The Role of Chloride Ions during the Formation of Akaganéite Revisited
Abstract
:1. Introduction
2. Experimental Section
2.1. General
2.2. Titrations
2.3. Isolation and Characterization of Precipitates
3. Results and Discussion
3.1. Binding of Chloride Ions during the Early Stages of Hydrolysis
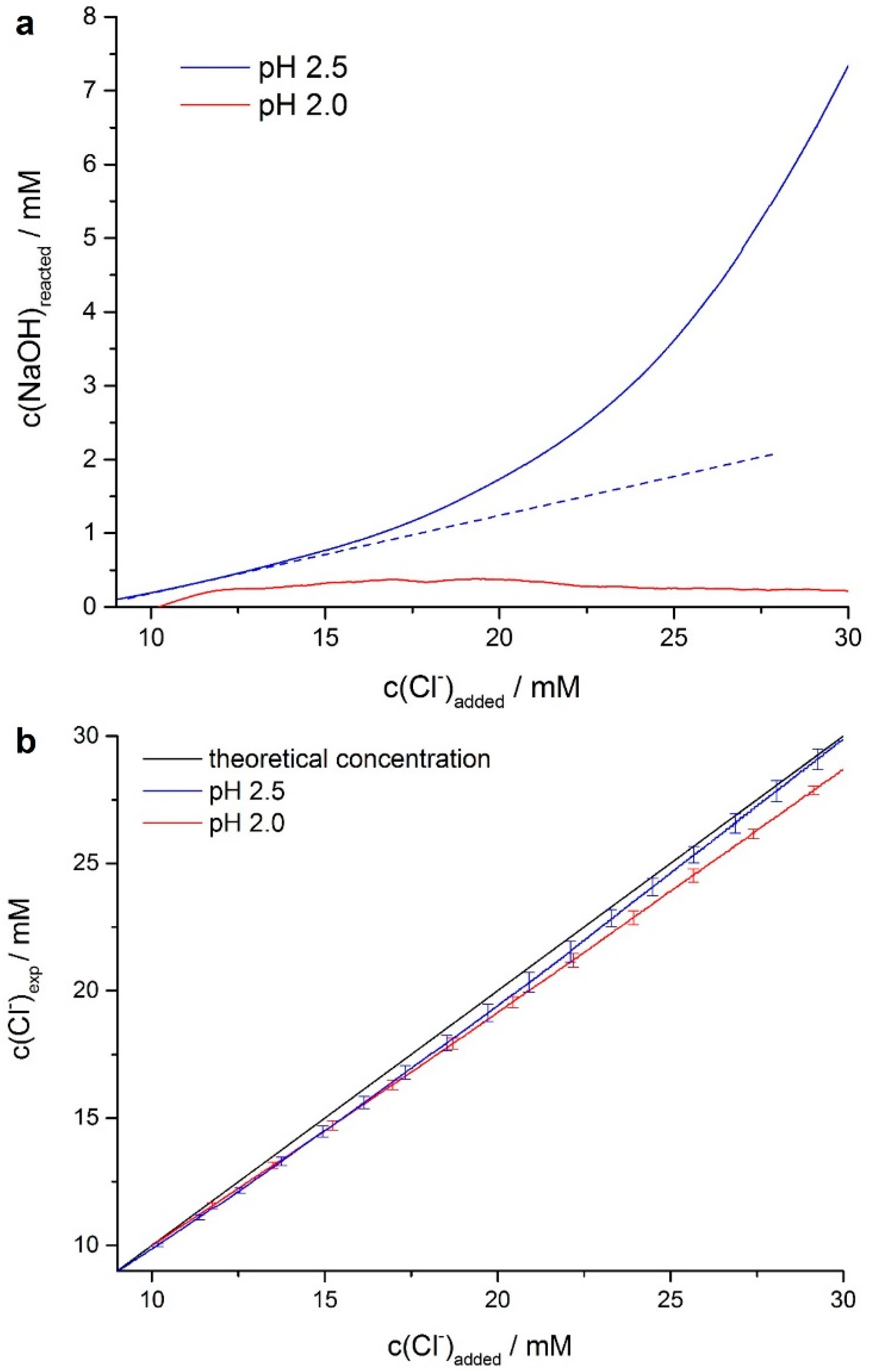
3.2. Chloride Content of the Formed Precipitates

3.3. Influence of Chloride Ions on the Mechanism of Hydrolysis

4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cornell, R.M.; Schwertmann, U. The Iron Oxides, 1st ed.; Wiley-VCH: Weinheim, Germany, 1997; pp. 488–495. [Google Scholar]
- Blesa, M.A.; Matijevic, E. Phase transfomations of iron oxides, oxohydroxides, and hydrous oxides in aqueous media. Adv. Colloid Interface Sci. 1989, 29, 173–221. [Google Scholar] [CrossRef]
- Murphy, P.J.; Posner, A.M.; Quirk, J.P. Characterization of partially neutralized ferric chloride solutions. J. Colloid Interface Sci. 1976, 56, 284–297. [Google Scholar] [CrossRef]
- Murphy, P.J.; Posner, A.M.; Quirk, J.P. Characterization of partially neutralized ferric perchlorate solutions. J. Colloid Interface Sci. 1976, 56, 298–311. [Google Scholar] [CrossRef]
- Murphy, P.J.; Posner, A.M.; Quirk, J.P. Characterization of hydrolyzed ferric ion solutions a comparison of the effect of various anions on the solutions. J. Colloid Interface Sci. 1976, 56, 312–319. [Google Scholar] [CrossRef]
- Jolivet, J.P.; Chaneac, C.; Tronc, E. Iron oxide chemistry. From molecular clusters to extended solid networks. Chem. Commun. 2004, 5, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Flynn, C.M. Hydrolysis of inorganic iron(III) salts. Chem. Rev. 1984, 84, 31–41. [Google Scholar] [CrossRef]
- Schneider, W. Hydrolysis of iron(III)… Chaotic olation versus nucleation. Comments Inorg. Chem. 1984, 3, 205–223. [Google Scholar] [CrossRef]
- Schwertmann, U.; Friedl, J.; Pfab, G. A New Iron(III) Oxyhydroxynitrate. J. Solid State Chem. 1996, 126, 336. [Google Scholar] [CrossRef]
- Schwertmann, U.; Friedl, J.; Stanjek, H. From Fe(III) Ions to Ferrihydrite and then to Hematite. J. Colloid Interface Sci. 1999, 209, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.; Vantelon, D.; Montargès-Pelletier, E.; Villain, F.; Gardoll, O.; Razafitianamaharavo, A.; Jaafar, G. Interaction of Fe(III) and Al(III) during hydroxylation by forced hydrolysis: The nature of Al–Fe oxyhydroxy co-precipitates. J. Colloid Interface Sci. 2013, 407, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Seder-Colomina, M.; Morin, G.; Benzerara, K.; Ona-Nguema, G.; Pernelle, J.-J.; Esposito, G.; van Hullebusch, E.D. Sphaerotilus natans, a Neutrophilic Iron-Related Sheath-Forming Bacterium: Perspectives for Metal Remediation Strategies. Geomicrobiol. J. 2014, 31, 64–75. [Google Scholar] [CrossRef]
