The Effect of Chloride Ions on the Activity of Cerussite Surfaces
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Dissolution Experiments
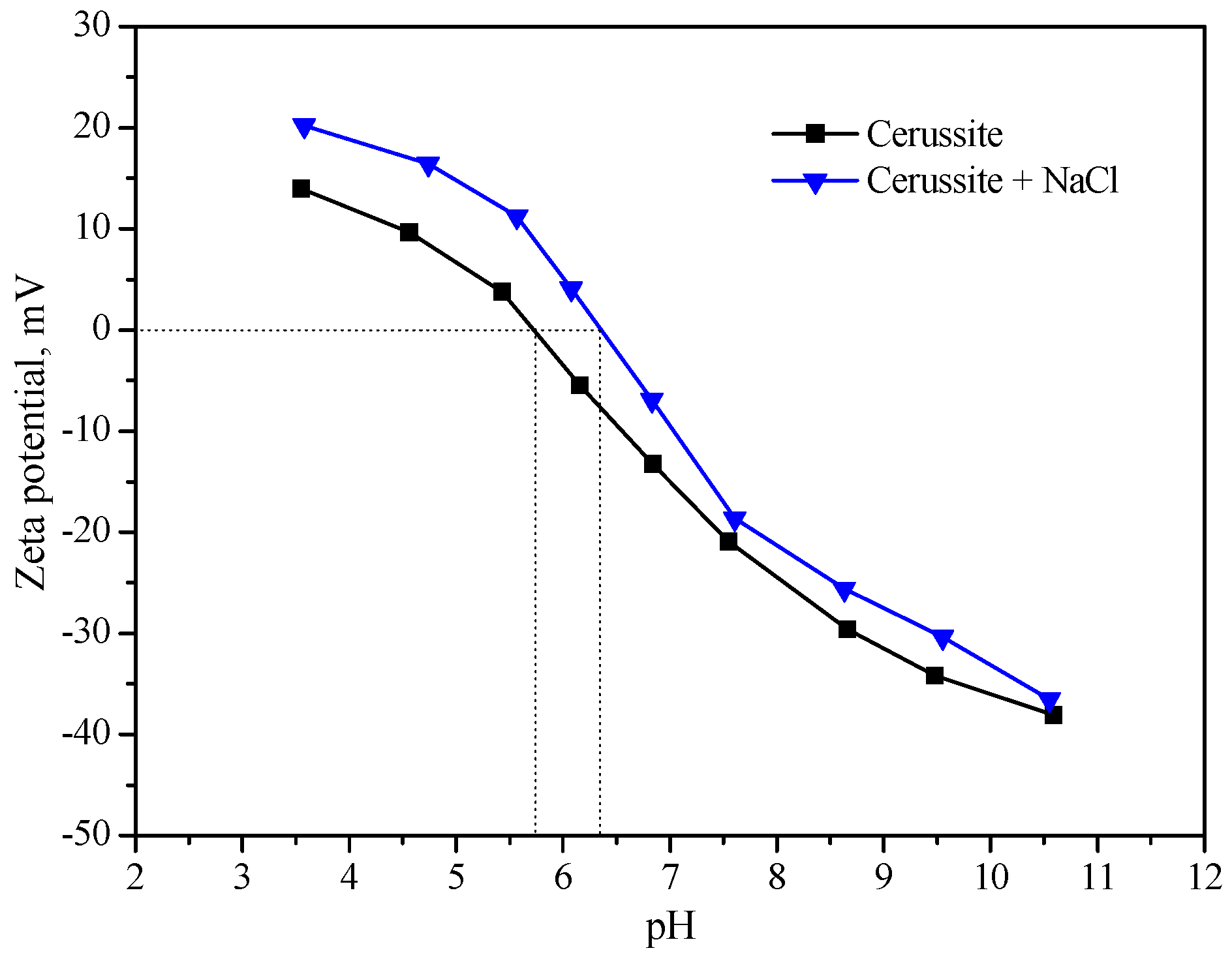
2.3. Zeta Potential Measurements
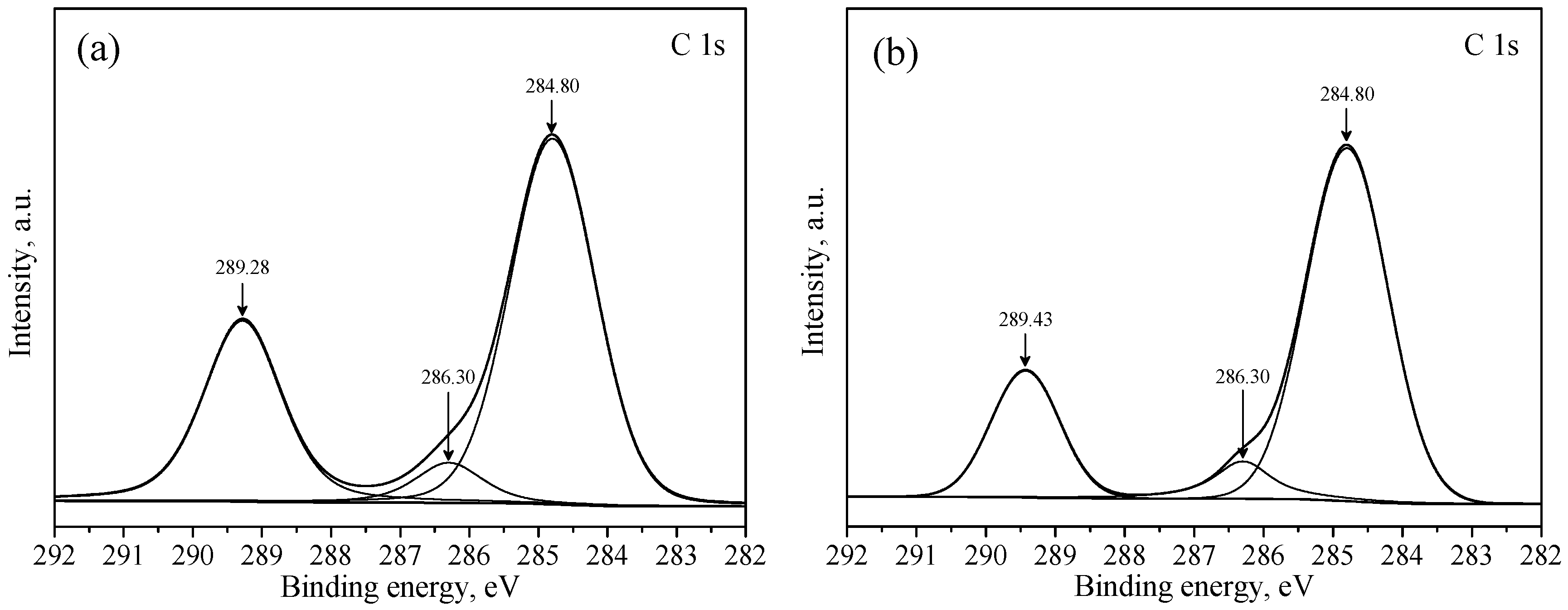
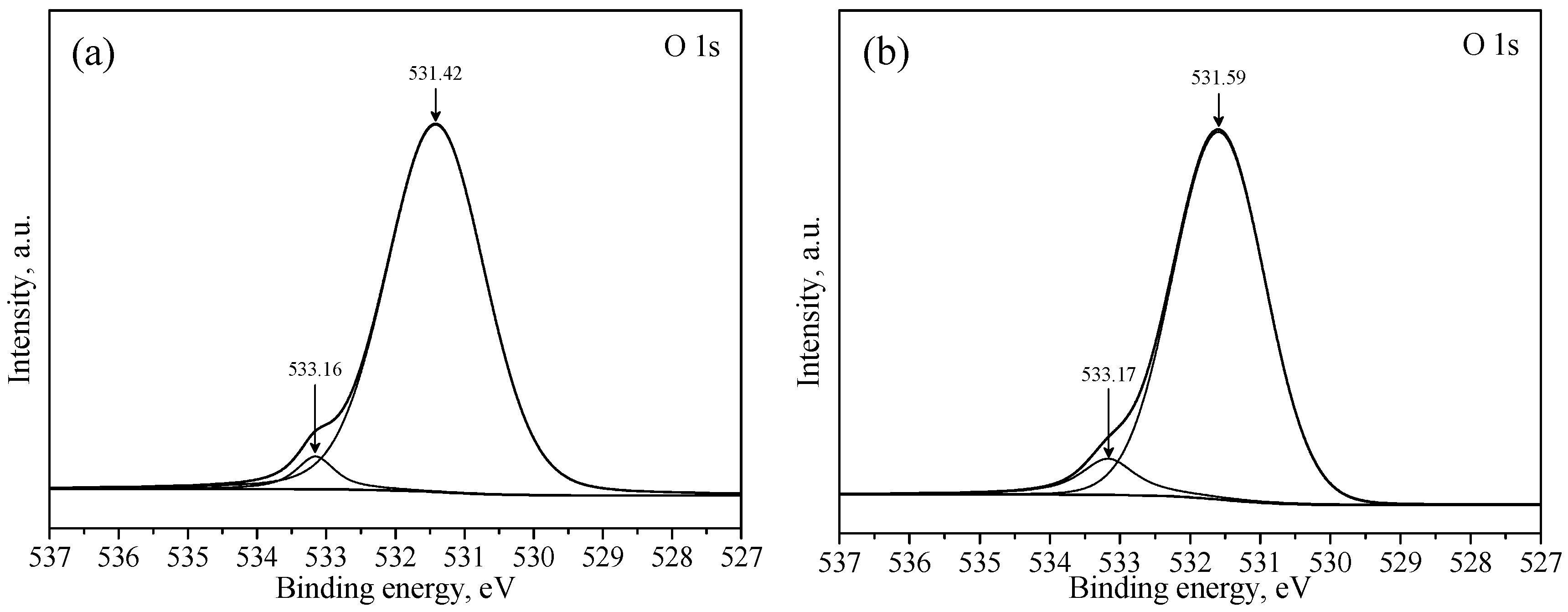
2.4. XPS Analysis
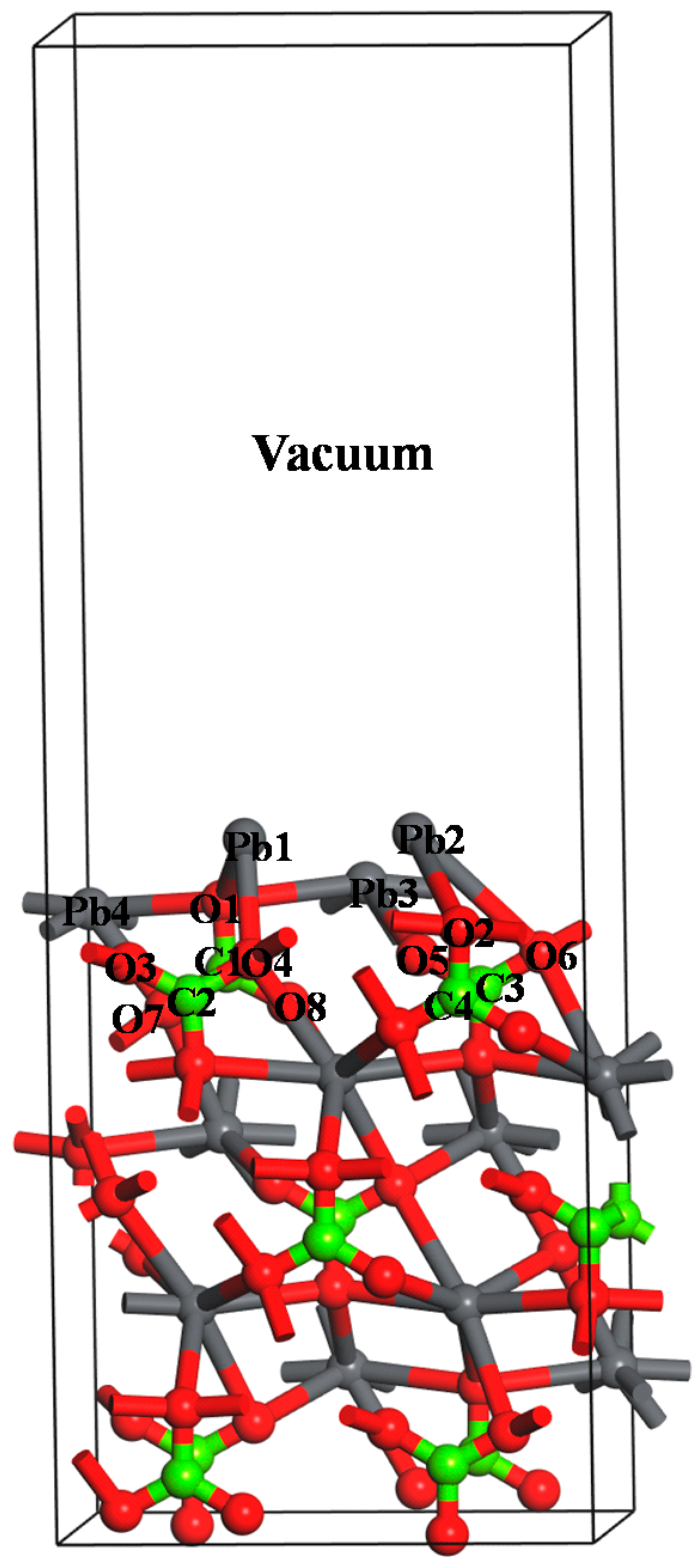
2.5. Computational Methods
3. Results and Discussion
3.1. Dissolution Experiments
3.2. Zeta Potential Measurements
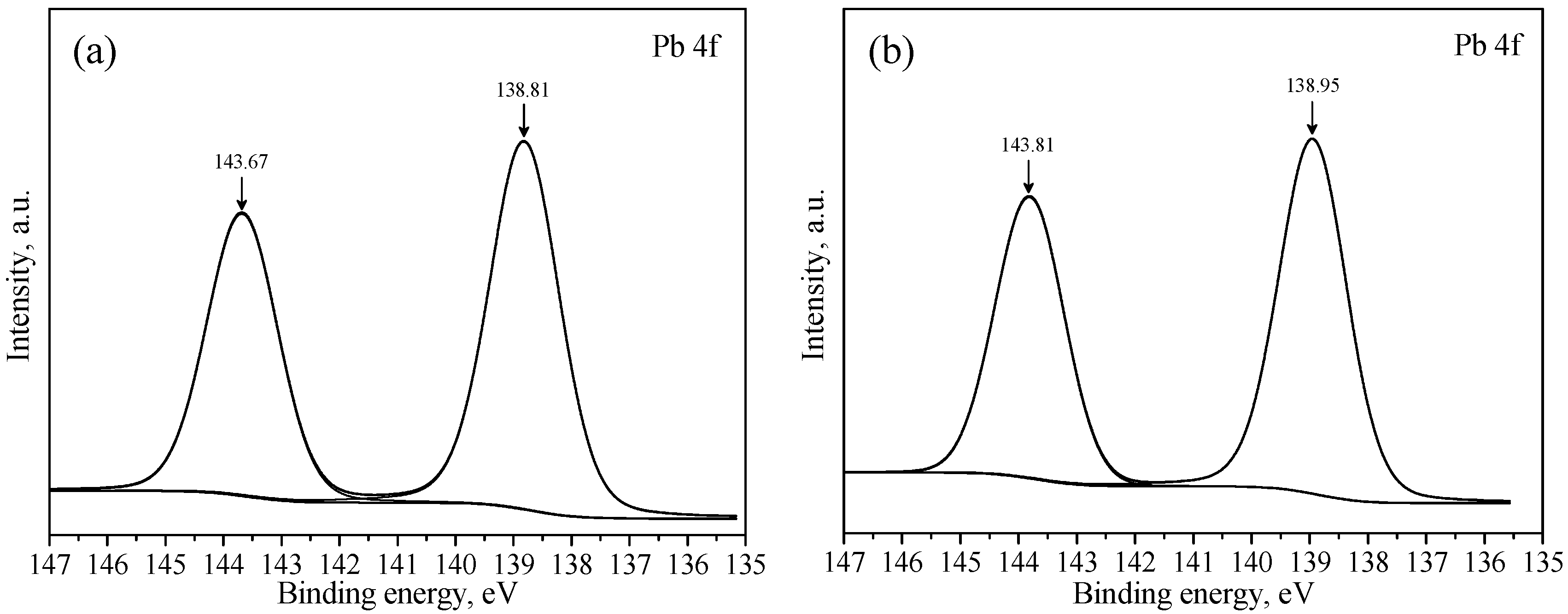
3.3. XPS Studies
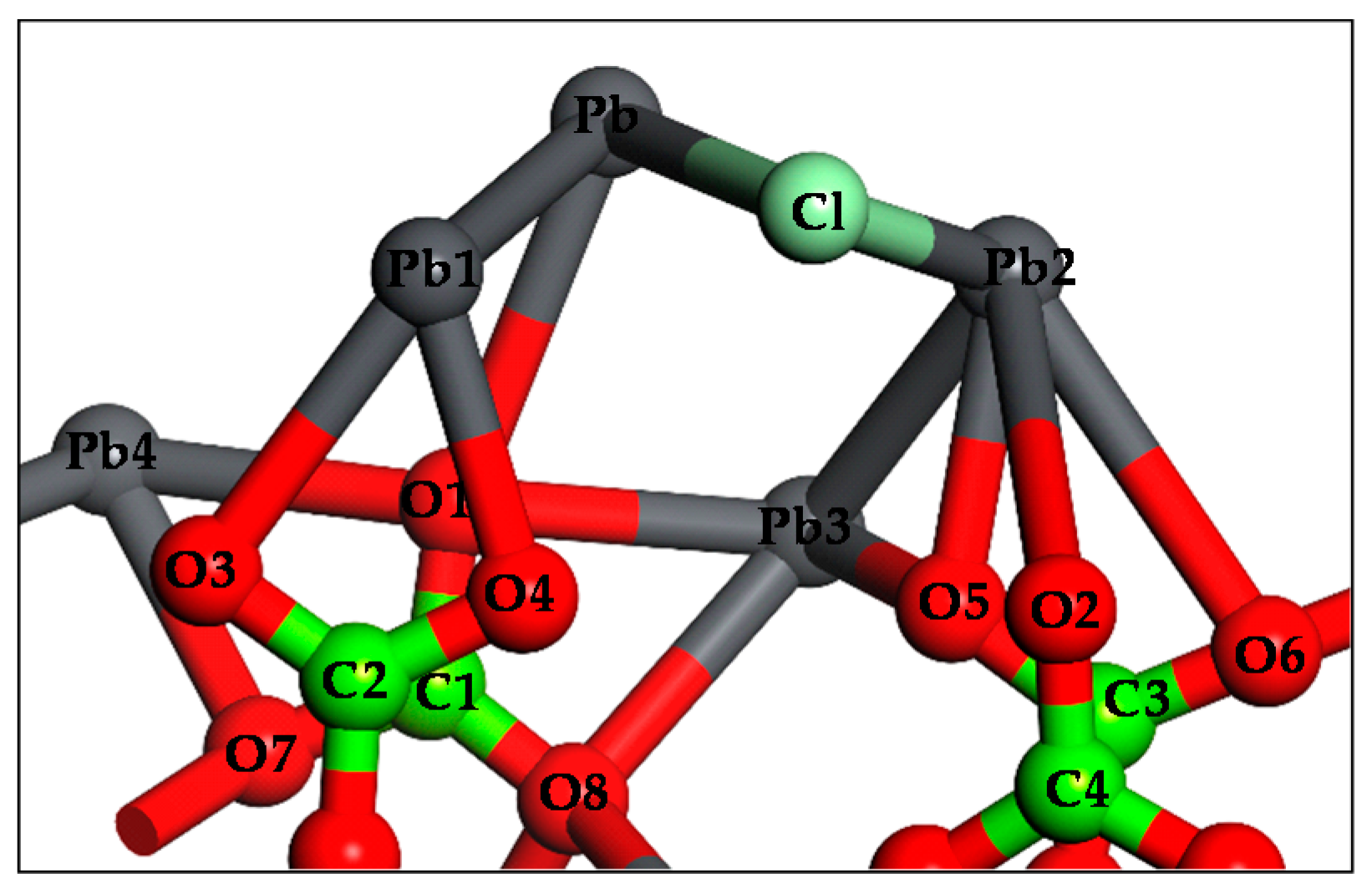
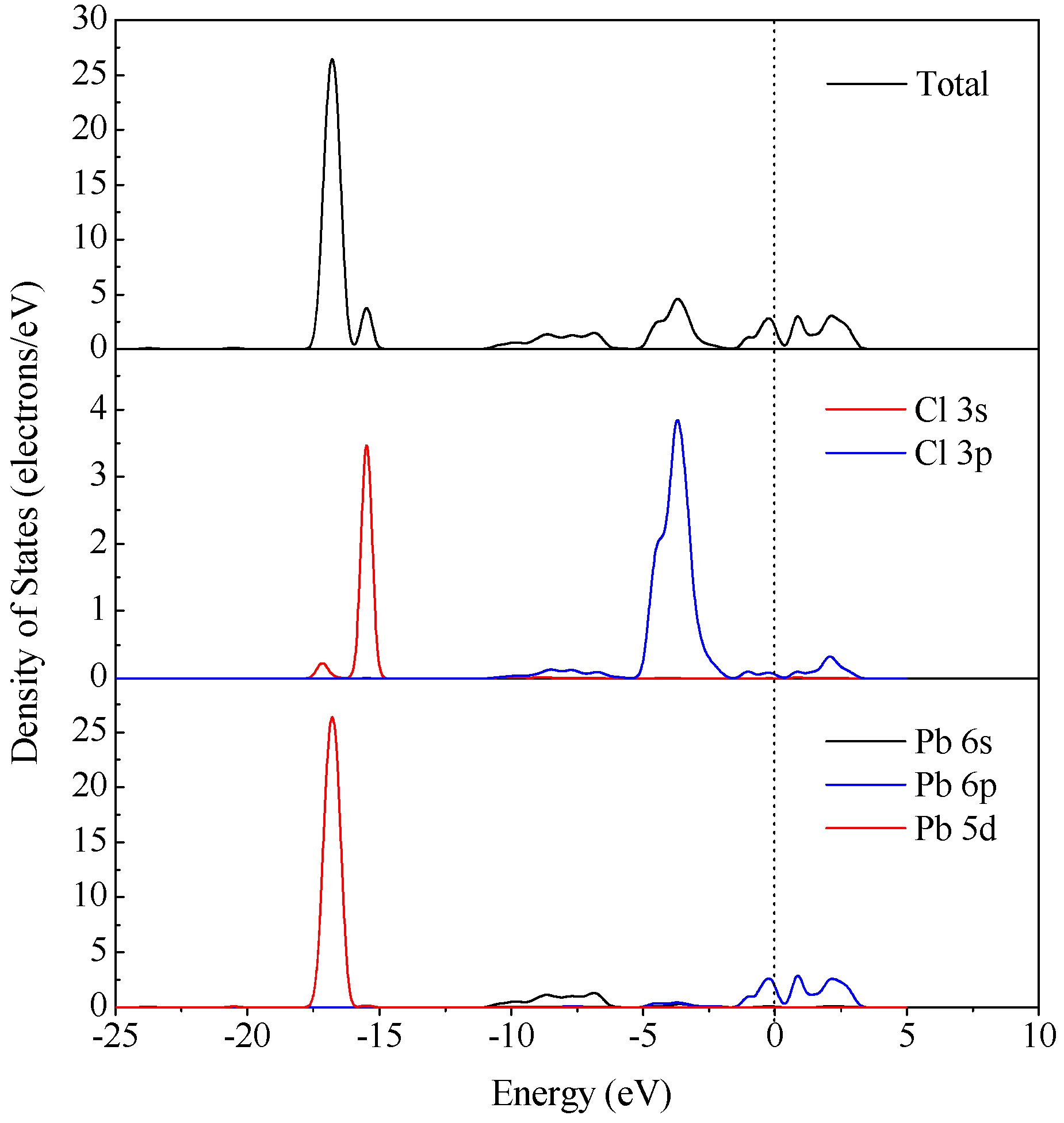
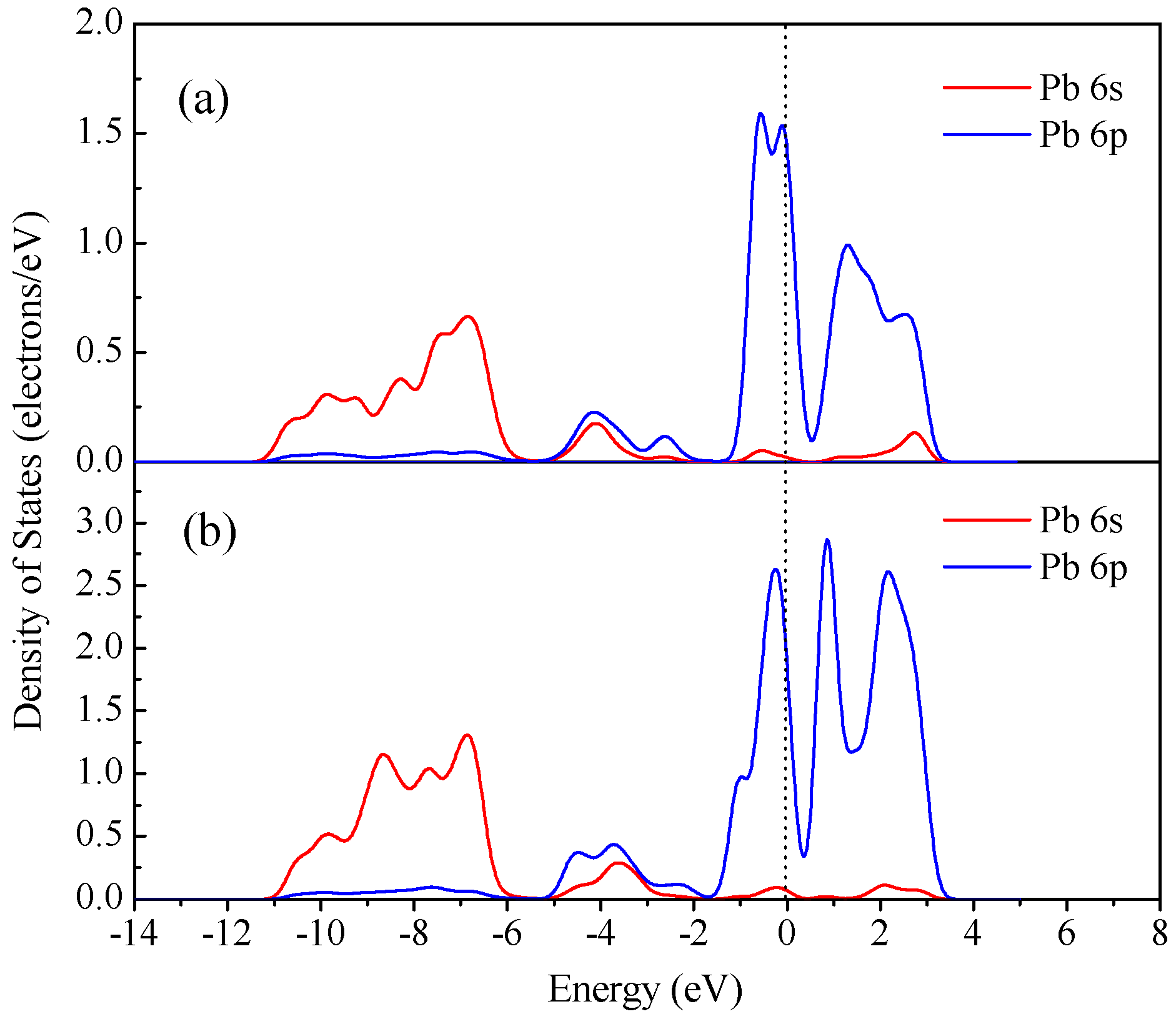
3.4. DFT Computation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, Y.; Wang, J.K.; Wei, C.; Liu, C.X.; Jiang, J.B.; Wang, F. Sulfidation roasting of low grade lead-zinc oxide ore with elemental sulfur. Miner. Eng. 2010, 23, 563–566. [Google Scholar] [CrossRef]
- Li, C.X.; Wei, C.; Deng, Z.G.; Li, X.B.; Li, M.T.; Xu, H.S. Hydrothermal sulfidation and flotation of oxidized zinc-lead ore. Metall. Mater. Trans. B 2014, 45, 833–838. [Google Scholar] [CrossRef]
