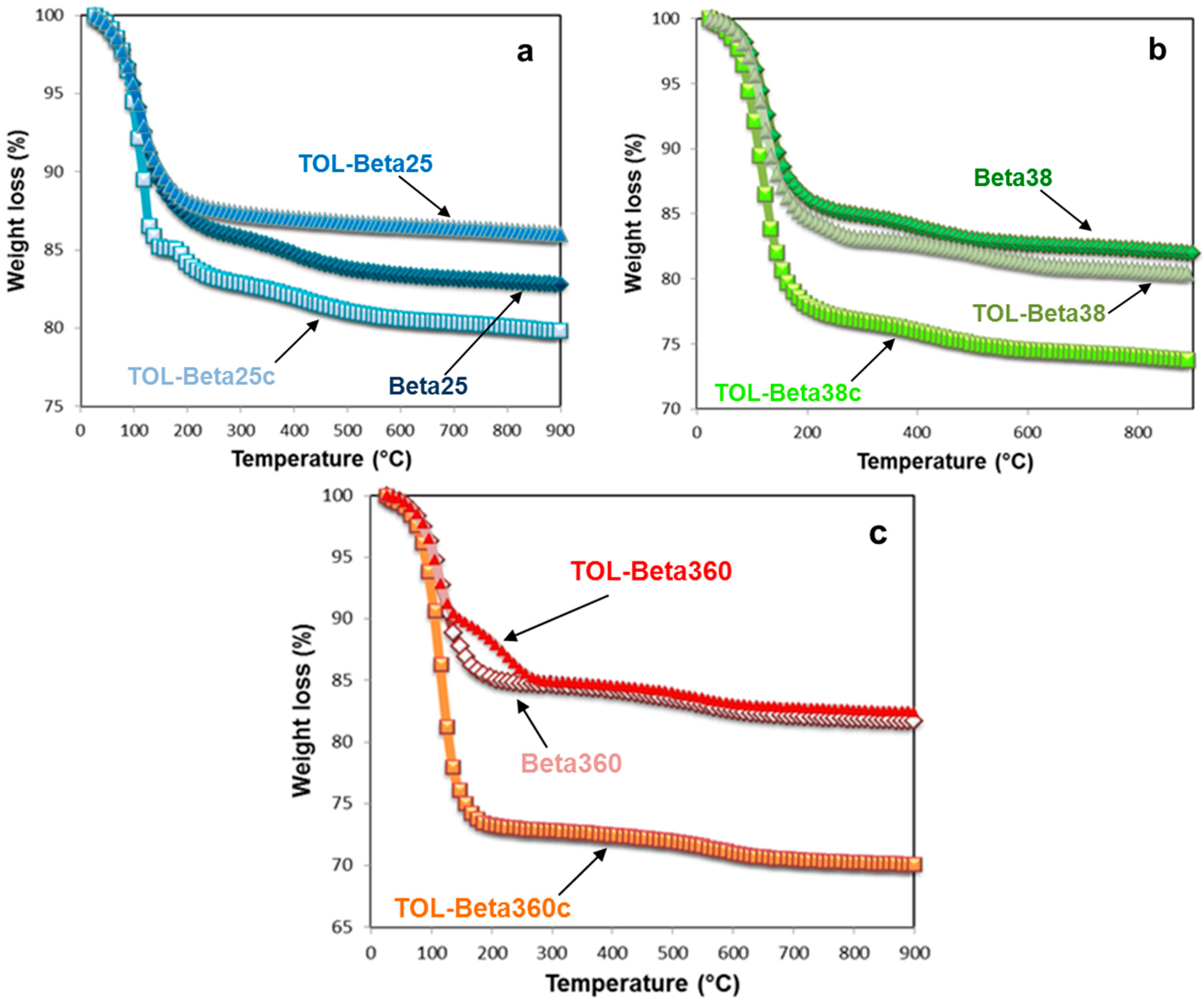
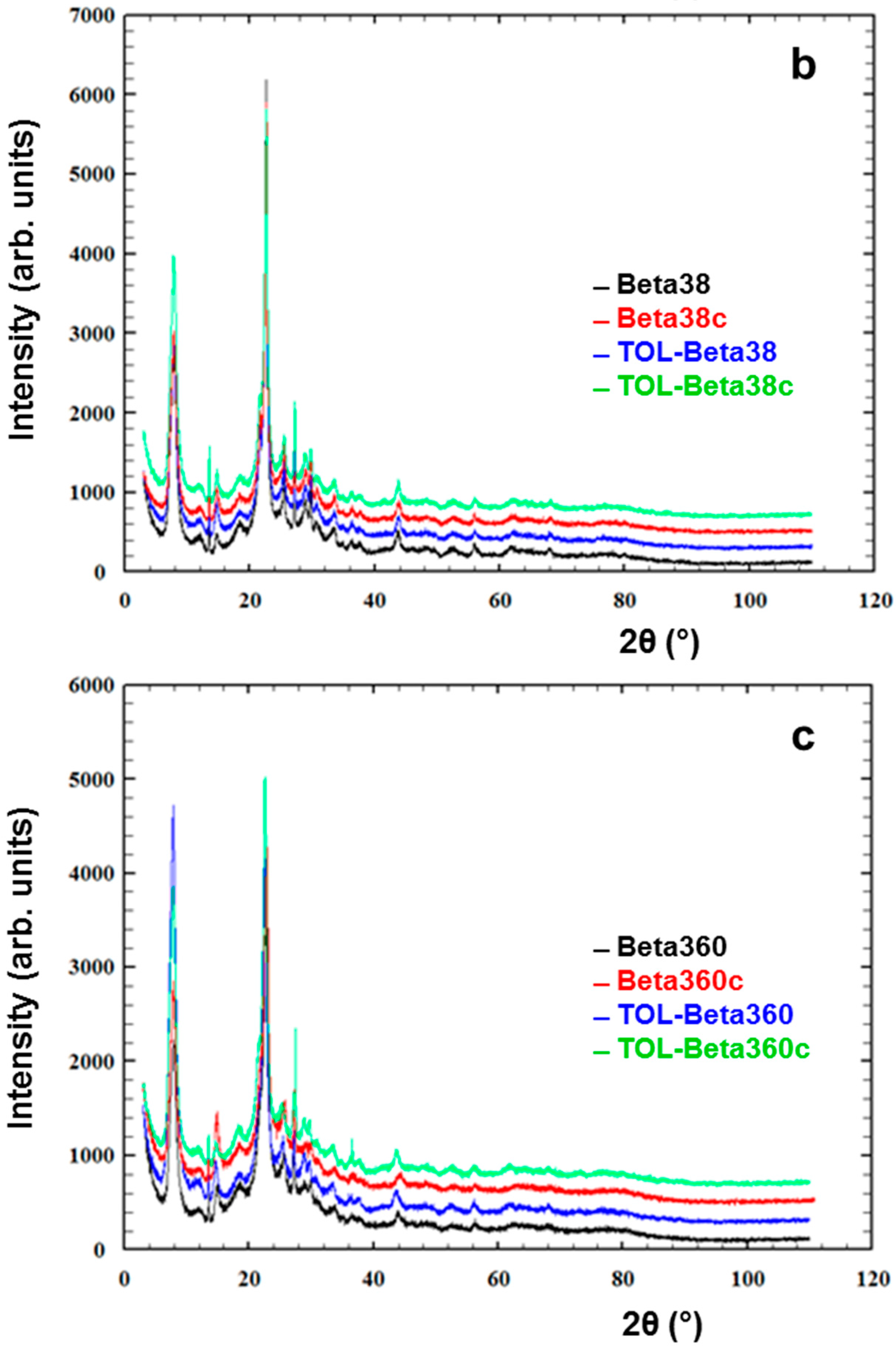
3.2. Thermal and Structural Analyses
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Aivalioti, M.; Vamvasakis, I.; Gidarakos, E. BTEX and MTBE adsorption onto raw and thermally modified diatomite. J. Hazard. Mater. 2010, 178, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Qu, F.; Zhu, L.; Yang, K. Adsorption behaviors of volatile organic compounds (VOCs) on porous clay heterostructures (PCH). J. Hazard. Mater. 2009, 170, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Verma, N. Removal of volatile organic compounds by cryogenic condensation followed by adsorption. Chem. Eng. Sci. 2002, 57, 2679–2696. [Google Scholar] [CrossRef]
- Arletti, R.; Martucci, A.; Alberti, A.; Pasti, L.; Nassi, M.; Bagatin, R. Location of MTBE and toluene in the channel system of the zeolite mordenite: Adsorption and host–guest interactions. J. Solid State Chem. 2012, 194, 135–142. [Google Scholar] [CrossRef]
- Martucci, A.; Pasti, L.; Marchetti, N.; Cavazzini, A.; Dondi, F.; Alberti, A. Adsorption of pharmaceuticals from aqueous solutions on synthetic zeolites. Microporous Mesoporous Mater. 2012, 148, 174–183. [Google Scholar] [CrossRef]
- Pasti, L.; Sarti, E.; Cavazzini, A.; Marchetti, N.; Dondi, F.; Martucci, A. Factors affecting drug adsorption on beta zeolites. J. Sep. Sci. 2013, 36, 1604–1611. [Google Scholar] [CrossRef] [PubMed]
- Braschi, I.; Blasioli, S.; Gigli, L.; Gessa, C.E.; Alberti, A.; Martucci, A. Removal of sulfonamide antibiotics from water: Evidence of adsorption into an organophilic zeolite Y by its structural modifications. J. Hazard. Mater. 2010, 17, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.A.; Wilson, W.B.; Wang, H.; Campiglia, A.D.; Dias, J.A.; Dias, S.C.L. Comparison of BEA, USY and ZSM-5 for the quantitative extraction of polycyclic aromatic hydrocarbons from water samples. Microporous Mesoporous Mater. 2012, 149, 186–192. [Google Scholar] [CrossRef]
- Khalid, M.; Joly, G.; Renaud, A.; Magnoux, P. Removal of phenol from water by adsorption using zeolites. Ind. Eng. Chem. Res. 2004, 43, 5275–5280. [Google Scholar] [CrossRef]
