The Effect of Ca2+ and Mg2+ on the Dispersion and Flocculation Behaviors of Muscovite Particles
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Methods
2.2.1. Sedimentation Test
2.2.2. Zeta Potentials Measurement
2.2.3. Calculation of Interfacial Energy between Particles
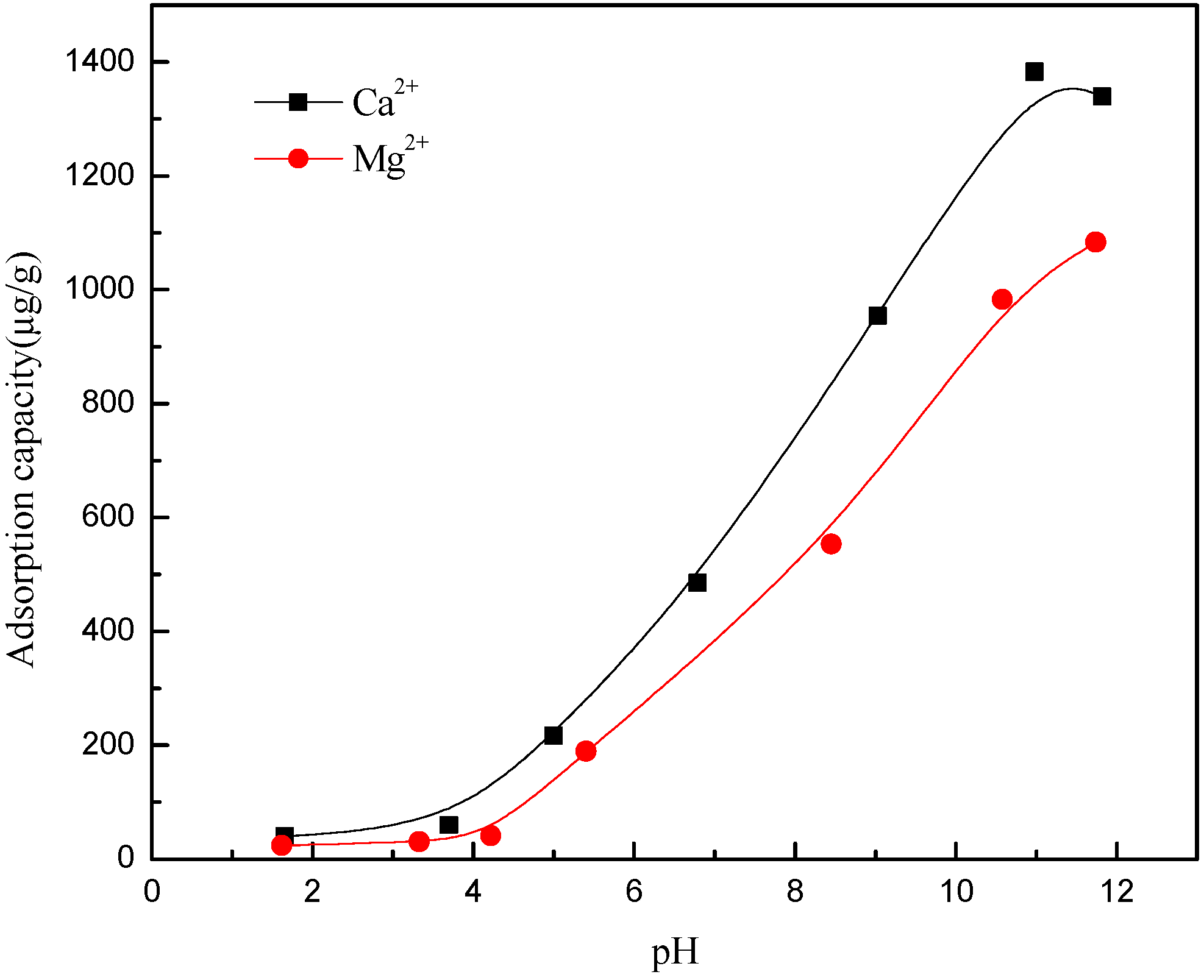
2.2.4. Adsorption Capacity Measurement
3. Results and Discussion
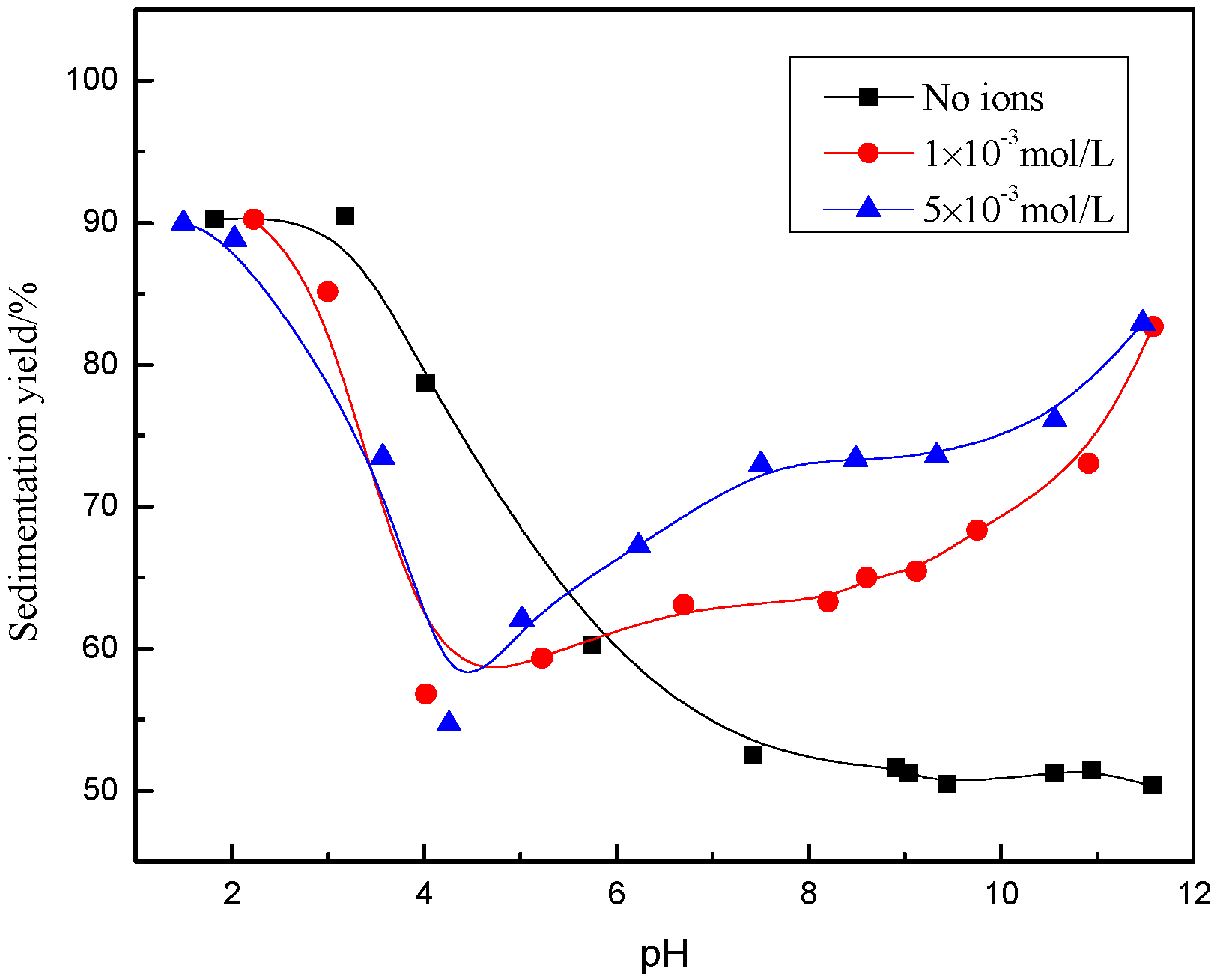
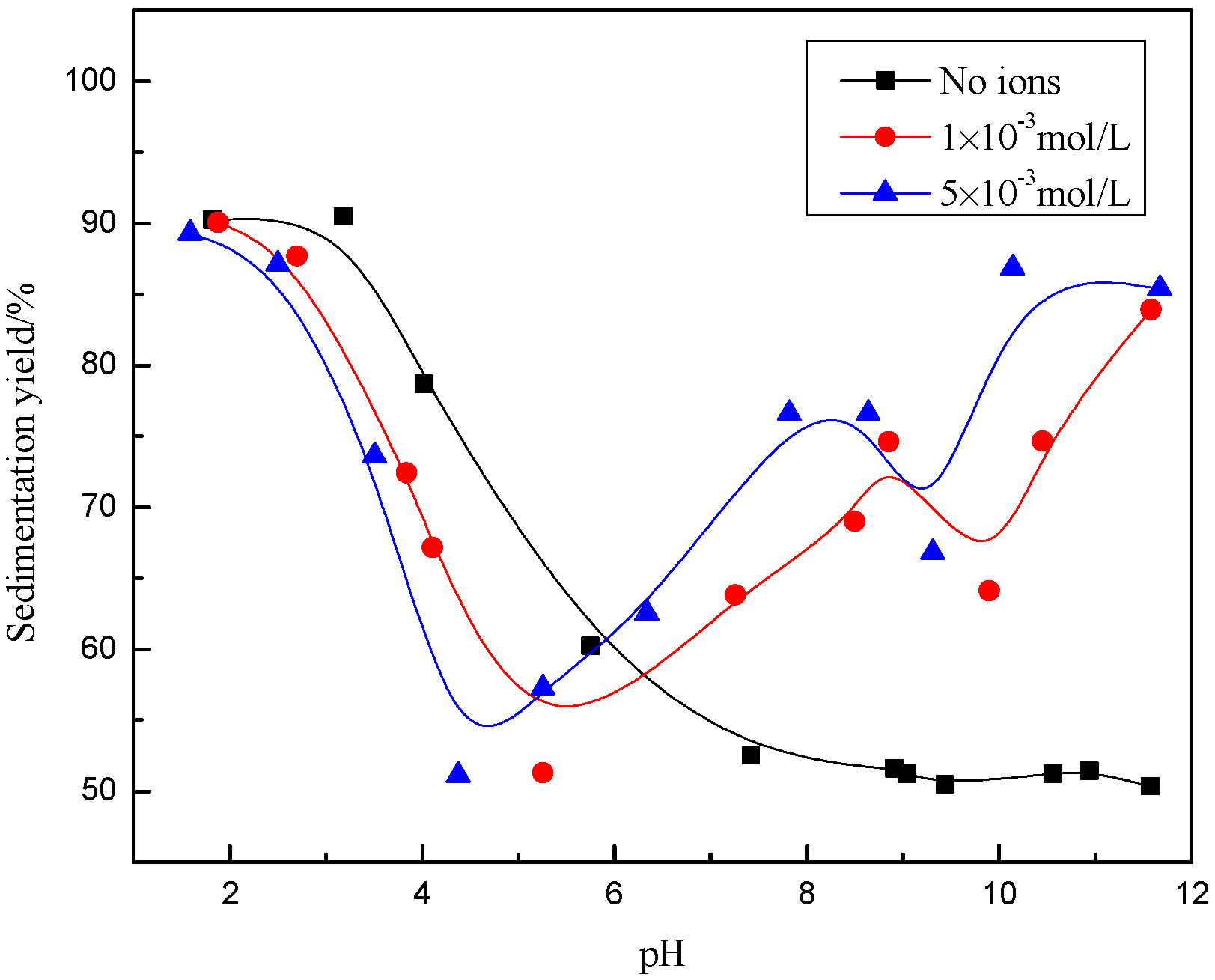