- Feng, Q.C.; Wen, S.M.; Wang, Y.J.; Zhao, W.J.; Deng, J.S. Investigation of leaching kinetics of cerussite in sodium hydroxide solutions. Physicochem. Probl. Miner. Process. 2015, 51, 491–500. [Google Scholar]
- Fa, K.Q.; Miller, J.D.; Jiang, T.; Li, G.H. Sulphidization flotation for recovery of lead and zinc from oxide-sulfide ores. Trans. Nonferr. Met. Soc. China 2005, 15, 1138–1144. [Google Scholar]
- Gush, J.C.D. Flotation of oxide minerals by sulphidization—The development of a sulphidization control system for laboratory testwork. J. S. Afr. Inst. Min. Metall. 2005, 105, 193–197. [Google Scholar]
- Feng, Q.C.; Wen, S.M.; Zhao, W.J.; Liu, J.; Liu, D. Effect of pH on surface characteristics and flotation of sulfidized cerussite. Physicochem. Probl. Miner. Process. 2016, 52, 676–689. [Google Scholar]
- Yuan, W.Y.; Li, J.H.; Zhang, Q.W.; Saito, F. Mechanochemical sulfidization of lead oxides by grinding with sulfur. Powder Technol. 2012, 230, 63–66. [Google Scholar] [CrossRef]
- Ke, Y.; Chai, L.Y.; Liang, Y.J.; Min, X.B.; Yang, Z.H.; Chen, J.; Yuan, S. Sulfidation of heavy-metal-containing metallurgical residue in wet-milling processing. Miner. Eng. 2013, 53, 136–143. [Google Scholar] [CrossRef]