- Rodeghero, E.; Martucci, A.; Cruciani, G.; Bagatin, R.; Sarti, E.; Bosi, V.; Pasti, L. Kinetics and dynamic behaviour of toluene desorption from ZSM-5 using in situ high-temperature synchrotron powder X-ray diffractionand chromatographic techniques. Catal. Today 2016, 227, 118–125. [Google Scholar] [CrossRef]
- Martucci, A.; Braschi, I.; Bisio, C.; Sarti, E.; Rodeghero, E.; Bagatin, R.; Pasti, L. Influence of water on the retention of methyl tertiary-butyl ether by high silica ZSM-5 and Y zeolites: A multidisciplinary study on the adsorption from liquid and gas phase. RSC Adv. 2015, 5, 86997–87006. [Google Scholar] [CrossRef]
- Pasti, L.; Martucci, A.; Nassi, M.; Cavazzini, A.; Alberti, A.; Bagatin, R. The role of water in DCE adsorption from aqueous solutions onto hydrophobic zeolites. Microporous Mesoporous Mater. 2012, 160, 182–193. [Google Scholar] [CrossRef]
- Weitkamp, J. Zeolites and catalysis. Solid State Ion. 2000, 131, 175–188. [Google Scholar] [CrossRef]
- Aivalioti, M.; Pothoulaki, D.; Papoulias, P.; Gidarakos, E. Removal of BTEX, MTBE and TAME from aqueous solutions by adsorption onto raw and thermally treated lignite. J. Hazard. Mater. 2012, 207, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Martucci, A.; Rodeghero, E.; Pasti, L.; Bosi, V.; Cruciani, G. Adsorption of 1,2-dichloroethane on ZSM-5 and desorption dynamics by in situ synchrotron powder X-ray diffraction. Microporous Mesoporous Mater. 2015, 215, 175–182. [Google Scholar] [CrossRef]
- Martucci, A.; Leardini, L.; Nassi, M.; Sarti, E.; Bagatin, R.; Pasti, L. Removal of emerging organic contaminants from aqueous systems: Adsorption and location of methyl-tertiary-butylether on synthetic ferrierite. Mineral. Mag. 2014, 78, 1161–1175. [Google Scholar] [CrossRef]
- Baerlocher, C.; Meir, W.M.; Olson, O.H. Atlas of Zeolite Framework Types, 5th revised ed.; Elsevier Science: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Jansen, J.C.; Creyghton, E.J.; Njo, S.L.; van Koningsveld, H.; van Bekkum, H. On the remarkable behaviour of zeolite Beta in acid catalysis. Catal. Today 1997, 38, 205–212. [Google Scholar] [CrossRef]