3.1. Sedimentation Behavior of Muscovite
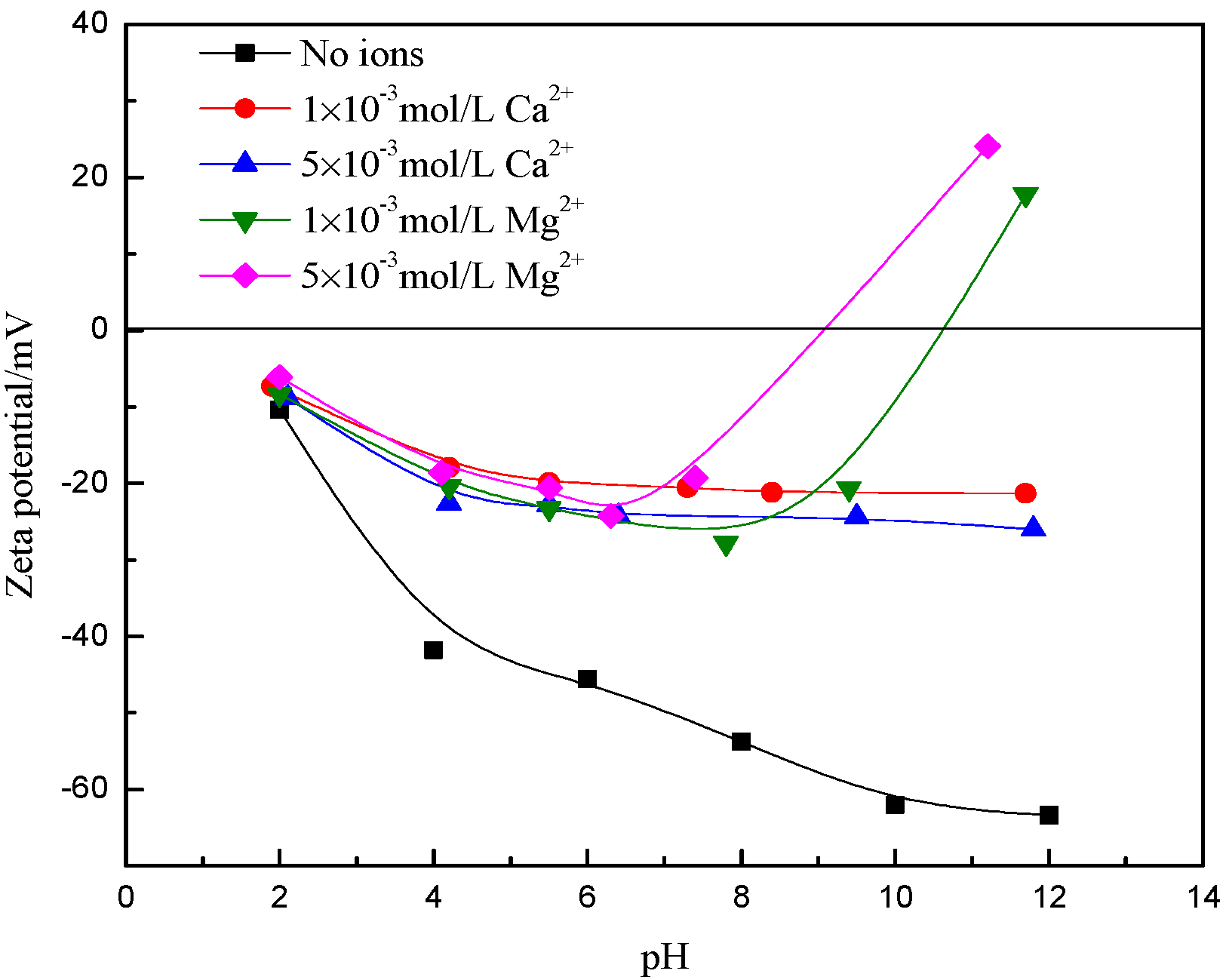
3.2. Zeta Potential Analysis
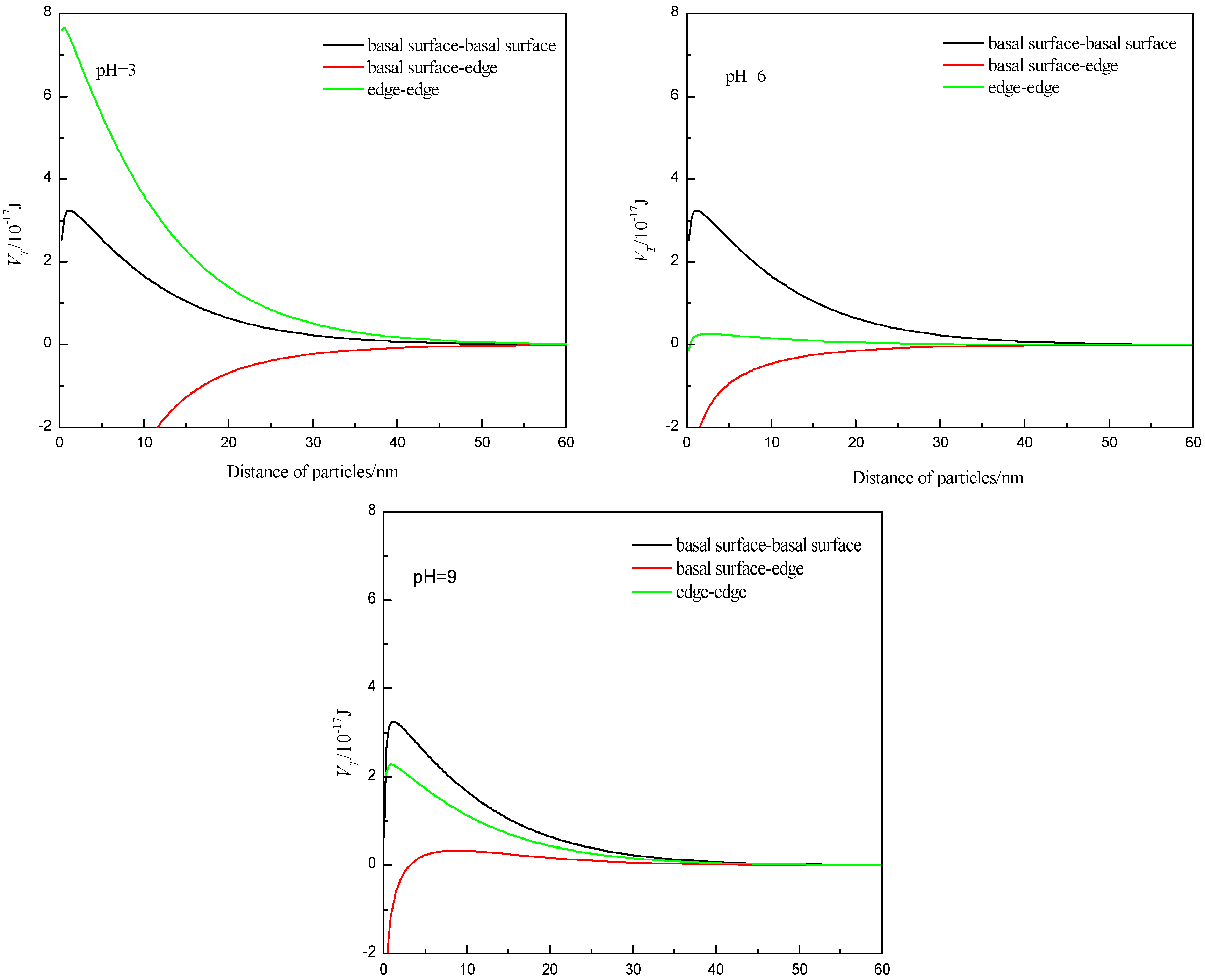
3.3. Calculation of Surface Energy of Particles Using Classic DLVO Theory