- Ke, Y.; Chai, L.Y.; Min, X.B.; Tang, C.J.; Zhou, B.S.; Chen, J.; Yuan, C.Y. Behavior and effect of calcium during hydrothermal sulfidation and flotation of zinc-calcium-based neutralization sludge. Miner. Eng. 2015, 74, 68–78. [Google Scholar] [CrossRef]
- Han, H.S.; Sun, W.; Hu, Y.H.; Jia, B.L.; Tang, H.H. Anglesite and silver recovery from jarosite residues through roasting and sulfidization-flotation in zinc hydrometallurgy. J. Hazard. Mater. 2014, 278, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.C.; Wen, S.M.; Zhao, W.J.; Wang, Y.J.; Cui, C.F. Contribution of chloride ions to the sulfidization flotation of cerussite. Miner. Eng. 2015, 83, 128–135. [Google Scholar] [CrossRef]
- Bargar, J.R.; Brown, G.E.; Parks, G.A. Surface complexation of Pb(II) at oxide-water interfaces: III. XAFS determination of Pb(II) and Pb(II)-chloro adsorption complexes on goethite and alumina. Geochim. Cosmochim. Acta 1998, 62, 193–207. [Google Scholar] [CrossRef]
- Guo, Y.B.; Li, C.; Liu, Y.C.; Yu, L.M.; Ma, Z.Q.; Liu, C.X.; Li, H.J. Effect of microstructure variation on the corrosion behavior of high-strength low-alloy steel in 3.5 wt % NaCl solution. Int. J. Miner. Metall. Mater. 2015, 22, 604–612. [Google Scholar] [CrossRef]