- Higgins, J.B.; LaPierre, R.B.; Schlenker, J.L.; Rohrman, A.C.; Wood, J.D.; Kerr, G.T.; Rohrbaugh, W.J. The framework topology of zeolite beta. Zeolites 1988, 8, 446–452. [Google Scholar] [CrossRef]
- Newsam, J.M.; Treacy, M.M.J.; Koetsier, W.T.; De Gruyter, C.B. Structural characterization of zeolite beta. Proc. R. Soc. Lond. Ser. A 1988, 420, 375–405. [Google Scholar] [CrossRef]
- Trombetta, M.; Busca, G.; Storaro, L.; Lenarda, M.; Casagrande, M.; Zambon, A. Surface acidity modifications induced by thermal treatments and acid leaching on microcrystalline H-BEA zeolite. A FTIR, XRD and MAS-NMR study. Phys. Chem. Chem. Phys. 2000, 2, 3529–3537. [Google Scholar] [CrossRef]
- Beyer, H.K.; Nagy, J.B.; Karge, H.G.; Kiricsi, I. Catalysis by Microporous Materials; Elsevier: Amsterdam, The Netherlands, 1995. [Google Scholar]
- Otomo, R.; Yokoi, T.; Kondo, J.N.; Tatsumi, T. Dealuminated Beta zeolite as effective bifunctional catalyst for direct transformation of glucose to 5-hydroxymethylfurfural. Appl. Catal. A Gen. 2014, 470, 318–326. [Google Scholar] [CrossRef]
- Čejka, J.; van Bekkum, H.; Corma, A.; Schüth, F. Introduction to Zeolite Science and Practice, 3rd revised ed.; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Al-Khattaf, S.; Ali, S.A.; Aitani, A.M.; Žilková, N.; Kubička, D.; Čejka, J. Recent advances in reactions of alkylbenzenes over novel zeolites: The effects of zeolite structure and morphology. Catal. Rev. 2014, 56, 333–402. [Google Scholar] [CrossRef]
- Dědeček, J.; Sobalík, Z.; Wichterlová, B. Siting and distribution of framework aluminium atoms in silicon-rich zeolites and impact on catalysis. Catal. Rev. 2012, 54, 135–223. [Google Scholar] [CrossRef]
- Krishna, R.; van Baten, J.M. Hydrogen bonding effects in adsorption of water–alcohol mixtures in zeolites and the consequences for the characteristics of the Maxwell–Stefan diffusivities. Langmuir 2010, 26, 10854–10867. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wu, Z.; Tan, T. Sorption equilibrium, mechanism and thermodynamics studies of 1,3-propanediol on beta zeolite from an aqueous solution. Bioresour. Technol. 2013, 145, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Magriotis, Z.M.; Leal, P.V.B.; de Sales, P.F.; Papini, R.M.; Viana, P.R.M.; Augusto Arroyo, P. A comparative study for the removal of mining wastewater by kaolinite, activated carbon and beta zeolite. Appl. Clay Sci. 2014, 91, 55–62. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Dehkordi, A.M.; Khademi, M. Adsorption of xylene isomers on Na-BETA zeolite: Equilibrium in batch adsorber. Microporous Mesoporous Mater. 2013, 172, 136–140. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 385–471. [Google Scholar]
- Tóth, J. State equations of the solid gas interface layer. Acta Chem. Acad. Sci. Hung. 1971, 69, 311–317. [Google Scholar]
- Camblor, M.A.; Pérez-Pariente, J. Crystallization of zeolite beta: Effect of Na and K ions. Zeolites 1991, 11, 202–210. [Google Scholar] [CrossRef]
- Kunkeler, P.J.; Zuurdeeg, B.J.; van der Waal, J.C.; van Bokhoven, J.A.; Koningsberger, D.C.; van Bekkum, H. Zeolite Beta: The relationship between calcination procedure, aluminum configuration, and lewis acidity. J. Catal. 1998, 180, 234–244. [Google Scholar] [CrossRef]
- Lohse, U.; Altrichter, B.; Fricke, R.; Pilz, W.; Schreier, E.; Garkisch, C.; Jancke, K. Synthesis of zeolite beta Part 2—Formation of zeolite beta and titanium-beta via an intermediate layer structure. J. Chem. Soc. Faraday Trans. 1997, 93, 505–512. [Google Scholar] [CrossRef]
- Kiricsi, I.; Flego, C.; Pazzuconi, G.; Parker, W.O.; Millini, R.; Perego, C.; Bellussi, G. Progress toward understanding zeolite β acidity: An IR and 27Al NMR spectroscopic study. J. Phys. Chem. 1994, 98, 4627–4634. [Google Scholar] [CrossRef]
- Maretto, M.; Vignola, R.; Williams, C.D.; Bagatin, R.; Latini, A.; Petrangeli Papini, M. Adsorption of hydrocarbons from industrial wastewater onto a silica mesoporous material: Structural and thermal study. Microporous Mesoporous Mater. 2015, 203, 139–150. [Google Scholar] [CrossRef]
- Burke, N.R.; Trimm, D.L.; Howe, R.F. The effect of silica:alumina ratio and hydrothermal ageing on the adsorption characteristics of BEA zeolites for cold start emission control. Appl. Catal. B Environ. 2003, 46, 97–104. [Google Scholar] [CrossRef]