- Straub, K.L.; Benz, M.; Schink, B. Iron metabolism in anoxic environments at near neutral pH. FEMS Microbiol. Ecol. 2001, 3, 181–186. [Google Scholar] [CrossRef]
- Konhauser, K.O. Diversity of bacterial iron mineralization. Earth-Sci. Rev. 1997, 43, 91–121. [Google Scholar] [CrossRef]
- Faivre, D.; Godec, T.U. From Bacteria to Mollusks: The Principles Underlying the Biomineralization of Iron Oxide Materials. Angew. Chem. Int. Ed. 2015, 54, 4728–4747. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.; Fakra, S.C.; Edwards, D.C.; Emerson, D.; Banfield, J.F. Iron oxyhydroxide mineralization on microbial extracellular polysaccharides. Geochim. Cosmochim. Acta 2009, 73, 3807–3818. [Google Scholar] [CrossRef]
- Brayner, R.; Yéprémian, C.; Djediat, C.; Coradin, T.; Herbst, F.; Livage, J.; Fiévet, F.; Couté, A. Photosynthetic Microorganism-Mediated Synthesis of Akaganeite (β-FeOOH) Nanorods. Langmuir 2009, 25, 10062–10067. [Google Scholar] [CrossRef] [PubMed]
- Warren, L.; Ferris, F.G. Continuum between Sorption and Precipitation of Fe(III) on Microbial Surfaces. Environ. Sci. Technol. 1998, 32, 2331–2337. [Google Scholar] [CrossRef]
- Bäuerlein, E. Biomineralization of Unicellular Organisms: An Unusual Membrane Biochemistry for the Production of Inorganic Nano- and Microstructures. Angew. Chem. Int. Ed. 2003, 42, 614–641. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Lee, I.; Roh, Y. Biomineralization of a poorly crystalline Fe(III) oxide, akaganeite, by an anaerobic Fe(III)-reducing bacterium (Shewanella alga) isolated from marine environment. Geosci. J. 2003, 7, 217–226. [Google Scholar] [CrossRef]
- Atkinson, R.J.; Posner, A.M.; Quirk, J.P. Crystal nucleation and growth in hydrolysing iron(III) chloride solutions. Clays Clay Miner. 1977, 25, 49–56. [Google Scholar] [CrossRef]
- Cai, J.; Liu, J.; Gao, Z.; Navrotsky, A.; Suib, S.L. Synthesis and anion exchange of tunnel structure akaganeite. Chem. Mater. 2001, 15, 4595–4602. [Google Scholar] [CrossRef]
- Mazeina, L.; Deore, S.; Navrotsky, A. Energetics of bulk and nano-akaganeite, β-FeOOH: Enthalpy of formation, surface enthalpy, and enthalpy of water adsorption. Chem. Mater. 2006, 18, 1830–1838. [Google Scholar] [CrossRef]
- Kolbe, F.; Weiss, H.; Morgenstern, P.; Wennrich, R.; Lorenz, W.; Schurk, K.; Stanjek, H.; Daus, B. Sorption of aqueous antimony and arsenic species onto akaganeite. J. Colloid Interface Sci. 2011, 357, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Dousma, J.; van den Hoven, T.J.; De Bruyn, P.L. The influence of chloride ions on the formation of iron(III) oxyhydroxide. J. Inorg. Nucl. Chem. 1977, 40, 1089–1093. [Google Scholar] [CrossRef]