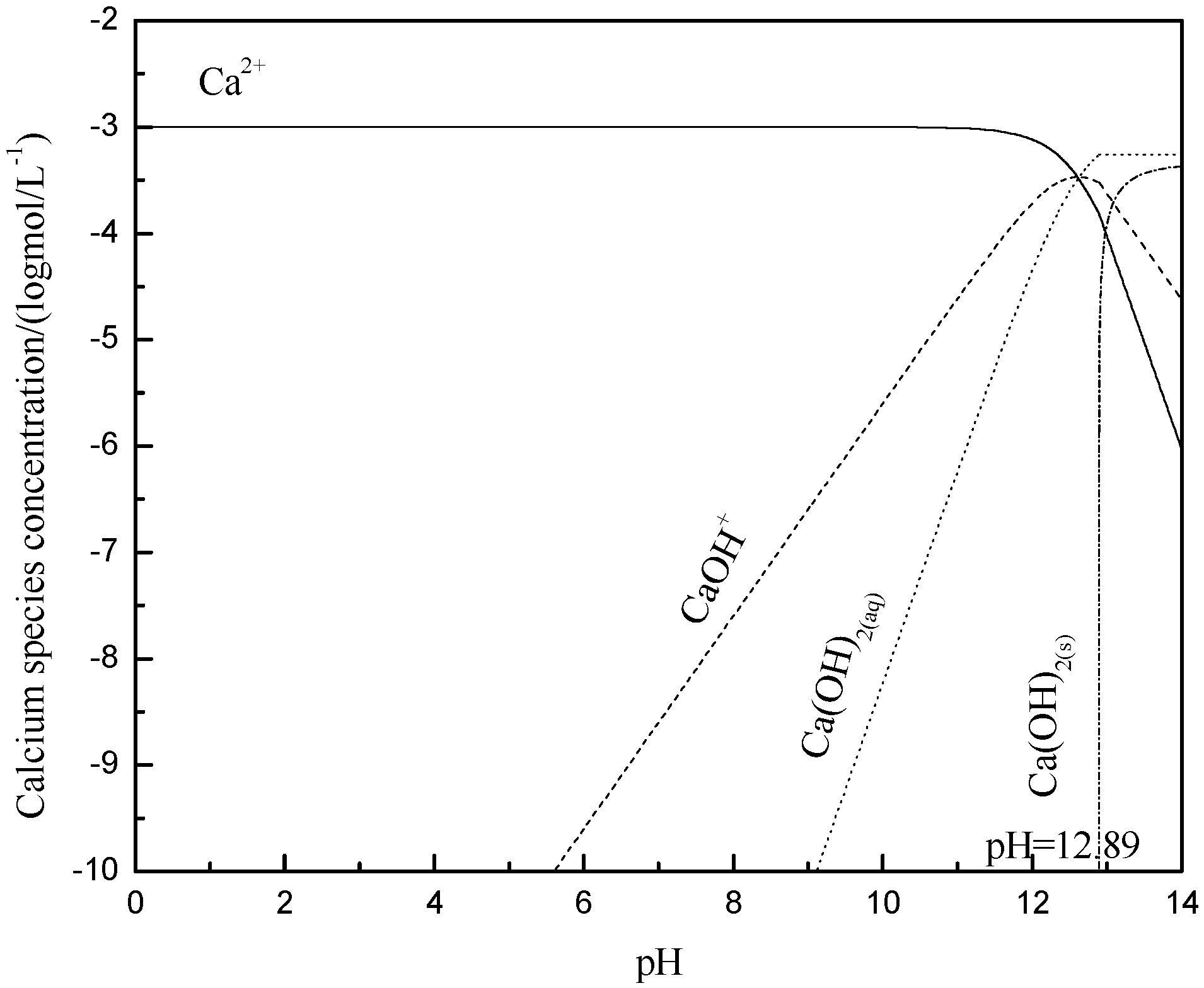
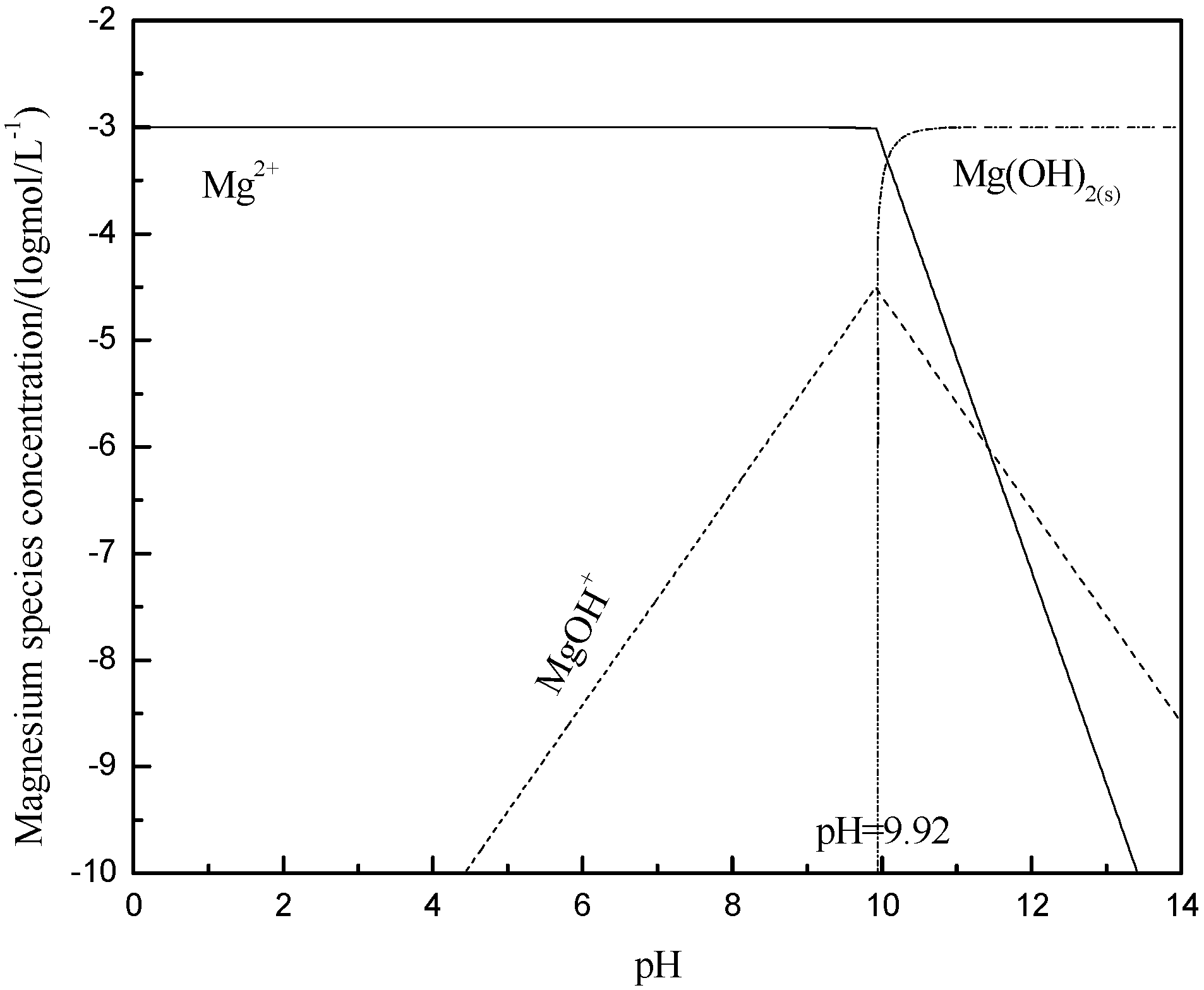
3.4. Metal Species in Different Solution
4. Conclusions
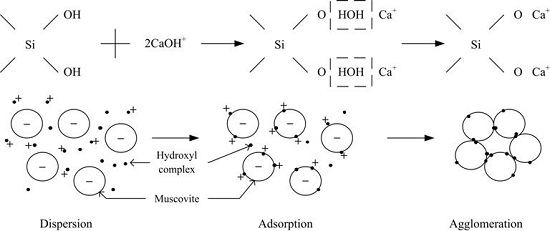
- The dispersion and flocculation behavior of muscovite in the absence of excess Ca2+ and Mg2+ agree with the classic DLVO theory based on the crystal anisotropy. The muscovite particles were aggregated in acidic suspension mainly by the form of basal surface-edge.