- Antao, S.M.; Hassan, I. The orthorhombic structure of CaCO3, SrCO3, PbCO3 and BaCO3: Linear structural trends. Can. Mineral. 2009, 47, 1245–1255. [Google Scholar] [CrossRef]
- Payne, M.C.; Teter, M.P.; Allan, D.C.; Arias, T.A.; Joannopoulos, J.D. Iterative minimization techniques for ab initio total-energy calculations: Molecular dynamics and conjugate gradients. Rev. Mod. Phys. 1992, 64, 1045–1097. [Google Scholar] [CrossRef]
- Perdew, J.P.; Ruzsinszky, A.; Csonka, G.I.; Vydrov, O.A.; Scuseria, G.E.; Constantin, L.A.; Zhou, X.L.; Burke, K. Restoring the density-gradient expansion for exchange in solids and surfaces. Phys. Rev. Lett. 2008, 100, 136406. [Google Scholar] [CrossRef] [PubMed]
- Powell, K.J.; Brown, P.L.; Byrne, R.H.; Gajda, T.; Hefter, G.; Leuz, A.K.; Sjöberg, S.; Wanner, H. Chemical speciation of environmentally significant metals with inorganic ligands. Part 3: The Pb2+ + OH−, Cl−, CO32−, SO42−, and PO43− systems (IUPAC Technical Report). Pure Appl. Chem. 2009, 81, 2425–2476. [Google Scholar] [CrossRef]