- Bottero, J.Y.; Manceau, A.; Villieras, F.; Tchoubar, D. Structure and mechanisms of formation of FeOOH(Cl) polymers. Langmuir 1994, 10, 316–319. [Google Scholar] [CrossRef]
- Zhang, H.; Waychunas, G.A.; Banfield, J.F. Molecular dynamics simulation study of the early stages of nucleation of iron oxyhydroxide nanoparticles in aqueous solutions. J. Phys. Chem. B 2015, 119, 10630–10642. [Google Scholar] [CrossRef] [PubMed]
- Combes, J.M.; Manceau, A.; Calas, G.; Bottero, J.Y. Formation of ferric oxides from aqueous solutions: A polyhedral approach by x-ray absorption spectroscopy: I. Hydrolysis and formation of ferric gels. Geochim. Cosmochim. Acta 1989, 53, 583–594. [Google Scholar] [CrossRef]
- Manceau, A. Comment on “Direct observation of tetrahedrally coordinated Fe(III) in ferrihydrite”. Environ. Sci. Technol. 2012, 46, 6882–6884. [Google Scholar] [CrossRef] [PubMed]
- Brinker, C.J.; Scherer, W.G. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing; Gulf Professional Publishing: Houston, TX, USA, 1990. [Google Scholar]
- Kellermeier, M.; Picker, A.; Kempter, A.; Cölfen, H.; Gebauer, D. A straightforward treatment of activity in aqueous CaCO3 solutions and the consequences for nucleation theory. Adv. Mater. 2014, 26, 752–757. [Google Scholar] [CrossRef] [PubMed]
- Jolivet, J.-P.; Tronc, E.; Chanéac, C. Iron Oxides: From molecular clusters to solid. A nice example of chemical versatility. Comptes Rendus Geosci. 2006, 338, 488–497. [Google Scholar] [CrossRef]
- Baumgartner, J.; Faivre, D. Iron solubility, colloids and their impact on iron(oxyhydr)oxide formation from solution. Earth-Sci. Rev. 2015, 150, 520–530. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, W.G. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing; Academic Press: Boston, MA, USA, 1990; pp. 22–30. [Google Scholar]
- Gebauer, D.; Kellermeier, M.; Gale, J.D.; Bergström, L.; Cölfen, H. Pre-nucleation clusters as solute precursors in crystallization. Chem. Soc. Rev. 2014, 2348–2371. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scheck, J.; Lemke, T.; Gebauer, D. The Role of Chloride Ions during the Formation of Akaganéite Revisited. Minerals 2015, 5, 778-787. https://doi.org/10.3390/min5040524
Scheck J, Lemke T, Gebauer D. The Role of Chloride Ions during the Formation of Akaganéite Revisited. Minerals. 2015; 5(4):778-787. https://doi.org/10.3390/min5040524
Chicago/Turabian StyleScheck, Johanna, Tobias Lemke, and Denis Gebauer. 2015. "The Role of Chloride Ions during the Formation of Akaganéite Revisited" Minerals 5, no. 4: 778-787. https://doi.org/10.3390/min5040524