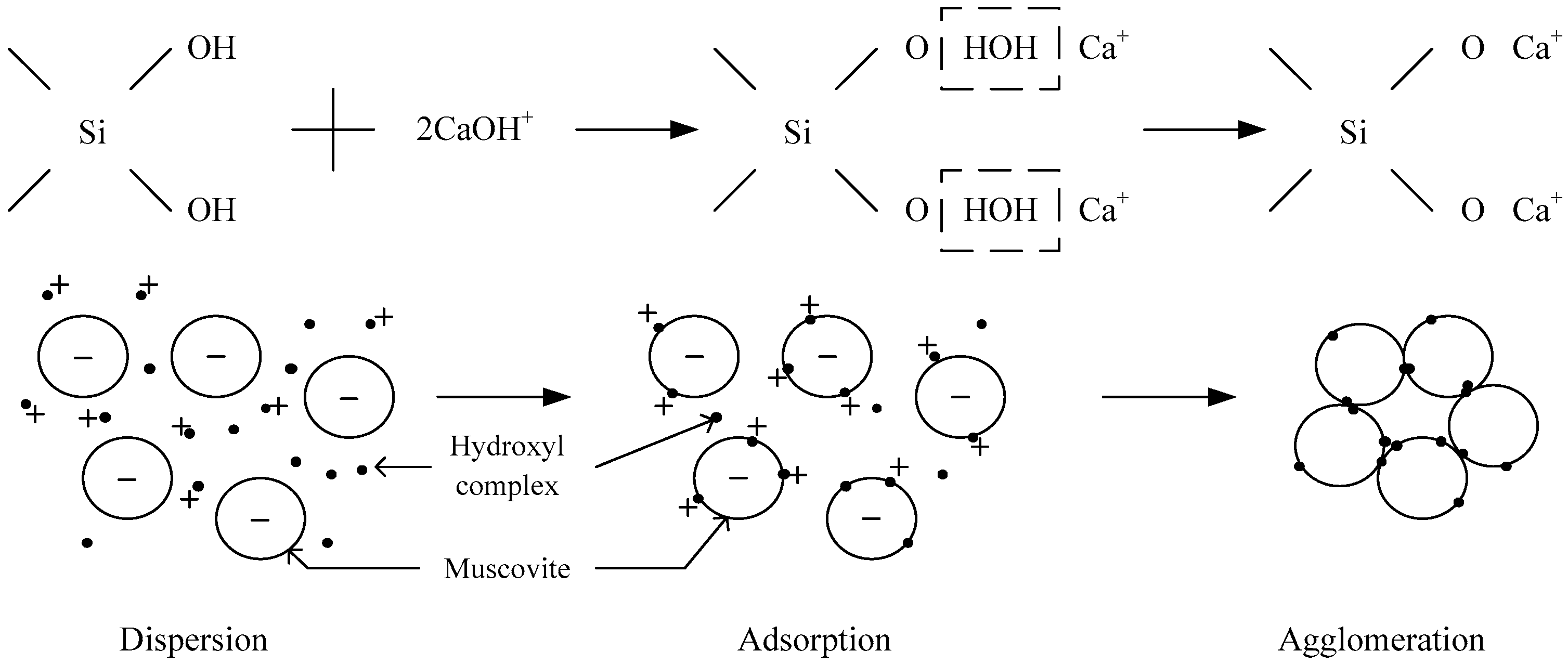
- The hydroxy complex of Ca2+ and Mg2+ was the main adsorption form on the mineral surface. The flocculation of muscovite was caused by a bridging effect of the hydroxy complex, which destroyed the excellent dispersion stability in alkaline suspension.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, Y.M.; Hu, Y.J.; Bao, S.X. Vanadium emission during roasting of vanadium-bearing stone coal in chlorine. Miner. Eng. 2012, 30, 95–98. [Google Scholar] [CrossRef]
- Zhu, X.B.; Zhang, Y.M.; Huang, J.; Liu, T.; Wang, Y. A kinetics study of multi-stage counter-current circulation acid leaching of vanadium from stone coal. Int. J. Miner. Process. 2012, 114–117, 1–6. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Zhang, Y.M.; Liu, T.; Chen, T.J.; Bian, Y.; Bao, S.X. Pre-concentration of vanadium from stone coal by gravity separation. Int. J. Miner. Process. 2013, 121, 1–5. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Zhang, Y.M.; Song, S.X.; Chen, T.J.; Bao, S.X. Behaviors of impurity elements Ca and Fe in vanadium-bearing stone coal during roasting and its control measure. Int. J. Miner. Process. 2016, 148, 100–104. [Google Scholar] [CrossRef]
- Valmacco, V.; Elzbieciak-Wodka, M.; Herman, D.; Trefalt, G.; Maroni, P.; Borkovec, M. Forces between silica particles in the presence of multivalent cations. J. Colloid Interface Sci. 2016, 472, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Ackler, H.D.; French, R.H.; Chiang, Y.M. Comparisons of Hamaker constants for ceramic systems with intervening vacuum or water: From force laws and physical properties. J. Colloid Interface Sci. 1996, 179, 460–469. [Google Scholar] [CrossRef]
- Elzbieciak-Wodka, M.; Popescu, M.N.; Ruiz-Cabello, F.J.M.; Trefalt, G.; Maroni, P.; Borkovec, M. Measurements of dispersion forces between colloidal latex particles with the atomic force microscope and comparison with Lifshitz theory. J. Chem. Phys. 2014, 140, 104906. [Google Scholar] [CrossRef] [PubMed]
- Morag, J.; Dishon, M.; Sivan, U. The governing role of surface hydration in ion specific adsorption to silica: An AFM-based account of the Hofmeister universality and its reversal. Langmuir 2013, 29, 6317–6322. [Google Scholar] [CrossRef] [PubMed]
- Popa, I.; Sinha, P.; Finessi, M.; Maroni, P.; Papastavrou, G.; Borkovec, M. Importance of charge regulation in attractive double-layer forces between dissimilar surfaces. Phys. Rev. Lett. 2010, 104, 228301. [Google Scholar] [CrossRef] [PubMed]
- Trefalt, G.; Behrens, S.H.; Borkovec, M. Charge Regulation in the Electrical Double Layer: Ion Adsorption and Surface Interactions. Langmuir 2015, 32, 380–400. [Google Scholar] [CrossRef] [PubMed]
- Dishon, M.; Zohar, O.; Sivan, U. From repulsion to attraction and back to repulsion: The effect of NaCl, KCl, and CsCl on the force between silica surfaces in aqueous solution. Langmuir 2009, 25, 2831–2836. [Google Scholar] [CrossRef] [PubMed]