- Shirota, Y.; Niki, K.; Shindo, H. Stabilities of crystal faces of aragonite-type strontianite (SrCO3) and cerussite (PbCO3) compared by AFM observation of facet formation in acid. J. Cryst. Growth 2011, 324, 190–195. [Google Scholar] [CrossRef]
- Feng, Q.C.; Wen, S.M.; Zhao, W.J.; Deng, J.S.; Xian, Y.J. Adsorption of sulfide ions on cerussite surfaces and implications for flotation. Appl. Surf. Sci. 2016, 360, 365–372. [Google Scholar] [CrossRef]
- Shi, Q.; Zhang, G.F.; Feng, Q.M.; Deng, H. Effect of solution chemistry on the flotation system of smithsonite and calcite. Int. J. Miner. Process. 2013, 119, 34–39. [Google Scholar] [CrossRef]
- Feng, B.; Feng, Q.M.; Lu, Y.P. The effect of lizardite surface characteristics on pyrite flotation. Appl. Surf. Sci. 2012, 259, 153–158. [Google Scholar] [CrossRef]
- Li, F.X.; Zhong, H.; Xu, H.F.; Jia, H.; Liu, G.Y. Flotation behavior and adsorption mechanism of α-hydroxyoctyl phosphinic acid to malachite. Miner. Eng. 2015, 71, 188–193. [Google Scholar] [CrossRef]
- Fuerstenau, M.C.; Olivas, S.A.; Herrera-Urbina, R.; Han, K.N. The surface characteristics and flotation behavior of anglesite and cerussite. Int. J. Miner. Process. 1987, 20, 73–85. [Google Scholar] [CrossRef]