- Krohn, J.E.; Tsapatsis, M. Amino acid adsorption on zeolite β. Langmuir 2005, 21, 8743–8750. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Gil, V.; Noya, E.G.; Sanz, A.; Khatib, S.J.; Guil, J.M.; Lomba, E.; Marguta, R.; Valencia, S. Experimental and simulation studies of the stepped adsorption of toluene on pure-silica MEL zeolite. J. Phys. Chem. C 2016, 120, 8640–8652. [Google Scholar] [CrossRef]
- Dong, J.; Tian, T.; Ren, L.; Zhang, Y.; Xu, J.; Cheng, X. CuO nanoparticles incorporated in hierarchical MFI zeolite as highlyactive electrocatalyst for non-enzymatic glucose sensing. Colloid Surf. B 2015, 125, 206–212. [Google Scholar] [CrossRef] [PubMed]

| Name | Product Code | SiO2/Al2O3 | Nominal Cation | Surface Area (m2·g−1) |
|---|---|---|---|---|
| Beta25 | CP814E | 25 | Ammonium | 680 |
| Beta38 | CP814C | 38 | Ammonium | 710 |
| Beta360 | CP811C-300 | 360 | Hydrogen | 620 |
| As-Received Materials | KF (mg·g−1)·(L·g−1)n | n | R2 |
|---|---|---|---|
| Beta25 | 5.2 (3.5, 7.0) | 0.86 (0.77, 0.95) | 0.9953 |
| Beta38 | 4.2 (2.5, 5.9) | 0.79 (0.69, 0.89) | 0.9936 |
| Beta360 | 4.1 (2.8, 5.3) | 0.93 (0.72, 1.1) | 0.9956 |
| Calcined Materials | qs (mg·g−1) | b (L·mg−1) | v | R2 |
|---|---|---|---|---|
| Beta25c | 234 (193, 275) | 0.073 (0.043, 0.10) | 0.96 (0.70, 1.2) | 0.9584 |
| Beta38c | 224 (198, 250) | 0.10 (0.075, 0.13) | 0.91 (0.72, 1.1) | 0.9688 |
| Beta360c | 241 (201, 280) | 0.55 (0.30, 0.80) | 0.84 (0.62, 1.0) | 0.9667 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarti, E.; Chenet, T.; Pasti, L.; Cavazzini, A.; Rodeghero, E.; Martucci, A. Effect of Silica Alumina Ratio and Thermal Treatment of Beta Zeolites on the Adsorption of Toluene from Aqueous Solutions. Minerals 2017, 7, 22. https://doi.org/10.3390/min7020022
Sarti E, Chenet T, Pasti L, Cavazzini A, Rodeghero E, Martucci A. Effect of Silica Alumina Ratio and Thermal Treatment of Beta Zeolites on the Adsorption of Toluene from Aqueous Solutions. Minerals. 2017; 7(2):22. https://doi.org/10.3390/min7020022
Chicago/Turabian StyleSarti, Elena, Tatiana Chenet, Luisa Pasti, Alberto Cavazzini, Elisa Rodeghero, and Annalisa Martucci. 2017. "Effect of Silica Alumina Ratio and Thermal Treatment of Beta Zeolites on the Adsorption of Toluene from Aqueous Solutions" Minerals 7, no. 2: 22. https://doi.org/10.3390/min7020022