- Pashley, R.M.; Israelachvili, J.N. DLVO and hydration forces between mica surfaces in Mg2+, Ca2+, Sr2+, and Ba2+ chloride solutions. J. Colloid Interface Sci. 1984, 97, 446–455. [Google Scholar] [CrossRef]
- Ruiz-Cabello, F.J.M.; Trefalt, G.; Csendes, Z.; Sinha, P.; Oncsik, T.; Szilagyi, I.; Maroni, P.; Borkovec, M. Predicting aggregation rates of colloidal particles from direct force measurements. J. Phys. Chem. B 2013, 117, 11853–11862. [Google Scholar] [CrossRef] [PubMed]
- Zohar, O.; Leizerson, I.; Sivan, U. Short range attraction between two similarly charged silica surfaces. Phys. Rev. Lett. 2006, 96, 177802. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Wang, Y.; Wang, J.; Xie, Z. Investigation on different behavior and mechanism of Ca(II) and Fe(III) adsorption on spodumene surface. Physicochem. Probl. Miner. 2014, 50, 535–550. [Google Scholar]
- Potapova, E.; Grahn, M.; Holmgren, A.; Hedlund, J. The effect of calcium ions and sodium silicate on the adsorption of a model anionic flotation collector on magnetite studied by ATR-FTIR spectroscopy. J. Colloid Interface Sci. 2010, 345, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Potapova, E.; Yang, X.; Grahn, M.; Holmgren, A.; Forsmo, S.P.E.; Fredriksson, A.; Hedlund, J. The effect of calcium ions, sodium silicate and surfactant on charge and wettability of magnetite. Colloids Surf. A Physicochem. Eng. Asp. 2011, 386, 79–86. [Google Scholar] [CrossRef]
- Dos Santos, M.A.; Santana, R.C.; Capponi, F.; Ataíde, C.H.; Barrozo, M.A. Effect of ionic species on the performance of apatite flotation. Sep. Purif. Technol. 2010, 76, 15–20. [Google Scholar] [CrossRef]
- Dos Santos, M.A.; Santana, R.C.; Capponi, F.; Ataíde, C.H.; Barrozo, M.A. Influence of the water composition on the selectivity of apatite flotation. Sep. Purif. Technol. 2012, 47, 606–612. [Google Scholar] [CrossRef]
- Fornasiero, D.; Ralston, J. Cu(II) and Ni(II) activation in the flotation of quartz, lizardite and chlorite. Int. J. Miner. Process. 2005, 76, 75–81. [Google Scholar] [CrossRef]
- Feng, Q.M.; Feng, B.; Lu, Y.P. Influence of copper ions and calcium ions on adsorption of CMC on chlorite. Trans. Nonferr. Met. Soc. China 2013, 23, 237–242. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Hu, Y.H.; Wang, Y.H. Effect of metallic ions on dispersibility of fine diaspore. Trans. Nonferr. Met. Soc. China 2011, 21, 1166–1171. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Wang, Y.H.; Hu, Y.H.; Sun, D.X.; Yu, M.J. Influence of metal ions on floatability of diaspore and kaolinite. J. Cent. South Univ. 2009, 40, 268–274. [Google Scholar]
- Wang, Y.H.; Chen, X.H.; Zhou, Y.L. Effect of metal ions on dispersity of fine aluminum silicate minerals. Met. Mine 2007, 5, 38–43. [Google Scholar]
- James, R.O.; Healy, T.W. Adsorption of hydrolyzable metal ions at the oxide—Water interface. III. A thermodynamic model of adsorption. J. Colloid Interface Sci. 1972, 40, 65–81. [Google Scholar] [CrossRef]