- Popov, S.R.; Vučinić, D.R. The effect of prolonged agitation in lead ion solution on ethylxanthate adsorption and surface characteristics of cerussite. Int. J. Miner. Process. 1992, 35, 85–100. [Google Scholar] [CrossRef]
- Popov, S.R.; Vučinić, D.R. Kinetics of ethylxanthate adsorption on cerussite in alkaline media in presence or absence of dissolved lead ions. Int. J. Miner. Process. 1994, 41, 115–128. [Google Scholar] [CrossRef]
- Grano, S.R.; Prestidge, C.A.; Ralston, J. Sulphite modification of galena surfaces and its effect on flotation and xanthate adsorption. Int. J. Miner. Process. 1997, 52, 1–29. [Google Scholar] [CrossRef]
- Buckley, A.N.; Goh, S.W.; Lamb, R.N.; Woods, R. Interaction of thiol collectors with pre-oxidised sulfide minerals. Int. J. Miner. Process. 2003, 72, 163–174. [Google Scholar] [CrossRef]
- Nowak, P.; Laajalehto, K. On the interpretation of the XPS spectra of adsorbed layers of flotation collectors-ethyl xanthate on metallic lead. Physicochem. Probl. Miner. Pross. 2007, 41, 107–116. [Google Scholar]
- Cozza, C.; Castro, V.D.; Polzonetti, G.; Marabini, A.M. An X-ray photoelectron spectroscopy (XPS) study of the interaction of mercapto-benzo-thiazole with cerussite. Int. J. Miner. Process. 1992, 34, 23–32. [Google Scholar] [CrossRef]
- Chanturia, V.A.; Bunin, I.Z.; Ryazantseva, M.V.; Khabarova, I.A.; Koporulina, E.V.; Anashkina, N.E. Surface activation and induced change of physicochemical and process properties of galena by nanosecond electromagnetic pulses. J. Min. Sci. 2014, 50, 573–586. [Google Scholar] [CrossRef]
| Time, min | Concentrations of Pb in Various Systems, mg/L | |||
|---|---|---|---|---|
| Deionized Water | NaCl | Na2S | NaCl + Na2S | |
| 1 | 1.5166 | 1.4208 | 0.2579 | 0.0382 |
| 3 | 1.9698 | 1.7309 | 0.2356 | 0.0289 |
| 6 | 2.2364 | 2.0611 | 0.2033 | 0.0297 |
| 10 | 2.6481 | 2.7033 | 0.3154 | 0.0187 |
| 15 | 3.1456 | 2.9109 | 0.1197 | 0.0384 |
| 20 | 3.2942 | 3.1346 | 0.1245 | 0.0456 |
| 30 | 3.3803 | 3.2844 | 0.2336 | 0.0353 |
| 45 | 3.6342 | 3.1327 | 0.1355 | 0.0322 |
| 60 | 3.8023 | 3.0532 | 0.1639 | 0.0246 |
| Samples | Atomic Concentration, % | Atomic Concentration Ratio | |||
|---|---|---|---|---|---|