- Hu, Y.H.; Wang, D.Z. Mechanism of adsorption and activation flotation of metallic ion on oxide mineral-water interface. J. Cent. South Inst. Min. Metall. 1987, 5, 501–508. [Google Scholar]
- Yu, F.S.; Wang, Y.H.; Wang, J.M.; Xie, Z.F.; Zhang, L. First-principle investigation on mechanism of Ca ion activating flotation of spodumene. Rare Met. 2014, 33, 358–362. [Google Scholar] [CrossRef]
- Luo, Z.J.; Hu, Y.H.; Wang, Y.H.; Qiu, G.Z. Mechanism of dispersion and aggregation in reverse flotation for bauxite. Chin. J. Nonferr. Met. 2001, 11, 683–686. [Google Scholar]
- Qiu, G.Z.; Hu, Y.H.; Wang, D.Z. Interactions between Particles and Flotation of Fine Particles; Central South University: Changsha, China, 1993. [Google Scholar]
- Fuerstenau, D.W. Zeta potentials in the flotation of oxide and silicate minerals. Adv. Colloid Interface Sci. 2005, 114, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, S.; Tateyama, H.; Tsunematsu, K.; Jinnai, K. Zeta potential measurement of muscovite mica basal plane-aqueous solution interface by means of plane interface technique. J. Colloid Interface Sci. 1992, 152, 359–367. [Google Scholar] [CrossRef]
- Nosrati, A.; Addai-Mensah, J.; Skinner, W. Muscovite clay mineral particle interactions in aqueous media. Powder Technol. 2012, 219, 228–238. [Google Scholar] [CrossRef]
- Xu, L.; Wu, H.; Dong, F.; Wang, L.; Wang, Z.; Xiao, J. Flotation and adsorption of mixed cationic/anionic collectors on muscovite mica. Miner. Eng. 2013, 41, 41–45. [Google Scholar] [CrossRef]
- Liu, X.W. Crystal Structure and Surface Property of Diaspore and Phyllosilicate Minerals. Ph.D. Thesis, Central South University, Changsha, China, 2003. [Google Scholar]
- Krishnan, S.V.; Iwasaki, I. Floc formation in quartz—Mg(OH)2 system. Colloids Surf. 1985, 15, 89–100. [Google Scholar] [CrossRef]
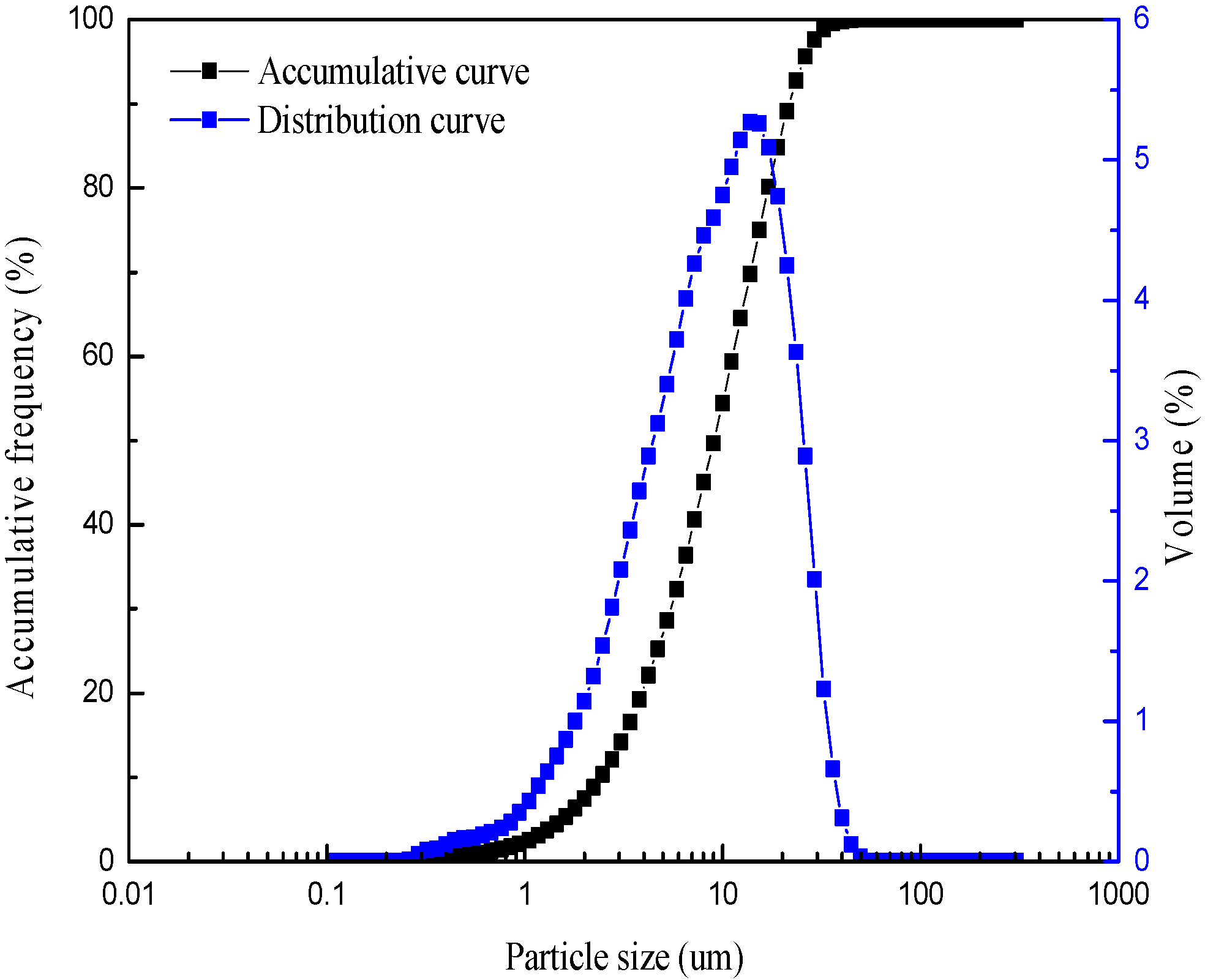

| Chemical Composition | Na2O | MgO | Al2O3 | SiO2 | K2O | Fe2O3 |
|---|---|---|---|---|---|---|
| Content | 0.50 | 0.92 | 30.64 | 46.95 | 10.57 | 5.12 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, J.; Zhang, Y.; Bao, S. The Effect of Ca2+ and Mg2+ on the Dispersion and Flocculation Behaviors of Muscovite Particles. Minerals 2016, 6, 93. https://doi.org/10.3390/min6030093
Tang J, Zhang Y, Bao S. The Effect of Ca2+ and Mg2+ on the Dispersion and Flocculation Behaviors of Muscovite Particles. Minerals. 2016; 6(3):93. https://doi.org/10.3390/min6030093
Chicago/Turabian StyleTang, Jiayan, Yimin Zhang, and Shenxu Bao. 2016. "The Effect of Ca2+ and Mg2+ on the Dispersion and Flocculation Behaviors of Muscovite Particles" Minerals 6, no. 3: 93. https://doi.org/10.3390/min6030093
APA StyleTang, J., Zhang, Y., & Bao, S. (2016). The Effect of Ca2+ and Mg2+ on the Dispersion and Flocculation Behaviors of Muscovite Particles. Minerals, 6(3), 93. https://doi.org/10.3390/min6030093