| C 1s | O 1s | Pb 4f | Pb/C | Pb/O | |
| a | 19.31 | 60.45 | 20.23 | 1.05 | 0.33 |
| b | 18.11 | 58.93 | 22.96 | 1.27 | 0.39 |
| Samples | Binding Energy, eV | Chemical Shift, eV | ||||
|---|---|---|---|---|---|---|
| C 1s | O 1s | Pb 4f | C 1s | O 1s | Pb 4f | |
| a | 289.28 | 531.42 | 138.81 | - | - | - |
| b | 289.43 | 531.59 | 138.95 | 0.15 | 0.17 | 0.14 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Q.; Wen, S.; Cao, Q.; Deng, J.; Zhao, W. The Effect of Chloride Ions on the Activity of Cerussite Surfaces. Minerals 2016, 6, 92. https://doi.org/10.3390/min6030092
Feng Q, Wen S, Cao Q, Deng J, Zhao W. The Effect of Chloride Ions on the Activity of Cerussite Surfaces. Minerals. 2016; 6(3):92. https://doi.org/10.3390/min6030092
Chicago/Turabian StyleFeng, Qicheng, Shuming Wen, Qinbo Cao, Jiushuai Deng, and Wenjuan Zhao. 2016. "The Effect of Chloride Ions on the Activity of Cerussite Surfaces" Minerals 6, no. 3: 92. https://doi.org/10.3390/min6030092
APA StyleFeng, Q., Wen, S., Cao, Q., Deng, J., & Zhao, W. (2016). The Effect of Chloride Ions on the Activity of Cerussite Surfaces. Minerals, 6(3), 92. https://doi.org/10.3390/min6030092






